Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.7 Texcoco Out./Nov. 2016
Animal science
Ovulation induction with male effect and a commercial energy tonic in prepubertal pelibuey ewes
1 Programa Educativo de Medicina Veterinaria y Zootecnia. Departamento de Agronomía. División de Ciencias de Vida. Campus Irapuato Salamanca. Universidad de Guanajuato. Exhacienda El Copal. km. 9. Carretera Irapuato-Silao. 3682. Irapuato, Guanajuato. (jahmarin@ugto.mx), (joseantonio@colpos.mx).
2 Ganadería, Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México.
3 Campus San Luis Potosí. Colegio de Postgraduados. 78622. Salinas de Hidalgo, San Luis Potosí México.
4 Campus Campeche. Colegio de Postgraduados. 24450. Champotón. Campeche.
5 Facultad de Agronomía, Universidad Autónoma de San Luis Potosí. km 14.5 Carretera San Luis Potosí, Matehuala, Ejido Palma de la Cruz, Soledad de Graciano Sánchez, San Luis Potosí. Apartado postal 32.
In animal reproduction, natural methods like male effect (ME) and the action of neuroexcitatory amino acids accelerate puberty and induce ovulation in ewe lambs. In this study it was found that the biostimulation of the ewe and the neurotransmitter action of amino acids of a commercial energy tonic (CET, Metabolase®) may change the pulse frequency of luteinizing hormone (LH) and induce ovulation in prepubertal ewes. The objective was to evaluate the ME and CET action to induce ovulation and characterize the preovulatory peak of LH in prepubertal Pelibuey ewes. The experimental design included two factors (CET and ME) on two levels each (without: wo, and with: w). The treatments were randomized to 46 ewes: T1 (n = 12) control group: ewes without CET and without ME (woCETwoME), T2 (n=12) ewes with CET and without ME (wCETwoME), T3 (n=10) ewes without CET and with ME (woCETwME) and T4 (n=12) ewes with CET and with ME (wCETwME). Ovarian activity (OA) and the ovulation rate (OR) were evaluated by laparoscopy. The characteristics of the preovulatory peak of LH were evaluated in response to ME to confirm the first ovulation. The action of the CET, the male effect as well as their interaction, did not increase (p>0.05) the number of ovarian follicles of different diameters. The male effect and its interaction with the energy tonic increased (p≤0.05) the OR (woCETwME: 0.70 and wCETwME: 0.75). The response of the preovulatory peak of LH was similar (p>0.05) in ewes with ME with or without CET (wCETwME: 91.7 % and woCETwME: 70.0 %). The male effect and its interaction with CET induce the preovulatory LH peak and the first ovulation in prepubertal Pelibuey ewes.
Key words: Biostimulation; neurotransmitters; Ovis aries; puberty; ovulation rate; LH
En la reproducción, los métodos naturales como el efecto macho (EM) y la acción de los aminoácidos neuroestimuladores aceleran la pubertad e inducen la ovulación en corderas. En el presente estudio se consideró que la bioestimulación del carnero y la acción neurotransmisora de los aminoácidos, en un reconstituyente energético comercial (REC, Metabolase®), podrían modificar la frecuencia de pulsos de la hormona luteinizante (LH) e inducir la ovulación en ovejas prepúebres. El objetivo fue evaluar el EM y la acción del REC para inducir la primera ovulación y caracterizar el pico preovulatorio de LH en ovejas Pelibuey prepúberes. El diseño experimental fue con un arreglo de dos factores (REC y EM) en dos niveles cada uno (sin: s; con: c) y los tratamientos fueron asignados al azar a 46 ovejas: T1 (n=12) testigo, ovejas sin REC y sin EM (sRECsEM); T2 (n=12) ovejas con REC y sin EM (cRECsEM); T3 (n=10) ovejas sin REC y con EM (sRECcEM); y T4 (n=12) ovejas con REC y con EM (cRECcEM). La actividad ovárica (AO) y la tasa ovulatoria (TO) se evaluaron por laparoscopía. Las características del pico preovulatorio de LH se evaluaron en respuesta al EM para confirmar la primera ovulación. La acción del REC, el efecto macho, así como su interacción, no aumentaron (p>0.05) la cantidad de folículos ováricos de diferente diámetro. El efecto macho y su interacción con el REC aumentaron (p≤0.05) la TO (sRECcEM: 0.70 y cRECcEM: 0.75). La respuesta del pico preovulatorio de LH fue similar (p>0.05) en las ovejas con EM con o sin REC (cRECcEM: 91.7 % y sRECcEM: 70.0 %). El efecto macho y su interacción con el REC inducen el pico preovulatorio de LH y la primera ovulación en ovejas Pelibuey prepúberes.
Palabras clave: Bioestimulación; LH; neurotransmisores; Ovis aries; pubertad; tasa ovulatoria
Introduction
The onset of puberty in ewes is characterized by the activation of the hypothalamic-pituitary-gonadal axis that precedes the cyclical ovarian activity (Amstalden et al., 2011). This can be modified by exogenous hormones to induce the preovulatory luteinizing hormone (LH) peak, ovulation and estrus (Letelier et al., 2011) or just to hasten its onset, synchronize and improve reproductive efficiency in prepubertal ewes (Abecia et al., 2012).
Reproductive events respond to environmental factors such as: photoperiod, nutrition, stress and biostimulation. Therefore, controlling some or all of these factors may allow sheep reproduction occur without applying exogenous hormones (Scaramuzzi and Martin, 2008). These factors may be based on the knowledge of socio-sexual and nutritional effects. (Scaramuzzi et al., 2014). The first set of effects describes as a sequence of physiological and endocrine events that occur to anestrous sheep in response to the pheromones of a sexually active ram (male effect). The outcome is ovulation and the formation of the corpus luteum (Hawken and Martin, 2012). Nutritional effects can be observed when supplementing the diet with neuroexcitatory amino acids (Wu, 2010), energy (Schneider et al., 2012) or offering a complete diet (Kara et al., 2010).
Nutritional mechanisms that regulate puberty onset involve the integration of metabolic sensors and effectors in the operation of the neurons that synthesize gonadotropin releasing hormone (GnRH) in the hypothalamus (Amstalden et al., 2011). This neuronal communication is carried out by neurotransmitters and increase with the supply of neuroexcitatory amino acids (Brann and Mahesh, 1995): arginine stimulates the secretion of LH in ewe lambs since it activates the hypothalamic-hypophysisgonadal axis during pubertal development (Recabarren et al., 1996); aspartate increases the secretion of LH and testosterone in males (Estienne et al., 2000); glutamate induces preovulatory LH and accelerates puberty and reproductive behavior onset (Mahesh and Brann, 2005). Glutamine, proline, and glycine are involved in regulating health, survival, growth, development, lactation and reproduction (Wu, 2010); these amino acids are also involved in gene expression, fertility, neurotransmission and immunity in animals (Wu, 2014).
Therefore, the objective of this study was to evaluate the response to the male effect and the action a commercial energy supplement has on inducing the first ovulation in prepubertal Pelibuey ewes as well as characterize their preovulatory LH peak.
Materiales and methods
Location of the study area
This study was conducted from October to December 2011 in the Sheep and Goat Reproduction Laboratory (LaROCa) at the Colegio de Postgraduados, Campus Montecillo, Texcoco, State of Mexico (19° 29’ N, 98° 53’ W, 2250 m altitude and Cb(wo)(w)(i’)g climate; these is temperate subhumid with summer rains, mean precipitation and temperature of 636.5 mm and 15.2 °C; García, 2004).
Experimental animals and handling
The study group consisted of 46 prepubertal Pelibuey ewes of 215±8.3 d age and weight of 24.40±3.54 kg. Each ewes daily consumed 2 kg of feed consisting of: 70 % milled oat hay, commercial supplement containing 15 % of crude protein and 2.9 Mcal metabolisable energy kg-1 (30 %), unrestricted access to mineral salts and water. They were weekly weighted in order to keep record and to know the weight of each ewe during their first ovulation.
Experimental protocol and treatments
The experimental design consisted of a two factor arrangement of two levels each (2 ́2): factor A, with (w) and without (wo) a commercial energetic tonic (CET, Metabolase®); factor B, with (w) and without (wo) male effect. The combinations resulted in the design of four treatments (T): T1 (n=12) control group: without CET and without the male effect (woCETwoME); T2 (n=12) ewes with a 100 mL CET every third day for 15 days and without ME (wCETwoME); T3 (n=10) ewes without CET and with the male effect for 56 h (woCETwME); and T4 (n=12) ewes with a 100 mL CET every third day for 15 d and the male effect for 56 h (wCETwME; Figure 1).

Figure 1 Protocol for ovulation induction in prepubertal Pelibuey ewes in response to the supply of the commercial energetic tonic (wCETwoME), response to the male effect (woCETwME) and the interaction of both factors (wCETwME) and the control group (woCETwoME).
The CET (Metabolase®; Schütze-Segen, Italy) contains in each 100 mL: L-carnitine (613.3 mg), thioctic acid (20 mg), pyridoxine (15 mg), cyanocobalamin (3 mg), acetyl methionine (2000 mg), L-arginine (240 mg), L-ornithine (153.2 mg), L-citrulline (120 mg), L-lysine (62.5 mg), glycine (150 mg), aspartic acid (150 mg), glutamic acid (150 mg), fructose (5000 mg) and sorbitol (8000 mg).
Seven weeks prior to the male effect stimuli (ME) and in order to potentiate it when the time arrived, all ewes were kept at a minimum distance of 100 m of the rams’s pen, avoiding visual, auditory and olfactory contact between them. After the seven weeks, sheep to be exposed to the ME were confined in an internal structure in the barnyard; this allowed the ewes and ram to have visual, olfactory and auditory contact, but no physical contact what so ever. The use of the structure facilitated the handling at the time of the ewe’s blood sampling. During the ME the exposed ram was replaced by a different one every 4 h for 56 h.
Ovarian activity Assessment
All ewes were explored by laparoscopy (Mellisho et al., 2006); at first, to diagnose the presence of the corpus luteum (CL) 2 d before the interaction with the ram, and repeated 9 d after in order to assess the response to the treatments (Figure 1).
Ovarian follicles were classified according to their approximate diameter (2 to 3 mm, 4 to 5 mm, and over 6 mm; Bartlewski et al., 2011). Ovulation rate (OR) was the total number of CL present in response to the treatments. The percentage of ovulating females was determined by laparoscopy and by the presence of the preovulatory LH peak.
Luteinizing hormone (LH) preovulatory peak determination
In ewes stimulated with the ME, 5 mL of blood were drawn via jugular puncture every 2 h, starting 6 h prior to the interaction with the ram, and finalizing in the 56th h in the ram’s presence (Figure 1). These, in order to observe the proportion of ewes that showed the preovulatory LH peak induced by the ME: 1) The beginning of the peak is the elapsed time since the ram introduction until the baseline average concentration of LH per ewe exceeded two standard deviations for 4 h (Van Cleeff et al., 1998); 2) The duration of the peak is the time elapsed from the beginning, or that remained for 4 h, and ended when the levels returned to baseline levels (Van Cleeff et al., 1998); 3) The peak amplitude is the difference between the maximum concentration of LH (ng mL-1) minus the basal concentration (BC) per ewe. The BC is the average of two samples: before and after the preovulatory peak (Mattioli et al., 1986). Blood samples were collected in 5 mL glass tubes, and centrifuged at 600 g for 15 min. Afterwards plasma was decanted into 5 mL plastic tubes and stored at -20 °C until the quantification of LH’s concentration was performed. The analysis was performed by radioimmunoassay, by means of the double antibody technique (Perera-Marin et al., 2005), which detects at least 0.03 ng mL-1 of LH and 5 % and 8 % of variation coefficients for the intraassay and the inter-assay.
Statistical analysis
Data was analyzed with the SAS® software (SAS Institute Inc., 2012). The number of ovarian follicles laparoscopically observed was compared with the NPAR1WAY procedure through the Kruskal-Wallis test. The ovulation rate was analyzed with the TTEST procedure through the paired t test. The duration and amplitude of the preovulatory peak was determined only in ewes subject to the ME, with a statistical analysis of repeated measurements for a complete design with randomized treatments done with the MIXED procedure (Littell et al., 1998); the sampling time was a fixed variable and the LH concentration the random variable.
Data of the sheep that presented the preovulatory LH peak was analyzed with a logistic regression with PROC LOGISTIC through the Wald test. Data of weight variation was analyzed with an ANOVA utilizing the MIXED procedure (Littell et al., 1998), LSMEANS was used to calculate the average of the minimum squares of the fixed effects, and ADJUST to arrange the multiple means test in pairs (Tukey, p≤0.05), with a mixed model in a 2 ́2 factorial arrangement, modified to observe the main effects and interactions in the evaluation period (weekly):
Yijkl=μ+Ai+Bj+(A*B)ij+dij+Pk+(A*P)ik+(B*P)jk +(A*B*P)ijk+Eijkl
where Yijkl: response variable, i: 1,2; j: 1,2; k: 1,2... 11; l: 1,2.; m: general average; Ai: effect of factor A, CET in i; Bj: effect of factor B, ME in j; Pk: effect of the period P in: dij: random error within main effects (treatments); (AB)ij (AP)ik, (BP)jk, (ABP)ijk, (AB)ij: effects of interactions; Eijkl: random error.
The weight of the sheep subject to ME during their first ovulation was determined through orthogonal contrast analysis with the GLM procedure.
Results and discussion
Ovarian follicles and ovulation rate
The action of the CET (wCETwoME), the ME (woCETwME) and their interaction (wCETwME) did not increase (p>0.05) the number of follicles of any size (2 to 3 mm, 4 to 5 mm, and bigger than 6 mm in diameter). The diameter of the majority of the follicles (p≤0.05) was between 4 and 5 mm and was observed in the control group (woCETwoME, Figure 2).

Figure 2 Ovarian follicles in prepubertal Pelibuey ewes in response to the action of the commercial energetic tonic (wCETwoME), the male effect (woCETwME), interaction of both factors (wCETwME) and control group (woCETwoME) ** p≤0.01.
Zavala-Elizarrarás et al. (2008) reported that the breed of the sheep has an impact in the number of ovarian follicles, but not in their follicular diameter or their ovulation rate. They reported 9.3 follicles of 1.5 mm in diameter and a 1.4 average ovulation rate in Pelibuey ewes. These values concurred with those reported by Pellicer-Rubio et al. (2013), but differed with the values obtained in prepubertal Pelibuey ewes in the present study. Perhaps, the response to the treatments that increase the number of ovarian follicles is related to the ovarian follicular development the female sheep presented at the beginning of the treatments. Due that follicular differentiation is observed at recruitment, where follicles that are sensitive to gonadotropins have 32 mm in diameter, and in the selection, 4 mm diameter ovarian follicles are dominant, and the others underwent atresia (Bartlewski et al., 2011).
The first laparoscopy showed that none of the ewes presented CL and the second showed tha: the CET (wCETwoME) did not increase the appearance of the CL (p>0.05); however the appearance of the CL did increase (p≤0.05) in ewes stimulated with ME (woCETwME) and with the combined treatment (wCETwME). These ewes had only one CL each; therefore, the ovulation rate was similar (p>0.05), but higher (p≤0.05) than the control group (woCETwoME) and those treated with CET (wCETwoME; Table 1).
Table 1 Quantity of corpora luteal present in prepubertal Pelibuey ewes in response to two levels of commercial energetic tonic and male effect.
| Tratamientos | n | Ovejas que ovularon (n) | Cuerpos lúteos (n) | Tasa ovulatoria |
| Ovejas testigo | 12 | 0 | 0 | 0.00 a |
| Ovejas con reconstituyente energético y sin “efecto macho” | 12 | 2 | 2 | 0.17 a |
| Ovejas sin reconstituyente energético y con “efecto macho” | 10 | 7 | 7 | 0.70 b |
| Ovejas con reconstituyente energético y con “efecto macho” | 12 | 9 | 9 | 0.75 b |
a, b: Values with distinct letter in a column are statistically different (p≤0.05).
Alcaraz-Romero et al. (2012) reported that 61.5% of prepubertal Pelibuey ewes ovulated with the male effect and presented ovulatory rates of 1.4±0.2. In contrast, findings of our study included 70 % of the ewes underwent ovulation due to the male effect (woCETwME) and presented an ovulatory rate of 0.7. When ovulation is induced with the ram presence, the luteal activity onset is also stimulated (Bartlewski et al., 2002). Therefore, in our study, the prepubertal Pelibuey ewes subject to ME, showed ovarian activity which in turn stimulated the production of GnRH and the secretion of the LH (Abecia et al., 2012). But, the similarity in the ovulation rate between the control group (woCETwoME) and the group treated only with CET without ME (wCETwoME) showed that its action is not enough to induce ovaria activity. This agrees with the similar effect in folliculogenesis and hormones concentrations of control ewes, as compared to those fed an extra 500 g d-1 of Lupinus luteus grains. (Somchit et al., 2007). But our result contrasts with the onset differences in puberty of ewes fed with a L-arginine supplement (Hamra et al., 2003), or the reproductive efficiency in female sheep that ingested more vitamins, minerals, neuroexitatory amino acids and sorbitol in their diet (Kara et al., 2010).
Pellicer-Rubio et al. (2013) found no a relationship between the male effect and follicular status on ewes in anestrous with ovulation capacity due to the ME. Hence, it is likely that the neuroexitatory capacity of glutamate, aspartate and arginine contained with the CET enhanced the response to the ME and it is showen in the ovulatory rate and in the induction of the preovulatory LH’s peak and ovulation itself (Estienne et al., 2000; Mahesh and Brann, 2005; Wu, 2010). The dosage and supply of each amino acid contained in CET as well as its individual use and function in stimulating ovarian activity in young ewes will be evaluated in subsequent studies (Wu, 2014).
Characterization of the preovulatory luteinizing hormone (LH) peak
The male effect (woCETwME) and its combination with CET (wCETwME) did not affect (p>0.05) the preovulatory LH peak characteristics (Table 2), the response rate or concentration (Figure 3) of preovulatory LH peak in prepubertal Pelibuey ewes.
Table 2 Characteristics of preovulatory LH peak in prepubertal Pelibuey ewes in response to the supply of commercial energetic tonic and male effect.
| Tratamiento | n | Pico (%) | Inicio† (h) | Ocurrencia† (h) | Duración† (h) | Amplitud† (ng mL-1) |
| Ovejas sin reconstituyente energético y con “efecto macho” | 10 | 70.0 | 12.0±4.4 | 21.1±3.3 | 11.5±1.0 | 17.6±0.8 |
| Ovejas con reconstituyente energético y con “efecto macho” | 12 | 91.7 | 15.8±2.8 | 23.2±2.6 | 11.2±1.8 | 17.5±0.8 |
There were no differences between treatments (p>0.05). †Mean ± standard error.

Figure 3 Quantity of LH in plasma of prepubertal Pelibuey ewes and to the interaction with male effect (woCETwME) in response to the effect of the commercial energetic tonic (wCETwME).
The sudden interaction of rams with nonovulating sheep modifies the sheep’s pulse frequency of GnRH and LH. The preovulatory LH peak occurs 3 to 30 h after the beginning of the interaction, and ovulation takes place between 24 and 60 h afterwards (Hawken and Martin, 2012). Knights et al. (2002) reported that all ewes exposed to the ram presented an LH’s preovulatory peak. This value resembles the results obtained in our study, and the similarity between treatments coincides with the characterization of preovulatory LH obtained by Camacho-Ronquillo et al. (2008); but differs from the values reported by Knights et al. (2002). The differences are because the ewes in anoestrous ovulate in response to the interaction with the male, even though the male effect does not stimulate the secretion of LH when the estrogen feedback is too strong (Martin and Cognie, 1984). The differences can also be attributed to the time of year when the LH’s peak was measured, because during breeding season a favorable response to male effect is expected (Chanvallon et al., 2011). Perhaps the continued presence of the ram is the main element that triggers the preovulatory LH peak, because if the ram is removed before the ewes ovulates, the frequency of the LH’s secretion pulse is reduced to baseline concentrations.
Body weight change
During the experimental period the male effect did not affect (p>0.05) the weight of the ewes, but the action of the CET and its interaction with male effect (wCETwME) influenced (p≤0.05) the weight change of the ewes (Table 3).
Table 3 Test for fixed effects and weekly interaction for weight change in Pelibuey ewes, in response to commercial energetic tonic and the male effect.
| Efecto | Periodo experimental (d) | p≤ | |||||||||||
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 63 | 70 | 77 | ||
| Reconstituyente energético*tiempo | NS | * | * | * | * | * | * | NS | * | ** | NS | ** | 0.0011 |
| “efecto macho” x tiempo | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.1237 |
| Interacción x tiempo | NS | NS | * | * | * | ** | * | NS | NS | ** | NS | * | 0.0041 |
NS non-significant (p>0.05); * p≤0.05; ** p≤0.01.
Ewes treated with the CET and male effect showed a higher weight gain than the control group (p≤0.05; wCETwME 25.0±0.68 kg; woCETwME: 22.2±0.68 kg). The ewes treated with male effect and CET during their first ovulation were weighted on the 63rd d of the experimental period and the weight was similar between treatments (p>0.05; woCETwME: 29.3 kg and wCETwME: 29.8 kg; Figure 4).
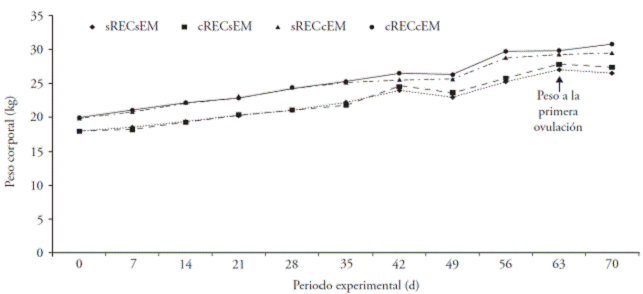
Figure 4 Body weight in prepubertal Pelibuey ewes in response to commercial energetic tonic (wCETwoME), the male effect (woCETwME), interaction of both factors (wCETwME) and control treatment (woCETwoME).
In our study, the weight on the first ovulation is similar to the 27.6±0.2 kg at the first CL reported by Zavala-Elizarrarás et al. (2008); but, differed from the 39 kg at first ovulation in ewes induced the preovulatory LH peak with male effect in reproductive season reported by Al-Mauly et al. (1991). Also, it was similar to the 31.2±0.7 kg at puberty in ewes receiving L-arginine (Hamra et al., 2003) and to the 32.5±1.4 kg in sheep subject to male effect (Alvarez and Andrade, 2008). These differences relate to nutrition and reproductive activity in ruminants and with variable and inconsistent evidence (Zavala-Elizarrarás et al., 2008).
Ewes received the same diet during the experiment; they did not show changes in body weight even though they underwent stressing techniques such as laparoscopy and frequent blood sampling. The conditions of animal handling may be considered stressful for reproductive events, in which weight loss and low body weight may be a consequence (Dobson et al., 2012). Therefore, the weight at puberty is considered as an indicative variable that is related to environmental factors, such as weather conditions, nutritional status, body condition and the stress level (Zavala-Elizarrarás et al.,
The differences found in the ovulation rate and absent in the ovaric activity of prepubertal Pelibuey ewes, in our study, may be associated with a major follicular atresia in ewes supplied with the CET and subject to male effect; that is the reason why we observed few follicles recruited and selected for growth, maturation and ovulation. Nevertheless, this hypothesis should be tested in future studies. But the response of ewes without CET and male effect served to compare the effects of the above treatments. The preovulatory LH peak occurred in sheep exposed to the ram, with or without CET supply. The handling of the sheep during laparoscopy and blood collection did not affect the body weight in the first ovulation of prepubertal Pelibuey CET.
Conclusions
The restorative action of the commercial energetic tonic, the male effect, and the interaction of both, do not increase the number of ovarian follicles of different diameter. But the male effect combined with commercial energetic tonic improves the ovulation rate, influences the response to the presentation of the preovulatory LH peak as well as the ovulation percentage.
The male effect does not cause weight fluctuation in prepubertal Pelibuey ewes; however, the combination of male effect and commercial energetic tonic during the evaluation period caused weight gain, though there is no effect in the weight at the first ovulation.
Literatura citada
Abecia, J. A., F. Forcada, and A. González-Bulnes. 2012. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 130: 173-179. [ Links ]
Alcaraz-Romero, R. A., J. A. Quintal-Franco, D. Hernández-Sánchez, T. Sánchez-Torres, E. Villagómez-Amezcua, J. Ramón-Ugalde, J. Baeza-Rodríguez, R. Bores-Quintero, and J. G. Cantón Castillo. 2012. Ovarian activity in F1 prepubertal ewe lambs under tropical conditions. Livest. Sci. 143: 24-28. [ Links ]
Al-Mauly, N. Z. N, M. J. Bryant, and F. J. Cunningham. 1991. Effect of the introduction of rams on the pulsatile release of luteinizing hormone and the onset of reproductive activity in ewe lambs. Anim. Prod. 53: 209-214. [ Links ]
Álvarez, L. and S. Andrade. 2008. The male effect reduces the age to first estrus and to ovulation in Pelibuey ewe lambs. Arch. Zoot. 57: 91-94. [ Links ]
Amstalden, M., B. R. C. Alves, S. Liu, R. C. Cardoso, and G. L. Williams. 2011. Neuroendocrine pathways mediating nutritional acceleration of puberty: insights from ruminant models. Front. Endocrinol. 2: 1-7. [ Links ]
Bartlewski, P. M., A. P. Beard, S. J. Cook, and N. C. Rawling. 2002. Ovarian activity during sexual maturation and following introduction of the ram to ewe lambs. Small Ruminant Res. 43: 37-44. [ Links ]
Bartlewski, P. M., T. E. Baby, and J. L. Giffin. 2011. Reproductive cycles in sheep. Anim. Rep. Sci. 124: 259-268. [ Links ]
Brann, D. W., and V. B. Mahesh. 1995. Glutamate: a major neuroendocrine excitatory signal mediating steroid effects on gonadotropin secretion. J. Steroid. Biochem. Molec. Biol. 53: 325-329. [ Links ]
Camacho-Ronquillo, J. C., J. C. Rodríguez-Castillo, J. E. Hernández-Hernández, A. Pro-Martínez, C. M. Becerril-Pérez y J. Gallegos-Sánchez. 2008. Características reproductivas de ovejas Pelibuey sincronizadas e inducidas a la pubertad. Arch. Lat. Prod. Anim. 16: 18-24. [ Links ]
Chanvallon, A., L. Sagot, E. Pottier, N. Debus, D. François, T. Fassier, R. J. Scaramuzzi, and C. Fabre-Nys. 2011. New insights into the influence of breed and time of the year on the response of ewes to the ‘ram effect’. Animal 5: 1594-1604. [ Links ]
Dobson, H., C. Fergani, J. E. Routly, and R. F. Smith. 2012. Effects of stress on reproduction in ewes. Anim. Rep. Sci. 130: 135-140. [ Links ]
Estienne, M. J., C. R. Barb, and R. R. Kraeling. 2000. Neuroendocrine regulation of reproduction in male domestic animal species: Role of excitatory amino acids. J. Anim. Sci. 77: 1-10. [ Links ]
García, E. 2004. Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana) 5a ed. Instituto de Geografía. UNAM. México. [ Links ]
Hamra, A. H., F. M. A. AI-Dabbas, and F. T. Awawdeh. 2003. Effect of arginine supplementation on puberty and some reproductive traits in female Awassi sheep. J. Agric. Inv. 20: 82-85. [ Links ]
Hawken, P. A. R., and G. B. Martin. 2012. Sociosexual stimuli and gonadotropin-releasing hormone/luteinizing hormone secretion in sheep and goats. Dom. Anim. Endocrinol. 43: 85-94. [ Links ]
Kara, Ç., A. Orman, E. Topal, and E. Çarkungöz. 2010. Effects of supplementary nutrition in Awassi ewes on sexual behaviors and reproductive traits. J. Biol. Environ. Sci. 4: 15-21. [ Links ]
Knights, M., Q. S. Baptiste, and P. E. Lewis. 2002. Ability of ram introduction to induce LH secretion, estrus and ovulation in fall-born ewe lambs during anestrus. Anim. Rep. Sci. 69: 199-209. [ Links ]
Letelier, C. A., I. Contreras-Solis, R. A. García-Fernández, M. A. Sánchez, P. García-Palencia, B. Sánchez, C. Ariznavarreta, J. A. F. Tresguerres, J. M. Flores, and A. Gonzalez-Bulnes. 2011. Effects of oestrus induction with progestagens or prostaglandin analogues on ovarian and pituitary function in sheep. Anim. Rep. Sci. 126: 61-69. [ Links ]
Littell, R. C., P. R. Henry, and C. B. Ammernan. 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76: 1216-1231. [ Links ]
Mahesh, V. B., and D. W. Braan. 2005. Regulatory role of excitatory amino acids in reproduction. Endocrine 28: 271-280. [ Links ]
Martin, G. B., and Y. Cognie. 1984. Induction of LH secretion and ovulation in anoestrous Romanov ewes by the introduction of rams. Appl. Anim. Behav. Sci. 13: 174. [ Links ]
Mattioli, M., F. Conte, G. Giovanna, and E. Seren. 1986. Effect of naloxone on plasma concentration of prolactin and LH in lactating sows. J. Rep. Fert. 76: 167-173. [ Links ]
Mellisho, S. E., H. R. Pinazo, F. L. Chauca, V. P. Cabrera, y P. V. Rivas. 2006. Inseminación intrauterina vía laparoscópica de ovejas Black Belly con semen congelado. Rev. Invest. Vet. Perú. 17: 131-136. [ Links ]
Pellicer-Rubio, M. T., J. L. Touzéa, G. Baril, and B. Malpaux. 2013. The luteal outcome of anoestrus ewes induced to ovulate by the male effect is not related to the population of ovarian antral follicles before male exposure. Anim. Rep. Sci. 137: 23-30. [ Links ]
Perera-Marín, G., C. Murcia, S. Rojas, J. Hernández-Cerón, and E. González-Padilla. 2005. Pattern of circulating luteinizing hormone isoforms during the estrous and luteal phases in Holstein heifers. Anim. Rep. Sci. 86: 53-69. [ Links ]
Recabarren, S. E., A. Jofré, A. Lobos, P. Orellana, and J. Parilo. 1996. Effect of arginine and ornithine infusions on luteinizing hormone secretion in prepubertal ewes. J. Anim. Sci. 74: 162-166. [ Links ]
SAS Institute Inc. 2012. SAS user ́s Guide Statistics, version 9.4 (TS1MO). Cary, N. C. USA. [ Links ]
Scaramuzzi, R. J., andG. B. Martin. 2008. The importance of interactions among nutrition, seasonality and socio-sexual factors in the development of hormone-free methods for controlling fertility. Reprod. Domest. Anim. 43: 129-136. [ Links ]
Scaramuzzi, R. J., L. Oujagir, J-B. Menassol, S. Freret, A. Piezel, H. M. Brown, J. Cognié, andC. Fabre-Nys . 2014. The pattern of LH secretion and the ovarian response to the ‘ram effect’ in the anoestrous ewe is influenced by body condition but not by short-term nutritional supplementation. Reprod. Fertil. Dev: 26: 1154-1165. [ Links ]
Schneider, J. E., C. M. Klingerman, and A. Abdulhay. 2012. Sense and nonsense in metabolic control of reproduction. Front. Endocrinol (Lausanne). 26: 1-21. [ Links ]
Somchit, A., B. K. Campbell, M. Khalid, N. R. Kendall, andR. J. Scaramuzzi. 2007. The effect of short-term nutritional supplementation of ewes with lupin grain (Lupinus luteus), during the luteal phase of the estrous cycle on the number of ovarian follicles and the concentrations of hormones and glucose in plasma and follicular fluid. Theriogenology 68: 1037-1046. [ Links ]
Van Cleeff, J., F. J. Karsh, and V. Padmanabhan. 1998. Characterization of endocrine events during the periestrous period in sheep after oestrous synchronization with controlled internal drug release (CIDR) device. Domest. Anim. Endocrin. 15: 23-24. [ Links ]
Wu, G. 2010. Functional amino acids in growth, reproduction, and health. Am. Soc. Nutr. Adv. Nutr. 1: 31-37. [ Links ]
Wu, G. 2014. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 5: 1-12. [ Links ]
Zavala-Elizarraráz, R., J. R. Ortiz-Ortiz, J. P. Ramón-Ugalde, P. M. Morales, A. Sierra-Vásquez, y J. R. Sanginés-García. 2008. Pubertad en hembras de cinco razas ovinas de pelo en condiciones de trópico seco. Zoot. Trop. 26: 465-473. [ Links ]
Received: July 2015; Accepted: April 2016





![Ocurrencia de micotoxinas en alfalfa (Medicago sativa L.), sorgo [Sorghum bicolor (L.) Moench] y zacate (Cenchrus ciliaris L.) en venta al menudeo en el estado de Nuevo León, México](/img/pt/next.gif)





 texto em
texto em 


