Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.50 no.6 Texcoco ago./sep. 2016
Crop Science
Iodine increases the growth and mineral concentration in sweet pepper seedlings
1Edafología y Estadística. Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México. México. (marinie@colpos.mx),
2Estrategias para el desarrollo agrícola regional (EDAR). Campus Puebla. Colegio de Postgraduados. México.
3Horticultura. Universidad Autónoma Agraria Antonio Narro. Saltillo Coahuila, México.
Iodine is a non-essential element for plants, despite inducing positive responses in their growth and metabolism. The aim of this study was to determine the feasibility of the use of iodine (as KI) via the leaves to promote growth of sweet pepper (Capsicum annum) seedlings. The hypothesis was that applying this element will increase the growth rate and the content of antioxidants in seedlings. A completely randomized experimental design was carried out with six treatments (0, 10, 15, 30, 45, 50 μM KI L-1) and three repetitions; the experimental unit was one tray with 50 cavities. Data were analyzed with an ANOVA and averages were compared with a Tukey test (p≤0.05). Four applications of KI were performed on the leaves: the first application when the first leaves appeared; the second one, 7 d later; and the third and fourth, in 14 d intervals. When taking samples, 10 seedlings were harvested for every treatment, in which their height was measured, along with the stem diameter, foliar area, and dry weight. Using these data, we calculated the growth indices: net assimilation rate, absolute growth rate, relative growth rate, and specific foliar area. The antioxidant activity and concentration of iodine and other mineral elements were also measured. Results showed significant increases in height, stem diameter, and dry weight with the application of 10 and 15 μM of KI. Antioxidant activity and iodine concentration in seedlings was proportional to the concentration of KI applied, whereas the concentration of other elements responded positively only with 10 and 15 μM of KI.
Key words: Seedlings; growth indices; sweet pepper; nurserybeds
El yodo es un elemento no esencial para las plantas pero induce respuestas positivas en el crecimiento y metabolismo. El objetivo de este estudio fue determinar la factibilidad de uso del yodo (como KI) por vía foliar para promover el crecimiento de las plántulas de pimiento morrón (Capsicum annum). La hipótesis fue que la aplicación de este elemento elevará la tasa de crecimiento y el contenido de antioxidantes en las plántulas. El diseño experimental fue completamente al azar con seis tratamientos (0, 10, 15, 30, 45, 50 μM KI L-1) y con tres repeticiones; la unidad experimental fue una charola de 50 cavidades. Los datos se analizaron con un ANDEVA y las medias se compararon con la prueba de Tukey (p≤0.05). Las aplicaciones de KI por vía foliar fueron cuatro: la primera al aparecer las primeras hojas, la segunda 7 d después, y la tercera y cuarta en intervalos de 14 d. En los muestreos se cosecharon 10 plántulas por tratamiento y se midió altura de plántula, diámetro de tallo, área foliar y peso seco. Con esos datos se calcularon los índices de crecimiento: tasa de asimilación neta, tasa de crecimiento absoluto, tasa de crecimiento relativo y área foliar específica. También se midieron la actividad antioxidante, la concentración de yodo y otros elementos minerales. Los resultados mostraron aumentos significativos en la altura, diámetro de tallo y peso seco con la aplicación de 10 y 15 μM de KI. La actividad antioxidante y la concentración de yodo en las plántulas fue proporcional a la concentración de KI aplicado, mientras que la concentración de otros elementos tuvo respuesta positiva sólo con 10 y 15 μM de KI.
Palabras clave: Plántulas; índices de crecimiento; pimiento morrón; almácigos
Introduction
Iodine has not yet been found to have a specific metabolic function on terrestrial plants (Kabata-Pendias, 2011). However, iodine induces a greater presence of antioxidants in plants, providing an increase in tolerance to some adverse factors (Blasco et al., 2008; Blasco et al., 2011; Gupta et al., 2015). In marine species such as Laminaria digitata (Kelp) the tolerance induction process is relatively well understood, and the cell accumulation or volatilization of this element towards the atmosphere is related to the level of oxidative stress. Under conditions of high production of reactive oxygen species (ROS), iodine is volatilized, whereas with a low ROS concentration, iodine is stored for availability in case stress increases (Kupper et al., 2008). Venturi (2011) proposed the hypothesis that iodine was one of the first antioxidants used by photosynthetic organisms. According to La Barre et al. (2010), iodine is used by sea algae as an antioxidant during oxidative stress, which could be similar in terrestrial plants. However, the use of iodine in agricultural practice has been scarcely investigated.
The positive effects of the application of iodine in terrestrial plants are maintained when applying it in low concentrations and toxicity occurs when applying iodine in plants above certain concentrations (Caffaggni et al., 2011; Landini et al., 2011). These thresholds of benefit and toxicity are variable, depending on the species (Kabata-Pendias, 2011).
In Mexico, the sweet pepper (Capsicum annuum L.) is the fowrth most important crop (SIAP, 2010). Applying iodine on this crop could bring advantages such as an increase in the concentration of iodine in the plants, for biofortification purposes and improving their capacity of tolerance to strees caused by the increase in antioxidant capacity. However, there is no available information published about responses of sweet pepper plants to the applications of this element. Therefore studies are necessary to provide answers on the growth responses and nutritional quality of the due to iodine applications.
The aim of this study was to determine the feasibility of the use of iodine via the leaves to promote the growth of sweet pepper seedlings. The hypothesis was that the application of this element will increase the growth rate and the content of antioxidants in the seedlings.
Materials and methods
Plant material and growth conditions
The experiment was carried out in campus Montecillo, Colegio de Postgraduados, Montecillo, Estado de Mexico. The seedbeds for the seed germination and plantlet growth were established in a controlled environment chamber, with temperatures between 23 and 25 °C and a relative humidity of 50 %, with a light period of 14 h (fluorescent lamps) and 10 h of darkness. For the seedbeds, we used plastic trays with 50 cavities. Each tray was filled with the substrate peat-moss Sungro®; one seed was placed in each cavity and covered with a thin layer of vermiculite. The sweet pepper variety planted was Sidenca, of Enza Zaden company. Irrigation was carried out daily using a hand-held sprinkler to maintain the humidity of the substrate. Nutrition was provided wit Steiner solution at 25 % [0.147 mM Ca(NO3)2, 0.034 mM KH2 PO4, 0.076 mM K2 SO4, 0.176 mM KNO3, 0.068 mM MgSO4·7 H2O, micronutrients, pH 5.5; it began once the first true leaves appeared, 7 d later, the concentration was increased to 50 % and 7 d later, to 75 % until transplant.
Foliar application of iodine
When seedlings presented the first true leaves, the foliar applications with iodine were performed, with intervals of 14 d. The treatments were 10, 15, 30, 45, and 50 μM KI L-1 and a control (0 iodine), with three repetitions. Foliar applications were sprayed using a low-volume spray outside the growth chamber to avoid contamination between treatments. The experimental unit was a tray with 50 cavities, and the experimental design was completely randomized.
Measurement of variables during crop development
Samples were obtained 7 d after each foliar application of iodine, collecting 10 seedlings per experimental unit to measure: plan height (cm), from point zero on the surface of the substrate to the apex; stem diameter (mm), with a digital Truper brand vernier; foliar area (cm2), obtained by planimetry (scanning and analysis of images and estimation from the length and width of leaf), using the method reported by Melgarejo et al. (2011). Seedlings were dried for 72 h in a continuous circulation oven at 70 °C and weighed to obtain dry weight in g.
Determining growth indixes
Data on dry matter (DM) and foliar area were used to determine growth indixes for sweet pepper: net assimilation rate (TAN= LnA2-LnA1/ t2-t1); absolute growth rate (TCA= P2-P1/t2-t1); relative growth rate (TCR= LnP2-LnP1/t2-t1); specific leaf area (AFE= AF/ leaf biomass). Ln A= natural foliar area logarithm (cm2); t= time (d); P= total dry weight (g); Ln P= natural logarithm of total DM (g) (Amaro et al., 2004).
Determining total antioxidant activity (DPPH method)
The total antioxidant activity was determined using the DPPH method (Scherer and Texeira 2009). The reaction is based on a reduction in color that takes place when the electron of a nitrogen atom in DPPH is reduced by receiving a hydrogen atom from the antioxidant compounds. In 10 repetitions per treatment, the plant material was softened. Once it was homogenized, 100 mg were weighed and placed in 2 mL microtubes. We added 1.5 mL of ethanol at 60 % and let it settle fro 24 h. Samples were centrifuged for 15 min at 9 000 rpm, 400 mL of the supernatant were taken from each sample and placed in a 2 mL microtube toadd600mLofmethanolat80%and1mLoftheDPPH solution. The samples were settled for 15 min and a reading was taken on the spectrophotometer at 517nm. The reading of each sample was repeated after 30 and 60 min. The results are expressed as a % of the DPPH inhibited.
Content of iodine and minerals content in seedlings
The iodine content was determined using the alkaline ash technique (Fisher et al. 1986). The ten seedlings sampled for each treatment were placed in a continuous circulation oven at 70 °C for 72 h, ground, and 0.5 g of the dry plant tissue were weighed. The samples were placed in porcelain crucibles, KOH and KNO3 were added, the crucibles were placed in a muffle at 580 °C for 3 h, they were then cooled at room temperature and the ashes were transferred to conical flasks for extraction with KOH. The ashes of the tubes were centrifuged at 7200 rpm for 15 min. Finally, 1 mL of the supernatant decanted and graduated at 10 mL with KOH 2M and readings were taken for quantification of iodine in the inductively coupled plasma optical emission spectrometry (ICP-OES, Varian 725-OES, Australia).
The contents of P, K, Ca, Mg, S, B, Cu, Fe, Mn, and Zn in the aerial section were analyzed using the humid digestion technique and read in the ICP, and N was determined using the Kjeldahl method (Bremner, 1965).
Results and discussion
Measuring crop development
The sweet pepper seedlings showed a significant response (p≤0.05) to the foliar iodine applications for plant height, stem diameter, foliar area, and DM for each treatment. Height increased with the foliar iodine application (10 μM L-1), and it was different to the other the treatments (Table 1; p≤0.05). The results show that increasing the concentration of iodine reduced plant height and, at the highest concentrations, the edges of the leaves displayed symptoms of toxicity. The positive response in plant height could be due to an antioxidant effect of iodine, which would help the plant adapt to the growth environment. This antioxidant effect was mentioned by Venturi (2011), although it depends on the concentration of iodine in the growth medium, since it is toxic at a higher concentration (Caffaggni et al., 2011; Landini, et al., 2011). The same response was found in onion (Allium cepa L.), carrot (Daucus carota L.), celery (Apium graveolens L.), and spinach (Spinacea oleracea L.); with iodine applications higher than 5 μM L-1, and with 40 μM L-1 or more, plants were smaller and with symptoms of toxicity (Jiu et al., 2004).
Table 1 Effect of six doses of KI applied on the foliage of sweet pepper seedlings on plant height, stem diameter, foliar area, and production of dry matter.
| Aplicación foliar | Altura de plántula (cm) | Diámetro de tallo (mm) | Área foliar (cm 2 ) | Materia seca (g) |
|---|---|---|---|---|
| Testigo 0 μM | 12.81 c | 2.319 c | 68.43 c | 0.92 d |
| Yodo 10 μM | 17.63 a | 3.056 a | 93.06 a | 1.86 a |
| Yodo 15 μM | 14.06 b | 2.675 b | 74.79 b | 1.23 c |
| Yodo 30 μM | 14.03 b | 2.713 b | 76.82 b | 1.25 c |
| Yodo 45 μM | 14.55 b | 2.790 b | 78.61 b | 1.28 c |
| Yodo 50 μM | 13.02 c | 2.073 d | 55.59 d | 1.52 b |
| R2 | 0.8275 | 0.8486 | 0.9474 | 0.9492 |
| C.V. | 5.2535 | 5.5623 | 5.2077 | 5.4078 |
| DMS | 0.6627 | 0.2175 | 6.242 | 0.0992 |
Means with different letters are statistically different (p≤0.05). R2: Coefficient of determination; C.V.: Coefficient of variation; SMD: Significant minimum difference.
Stem diameter increased up to 32 % with respect to the foliar application of 10 μM L-1 (Table 1), and 20 % with 45 μM L-1. The thinnest stem was observed in seedlings with the highest iodine doses, along with burns on some leaf edges.
The foliar area was maintained in seedlings with iodine applications of medium concentrations (10 to 45 μM L-1) (Table 1). The application of 50 μM L-1 produced plants with smaller leaves and some symptoms of toxicity. The same trend is reported in the biofortification of lettuce (Lactuca sativa) with seven concentrations (10, 20, 30, 40, 80, 160, and 240 μM L-1 KI), since there is a reduction in plant growth and biomass production with doses greater than 20 μM L-1 of KI, and reduction is most notorious with 40 μM L-1 KI. The toxic effect of iodine in high concentrations is described for various species (Caffaggni et al., 2011; Landini, et al., 2011; Zhu and Liu, 2003), yet it is not properly understood. It is likely that the excess iodine interferes with the metabolism and cellular signaling of the ROS, causing negative responses on growth and other processes.
Dry matter was promoted when applying 10 μM L-1 of iodine on the leaves; the control seedlings showed the lowest dry weight (Table 1), and with intermediate doses, the values were lower. Jiang et al. (2001) report similar results, as well as a phytotoxic effect on plant growth, and it may be due to an excessive accumulation of iodine in the plant tissues, or that once absorbed, intracellular oxidation to I2 can occur, producing an inhibition of the photosynthetic process, which reflects on the growth of the plant.
Growth indices
Andrews et al. (2001) and Raven et al. (2005) indicate that levels of radiation, photoperiod, water and nutrients have a direct influence on the accumulation of DM and foliar area, which are determining factors in growth indices. And in leaves carbohydrates are synthesized, which will be distributed to plant organs (Amaro et al., 2004).
Applying iodine modified the dynamics of DM accumulation, changing the values of growth indices (Table 2). Data (Table 1) indicate that the 10 μM iodine treatment displayed the highest dry weight, but data shown in Table 2 indicate that the dynamic behavior of the accumulation was different in each case. According to these results, iodine application in different concentrations modify the strategy of the biomass distribution, its production efficiency and how it is used to create photosynthetic structures.
Table 2 Growth indices in sweet pepper seedlings with six foliar applications of KI.
| Aplicación foliar | TAN g cm 2 d -1 | TCA g d -1 | TCR g g -1 d -1 | AFE Cm 2 g -1 |
|---|---|---|---|---|
| Testigo 0 μM | 0.00011 d | 0.0385 c | 0.0101 a | 32.037 b |
| Yodo 10 μM | 0.00055 d | 0.0378 c | 0.0471 a | 19.441 a |
| Yodo 15 μM | 0.0359 c | 0.0427 b | 0.0424 f | 19.111 c |
| Yodo 30 μM | 0.0419 b | 0.0603 a | 0.0485 d | 24.861 c |
| Yodo 45 μM | 0.0514 a | 0.0616 a | 0.0086 e | 25.178 c |
| Yodo 50 μM | 0.00081 d | 0.0182 d | 0.0073 c | 14.906 d |
| R2 | 0.9991 | 0.9843 | 0.9988 | 0.9731 |
| C. V. | 3.0605 | 4.5303 | 2.4295 | 3.7328 |
| DMS | 0.0009 | 0.0026 | 0.0009 | 1.0337 |
Means with different letters are statistically different (p≤0.05); TAN: Net assimilation rate; TCA: Absolute growth rate; TCR: Relative growth rate; AFE: Specific leaf area; R2: Coefficient of determination; C.V.: Coefficient of variation; SMD: Significant minimum difference.
The TAN values in Table 2 indicate, for each treatment, the dry weight produced per unit of foliar area. Treatments with 10 and 15 μM produced less biomass per unit of foliar area, but they have the highest foliar area (Table 1) and accumulated a greater amount of dry biomass. The iodine treatments with 30, 45, and 50 μM were more effective than the control for biomass accumulation per unit of foliar area, and they accumulated more dry weight than the control. Changes in TAN indicate morphological, physiological, and biochemical modifications related to carbon metabolism (Shipley, 2002). In marine plants, iodine has a significant effect on the photosynthetic metabolism (Kupper et al., 2008), although we have no information available on this subject regarding terrestrial plants.
The results for TAC and TCR (Table 2) show a complex behavior towards iodine concentrations. The high TCA values in treatments with 30 and 45 μM, which did not have the highest final dry biomass (Table 1), show that seedlings did not grow uniformly, but rather had a combination of slow and rapid growth periods, which could identify conflicts between supply and demand. Nevertheless, they presented a greater biomass than the control. TCR was highest for treatments with 10, 15, and 30 μM, indicating that the highest efficiency in biomass accumulation took place with the intermediate iodine concentrations.
The data for AFE (Table 2) indicate the changes induced by the concentration of iodine in the strategy of the plant to distribute the dry biomass. AFE is an indicator of how the plant accumulates dry biomass in the foliar structures. The low AFE values in treatments with 10 and 15 μM show that plants had a high density of photosynthetic structures, evident due to the large amount of mass per unit of foliar area, as compared to control and the application of 30 and 45 μM KI. Potentially, this characteristic would relate to a higher efficiency of radiation absorption PAR per unit of foliar area. (Yao et al., 2016).
It is difficult to explain the above results in depth without more biochemical, anatomic, and genomic information. However, the results show a direction for research about the effect of iodine on crop plants.
Total antioxidant activity
Iodine applications, as potassium iodide, increase antioxidant capacity of plants tissues. In Figure 1 it is shown that all KI concentrations increased up to 12 % DPPH inhibition, even with the highest KI concentrations, which caused reductions in plants height as well as some toxicity symptoms. This finding is similar to that observed in lettuce leaves treated with KI doses lower than 40 μM (Leyva et al., 2011). In another experiment with lettuce, 10 to 40 μM does caused a clear increment of total antioxidant activity, mainly due to an increase in levels of ascorbic acid, in its reduced form, and this molecule has a high capacity to donate electrons and directly neutralize free radicals in enzymatic and non enzymatic reactions (Blasco, 2010). The antioxidant response obtained with these treatments is relevant because it increases plants tolerance to stress conditions (Stevens et al., 2008).
Content of iodine and minerals in the aerial section of seedlings
The concentration of iodine in sweet pepper seedlings was directly proportional to the foliar dose applied (Figure 2). There was a considerable increase in iodine concentration in treatment with 45 μM L-1, and threefold with 50 μM L-1 with regard to the treatments.
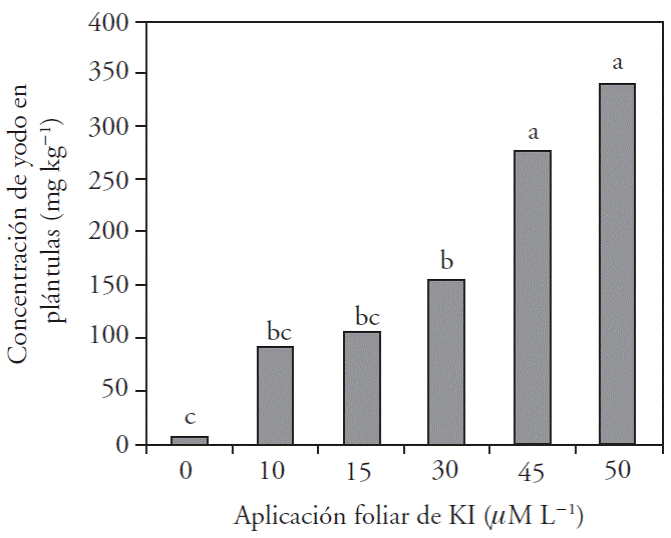
Figure 2 Concentration of iodine in sweet pepper sedlings tissues with foliar application of six KI doses.
The results of our study show that iodine absorption by sweet pepper seedlings is directly proportional to the concentrations applied and there is a similar trend for iodine in the aerial section and total antioxidants. These results are similar to those reported by Ujowondu et al. (2010) who biofortified different species of vegetables, native to Nigeria, with iodine and there was a higher concentration of iodine in plant tissues when the doses of potassium iodate or iodide increased in their treatments.
Jiu et al. (2006) point out that iodine concentration increases in leaves and roots of spinach when increasing the doses of potassium iodate and iodide. Besides, Zhu et al. (2003) report that the accumulation of iodine in foliar tissue of spinach is directly proportional to doses of iodine supplied.
For the nutritional concentration of the aerial section, results show that the application of iodine increased nitrogen concentration by an average of 5 %, except with 45 μM L-1, which reduced the amount of N, possible due to the effect of toxicity (Figure 3).
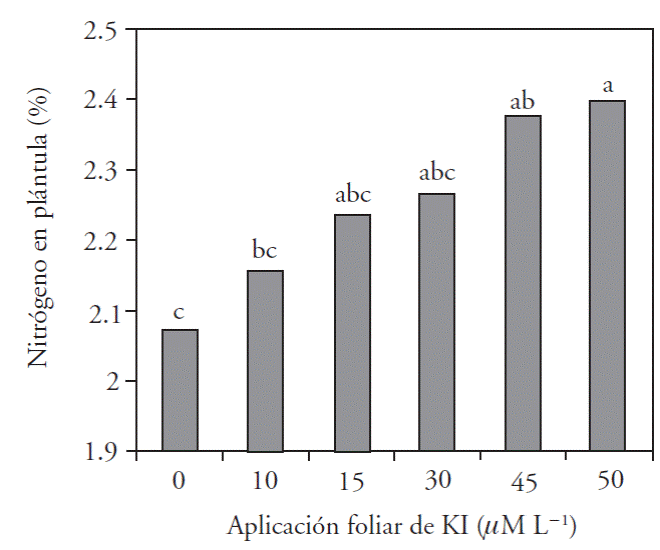
Figure 3 Concentration of total nitrogen in sweet pepper seedlings tissues with foliar application of six KI doses.
The lowest concentration of P, K, Ca, Mg, and S (Cuadro 3), was found in control plants. This chart shows that applying iodine in low and medium doses increase the concentrations of the elements quantified. Blasco et al. (2011) indicate that applying iodine on lettuce in doses of no more than 40 μM L-1 promotes nutritional quality without phytotoxic effects, and therefore iodine is considered a beneficial element for plants and can be used in these doses. Jiu et al. (2004), in a study of the effect of residual iodine in the soil, mentions that low concentrations of this element promote absorption and the use of essential elements in an important number of plant species.
Table 3 Minerals concentrations in aerial parts of sweet pepper seedlings with six foliar applications of KI.
| Aplicación foliar | P | K | Ca mg kg -1 | Mg | S |
|---|---|---|---|---|---|
| Testigo 0 μM L-1 | 517 a | 3593 a | 5715 b | 5874 | 1860 |
| 10 μM L-1 | 1288 a | 4371 a | 12973 ab | 9648 | 2347 |
| 15 μM L-1 | 1163 a | 4599 a | 16599 a | 9525 | 2210 |
| 30 μM L-1 | 1134 a | 4072 a | 12630 | 8882 | 2101 |
| 45 μM L-1 | 990 a | 4473 a | 12184 | 8549 | 2085 |
| 50 μM L-1 | 846 a | 3902 a | 12030 | 8005 | 1897 |
| R2 | 0.4932 | 0.1426 | 0.6358 | 0.3890 | 0.6227 |
| C.V. | 20.053 | 11.6432 | 19.8691 | 13.870 | 15.541 |
| LSD | 484.14 | 1199.9 | 6507.20 | 2952.5 | 747.48 |
Results (Table 2) indicate that there is an optimum dose of iodine application on leaves, of 10 to 15 μM L-1, since it promotes nutrient absorption. Besides, the results on the increase of foliar area by iodine absorption show that iodine promotes growth and minerals absorption in sweet pepper seedlings.
Conclusions
Foliar application of iodine as KI, on leaves of sweet pepper seedlings, improves minerals absorption, the amount and activity of antioxidants, as well as vigor of seedlings. Therefore addition of iodine in the production of seedbeds allows to obtain plant material of a higher quality in grafting.
Literatura citada
Amaro J. A., E. L. García y J. F. Henríquez. 2004. Análisis del crecimiento, área foliar específica y concentración de nitrógeno en hojas del pasto “mulato” (Brachiaria hibrido, cv.). Tec. Pecu. Mex. 42: 447-458. [ Links ]
Andrews M., J. A. Raven, and J. I. Sprent. 2001. Environmental effects on dry matter partitioning between shoot and root of crop plants: relations with growth and shoot protein concentration. Ann. Appl. Biol. 138: 57-68. [ Links ]
Blasco B. 2010. Biofortificación con yodo en plantas de lechuga (Lactuca sativa L.). implicaciones fisiológicas y nutricionales. Universidad de Granada. España. pp: 70-75. [ Links ]
Blasco B., J. Ríos J., L. Cervilla M., Sánchez-Rodríguez E., J. Ruiz M., and Romero L 2008. Iodine biofortification and antioxidant capacity of lettuce: potential benefits for cultivation and human health. Ann. Appl. Biol. 152: 289-299. [ Links ]
Blasco B. , J. Ríos J., Leyva R., L. Cervilla M., Sánchez, R. E., Rubio-Wilhelmi, Rosales M. M., M. Ruiz, and J. M. Romero. 2011. Does iodine biofortification affect oxidative metabolism in lettuce plants? Biol. Trace Elem. Res 142: 831-842. [ Links ]
Bremner J. M. 1965. Total nitrogen. Agronomy 9: 1149-1178. [ Links ]
Caffagni, A., Arru, L., Meriggi, P., Milc, J., Perata, P. & Pecchioni, N. 2011. Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun. Soil Sci. Plant Anal. 42: 706-718. [ Links ]
Fisher P. W. F., L’. Abbe M. R, and Giroux A. 1986. Colorimetric determination of total iodine in foods by iodide-catalyzed reduction of Ce+4 J. Assoc. Official Anal. Chem. 69: 687-689. [ Links ]
Gupta, N., Bajpai, M., Majumdar, R. & Mishra, P. 2015. Response of iodine on antioxidant levels of Glycine max L. grown under Cd+2 stress. Advan. Biol. Res. 1: 40-48. [ Links ]
Jiang X. M., X. Y. Cao, and J. Y. Jiang, 2001. Four-year experience in iodination of irrigation water in Hotien, Xinjiang province of China, Arch. Environ. Health 152: 399-408. [ Links ]
Jiu-Lan D., Z. Yong Guan, Min-Zhang, and Yi-Zhong H. 2004. Iodine-enriched selecting vegetables and the residual effect of iodate application to soil. Research Center for Eco-environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China; and College of Environment and Resource, Shandong Agricultural University, Tailan 271018, China. [ Links ]
Jiu-Lan D., Z. Yong-Guan, Min-Zhang, and Yi-Zhong H. 2006. Availability of iodide and iodateto spinach (Spinacia oleracea L.) in relation to the total iodine in soil solution. Plant Soil 289: 301-308. [ Links ]
Kabata-Pendias, A. 2011. Trace Elements in Soils and Plants (4th ed.) CRC Press, Taylor and Francis Group. New York. pp: 397-401. [ Links ]
Kanshe, F 1999. Iodine deficiency disorders: In: encyclopedia of human nutrition. Academic Press. London. pp: 1136-1153. [ Links ]
Kupper, F. C., Carpenter, L., Mcfiggans, G., Palmere, G., Waiteh, T., Bonebergb, E., Woitsch, S., Weiller, M., Abela, R., Grolimund, D., Potin, P., Butler, A., Luther, G., Kroneck, P., Meyer-Klaucke, W. & Feiters, M.C. 2008. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. PNAS. 105: 6954-6958. [ Links ]
La Barre, S., Potin, P. , Le blanc, C. & Delage, L. 2010. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs. 8: 988-1010. [ Links ]
Landini M., Gonzali S., and Perata P. 2011. Iodine biofortification in tomato. J. Plant Nutr. Soil Sci 174: 480-486. [ Links ]
Leyva, R., Sánchez-Rodríguez E. , Ríos J., Rubio-Wilhelmi M., Romero L ., Ruiz J. M., and Blasco B. 2011. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 181: 195-202. [ Links ]
Melgarejo M. L., Barrera J., y Suárez D. 2011. Experimentos en fisiología vegetal. Laboratorio de fisiología y bioquímica vegetal. Departamento de Biología. Universidad Nacional de Colombia. [ Links ]
Raven J. A., M. Andrews., and A. Quigg. 2005. The Evolution of Oligotrophy: Implications for the breeding of crop plants for low input agricultural systems. Ann. Appl. Biol. 146: 261-280. [ Links ]
Scherer R., and G. H. Teixeira 2009. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 112: 654-658. [ Links ]
Servicio de Información Agroalimentaria y Pesquera 2010. Un Panorama del cultivo del chile. Consultado 09/2016. http://infosiap.siap.gob.mx/images/stories/infogramas/100705monografia-chile.pdf [ Links ]
Shipley, B., 2002. Trade‐offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Functional Ecology, 16(5): 682-689. [ Links ]
Stevens R., Page D., Gouble B., Garchery C., Zarmir D., Causse M. 2008. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 31: 1086-1096. [ Links ]
Ujowundu C. O., Ukoha A. I., Agha C. N., Nwachukwu N., Igwe K .O. and Kalu F. N. 2010. Effects of potassium iodate application on the biomass and iodine concentration of selected indigenous nigerian vegetables. Afr. J. Bioltech. 9: 7141-7147. [ Links ]
Venturi, S. 2011. Evolutionary significance of iodine. Current Chem. Biol. 5: 155-168. [ Links ]
Yao, H., Zhang, Y., Yi, X., Zhang, X. and Zhang, W., 2016. Cotton responds to different plant population densities by adjusting specific leaf area to optimize canopy photosynthetic use efficiency of light and nitrogen. Field Crops Research, 188: 10-16. [ Links ]
Zhu, Y. G., Huang, Y. Z., Hu Y., Liu Y. X. 2003. Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: Effects of iodine species and solution concentrations. Environ. Int. 29: 33-37. [ Links ]
Received: August 01, 2015; Accepted: April 01, 2016











 texto en
texto en 



