Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.50 no.6 Texcoco ago./sep. 2016
Biotechnology
Phytotoxicity of ZnO nanoparticles on the aquatic fern Azolla filiculoides Lam
1Postgrado de Botánica, Unidad de Microscopía Electrónica. México.
2Área de Microbiología, Postgrado de Edafología, Colegio de Postgraduados. 56230. Montecillo, Estado de México. México. (arazavaleta@yahoo.com.mx).
3Facultad de Ciencias, Departamento de Biología Celular, Universidad Nacional Autónoma de México, Ciudad Universitaria. 04510. Ciudad de México, México.
Nanoparticles of ZnO are disposed of in the environment and abound in wastewater, but their toxicity to plants is little known. The objective of this study was to evaluate the physiological response of Azolla filiculoides to the presence of two ZnO particle sizes: analytical grade nanoparticles (NPs, 26.7±1 nm) and pharmaceutical grade submicrometric particles (SMPs, 238±30.7 nm), in three concentrations (100, 200, and 400 mg L-1), and a control. The experimental design was completely randomized, data were analyzed with an ANOVA and means were compared with Tukey (p≤0.05). Transmission Electron Microscopy (TEM) showed that the NPs were isodiametric, while the SMPs were elongated. Plants were incubated in Yoshida solution containing the NPs and SMPs in a greenhouse for 6 d. The small particles (NPs) and the concentration of 400 mg NPs ZnO L-1 reduced growth and concentration of chlorophylls (Chla, Chlb, Chl total) and of carotenes and xanthophylls (c+x). Chlorophyll fluorescence (Fv/Fm) was decreased in all tested concentrations compared with the control, especially with NPs, which were more toxic than SMPs. NPs reduced total antioxidant activity and total phenolic compounds, relative to SMPs. Nitrogenase activity decreased in all the ZnO particle concentrations regardless of size. The Zn element microanalysis with energydispersive X-ray spectroscopy on scanning microscopy (EDS-SEM) showed that Zn was more abundant on the abaxial side of the frond, in function of NPs concentration, in contrast with that of SMPs. In conclusion, ZnO particle size is more determinant in degree of toxicity than concentration. Chlorophyll fluorescence and nitrogenase activity were good indicators of toxicity in this species.
Key words: Phytotoxicity; water fern; nanoparticles; ZnO NPs
Las nanopartículas de ZnO son desechadas al ambiente y abundan en el agua residual, pero su fitotoxicidad es poco conocida. El objetivo de esta investigación fue evaluar la respuesta fisiológica de Azolla filiculoides a la presencia de dos tamaños de partículas de ZnO: nanopartículas grado analítico (NPs, 26.7±1nm) y partículas submicrométricas (SMPs, 238±30.7 nm) grado farmacéutico, en tres concentraciones (100, 200, y 400 mg L-1), y un testigo. El diseño experimental fue completamente al azar, los datos se analizaron con ANDEVA y las medias se compararon con Tukey (p≤0.05). El análisis con Microscopía Electrónica de Transmisión (MET) mostró que las NPs fueron isodiamétricas, mientras que las SMPs fueron alargadas. Las plantas se incubaron en solución Yoshida con las NPs y SMPs, en invernadero por 6 d. Las partículas pequeñas (NPs) y la concentración de 400 mg NPs ZnO L1 redujeron el crecimiento y la concentración de clorofilas (Chla, Chlb, Chl total) y carotenos y xantofilas (c+x). La fluorescencia de la clorofila (Fv/Fm) se redujo en todas las concentraciones de partículas probadas, comparadas con el testigo, especialmente con NPs que fueron más tóxicas que las SMPs. Las NPs redujeron la actividad antioxidante total y compuestos fenólicos totales con respecto a las SMPs. La actividad nitrogenasa disminuyó en todas las concentraciones de partículas de ZnO, e independiente del tamaño. El microanálisis elemental de Zn con espectroscopía dispersiva de rayos X y Microscopía de Barrido (EDS-MEB) mostró mayor abundancia de Zn en el envés de las frondas, la cual dependió de la concentración de NPs en contraste con las SMPs. En conclusión, el tamaño de las partículas de ZnO es más determinante en el grado de toxicidad, que la concentración. La fluorescencia de clorofila y actividad nitrogenasa fueron buenos indicadores de toxicidad en esta especie.
Palabras clave: Fitotoxicidad; helecho acuático; nanopartículas; NPs ZnO
Introduction
The size of a nanostructured material is intermediate between molecular and micrometric structures and its magnitude is measured on a nanometric scale. Nanoparticles (NPs) have unique physical-chemical and optical characteristics because of the high surface/ volume ratio. In this condition, a large number of atoms on the surface modify physical, magnetic, photoelectric and thermal properties (Hernando Grande, 2007). Nanotechnology has led to development of nanomaterials used in the car, paint, electronics, construction, aeronautics, cosmetics and pharmaceutical industries. In 2012, there were 1300 products with at least one NP component (Love et al., 2012).
The most common metal oxide particles are titanium oxide (TiO2) and zinc oxide (ZnO) NPs, which are the most used in the pharmaceutical industry (Huang et al., 2013). To evaluate the impact of exposure to these NPs on human health, it is necessary to detect and monitor nanomaterials in the air, water and soil, which requires several types of sensors (Maynard et al., 2006; Handy et al., 2008).
The mechanism by which a nanomaterial can become toxic and alter biological systems depends on its size, composition, shape, and surface properties (Dhawan et al., 2009, Hossain et al., 2015). Evaluation of toxicity of NPs that contain a heavy metal will vary depending on the size and shape of the NPs and of the toxicity of the metal ion that they can release (Perreault et al., 2010). The toxicity of a metal for live organisms is affected by its water solubility, its union to a specific biological site and its morphology. The toxic effects of a heavy metal are defined by changes in functionality and morphology in the human body when it is ingested, inhaled, absorbed or introduced through a biological agent (Panyala et al., 2008, Hossain, 2015).
Heavy metals are found in nature, but they can also be anthropogenic. In Europe, concentrations of ZnO NPs of 0.01 g L-1 were found in surface water and 0.432 g L-1 in wastewater, and in the United Kingdom they were less than 100 mg L-1 (Boxall et al., 2007; Gottschalk et al., 2009).
Plants are not exempt from exposure to NPs and their effects are little known. The mechanism of absorption, translocation and accumulation of NPs in plants depends on the plant species as well as on the size, type, chemical composition, functionalization and stability of the NPs (Rico et al., 2011). For example, exposure of Passiflora juliflora-velutina to ZnO NPs increased the antioxidant activity of catalase (CAT) and ascorbate peroxidase (APOX), especially in the root where greater NPs absorption was observed. Nevertheless, the plants did not exhibit chlorosis, necrosis or wilting, indicating certain tolerance to these NPs (Hernández et al., 2011). In contrast, the effect of ZnO NPs (17.4±4.9 nm) on maize (Zea mays L.) and cabbage (Brassica oleraceae var. capitata L.) was negative. In maize, meristematic cells of the roots exhibited structural damage without effect on germination, but in cabbage root elongation was not affected (Pokhrel et al., 2013). The toxic effect of ZnO and Zn+ on Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Z. mays and Cucumis sativus was assessed; a concentration of 2000 mg L-1 affected seed germination and plant growth in L. perenne and Z. mays (Lin et al., 2007). López-Moreno et al. (2010) found genotoxic effects of ZnO (8 nm) and of CeO2 (7 nm) NPs on Glycine max; accumulation of 4000 mg L-1 ZnO NPs in the tissues altered DNA stability and the antioxidant system.
Toxicity of ZnO NPs for aquatic plants is little known. There are studies on toxicity of ZnO, Ag, CuO and TiO NPs in Lemmna minor (Perreault et al., 2010), Spirodela polyrhiza (Jiang et al., 2012), Landoltia punctata (Shi et al., 2011) and Salvinia natans (Hu et al., 2013), but no information was found for other aquatic plants. Hossain et al. (2015) reviewed literature about plant proteomics to understand the response to stress caused by NPs with metals; they point out that studies carried out with Arabidopsis thaliana predominated and information on other species was scarce.
Azolla filiculoides is an aquatic fern used to clean surface water contaminated by organic and inorganic compounds; its tolerance to several metals (Cu and As) is outstanding; besides, this species grows rapidly and has a high rate of duplication. For these reasons, it is a good candidate for use in phytoremediation (Sánchez-Viveros et al., 2010, 2011). However, there are no reports on the effect of NPs on this floating fern. Therefore the objective of this study was to evaluate the toxic effect of size and concentration of ZnO NPs on the physiological response of the floating fern A. filiculoides. To this end, changes in growth, photosynthetic pigments, chlorophyll fluorescence, total antioxidant activity and nitrogenase activity, as well as Zn accumulation in A. filiculoides fronds were measured.
Materials and methods
Characterization of ZnO particles
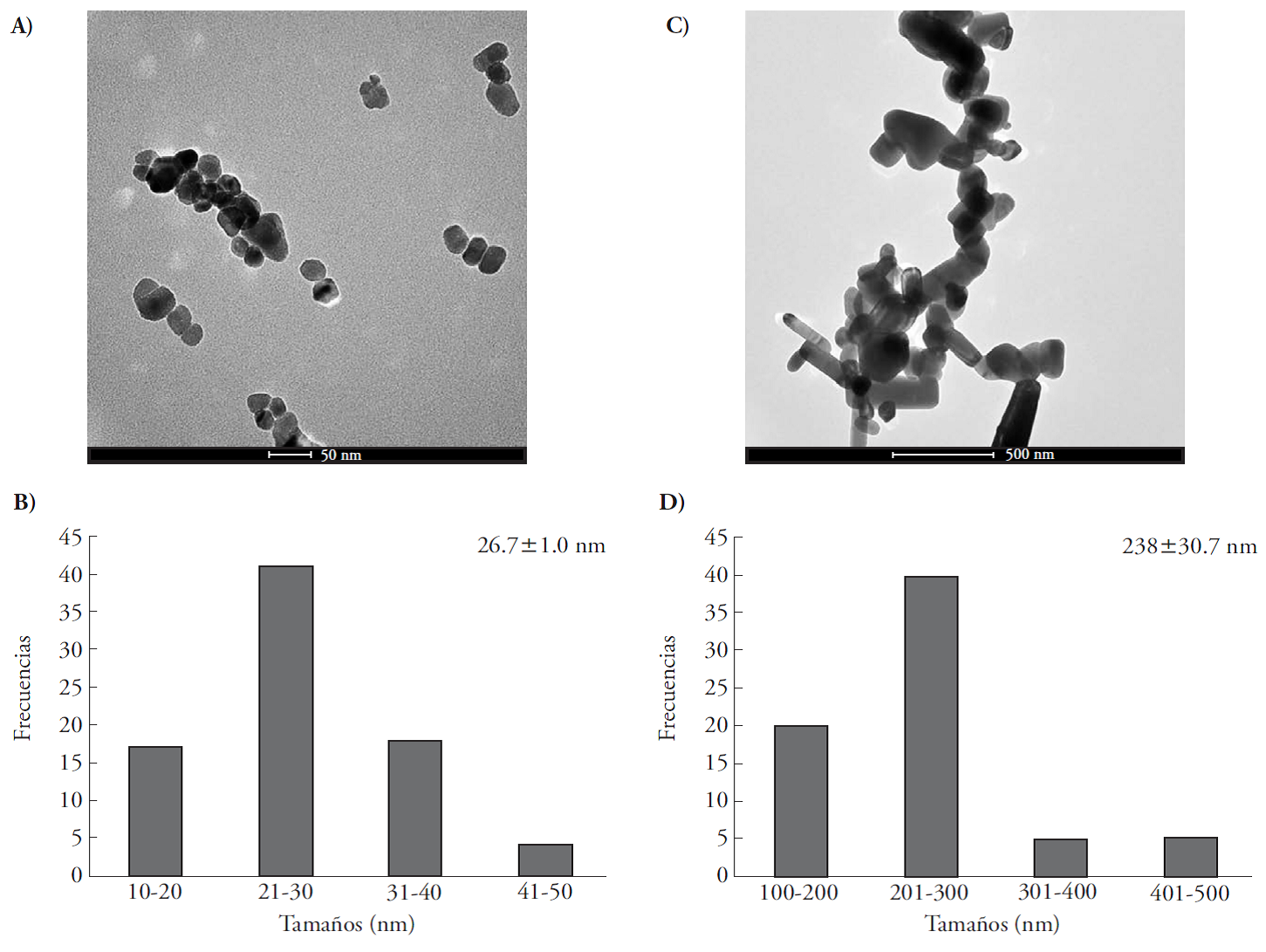
In this study, analytical grade ZnO NPs suspended in 40 % butylglycol (Sigma-Aldrich™, USA) and pharmaceutical grade ZnO particles (SMPs) in powder form (Farmacia Paris, México) were used. The butylglycol was eliminated by centrifuging 1 mL of the suspension at 18 000 g for 20 min and washing the NPs three times with de-ionized water. The dry NPs and SMPs were diluted in de-ionized water 1:100 (v:v) and sonicated (Ultrasonicador, Branson 1800™, Connecticut, USA) at 70 W and 70 Hz for 10 min. Morphology, roundness index (largest diameter/smallest diameter) and average size of both types of particles were examined with an transmission electron microscope (TEM) (Tecnai 2 Spirit, Fei Company, USA), operated at 120 Kv and, with the image processor TIA (Tecnai, Imagining & Analysis, New York USA) version 4.7 SP3 (1994-2014). The average diameter of the SMPs was 238±30.7 nm and that of NPs was 26.7±1 nm.
Biological material and experiment setup
Azolla filiculoides plants were obtained from the solarium of the area of Microbiology of the Graduate Program in Edaphology, Colegio de Postgraduados, campus Montecillo (19.52° N, 98.88° W, altitude 2250 m). One gram of fresh plants was placed in each plastic tray (12´6.5´4 cm) containing 200 mL nutritive solution, pH 5.5 (Yoshida et al., 1971) and kept in a greenhouse with an average diurnal temperature of 26 °C and nocturnal temperature of 19 °C.
The treatments exposure of fern to two particle sizes (NPs and SMPs) and three concentrations of ZnO particles (100, 200, and 400 mg L-1); the control contained no particles. The eight treatments were distributed in a completely randomized experimental design. The nutrient solution with NPs was sonicated for 10 min to disperse the particles before introducing the fern. The variables were measured after 6 d of exposure to the treatments.
Relative growth rate and quantification of photosynthetic pigments
The relative growth rate (RGR; g g·d-1) was calculated with the equation:
where Ln iw : natural logarithm of initial weight (iw); Ln fw : natural logarithm of final weight (fw); t: incubation time (d).
The photosynthetic pigments were extracted in 80 % acetone and the content of chlorophyll a (Chla), chlorophyll b (Chlb) and xanthophylls+carotenoids (x+c) were measured with UV/vis spectroscopy using the formula of Lichtenthaler and Wellburn (1983).
Total antioxidant activity and total soluble phenolic compounds
Total antioxidant activity was determined by the bleaching test of 1,1-diphenyl-2picrylhydrazyl radical (DPPH) (Matthäus, 2002). Extraction was obtained from 150-200 mg A. filiculoides fresh weight with 1 mL 80 % methanol at 4 °C; the extracts were centrifuged 15 min at 17000 g. To 30 mL extract, 235 mL DPPH solution was added. Initial absorbance readings and final readings (15 min later) were taken at a wavelength of 515 nm with a spectrophotometer (Synergy 2, Biotek Instruments™, USA). Antioxidant activity was calculated by applying aliquots of Trolox and known concentrations of 1,1-diphenyl-2picrylhydrazyl (DPPH) solution. The results were expressed in equivalent micromoles of Trolox per gram of fresh tissue (mM Trolox g-1 FW).
Content of total soluble phenolic compounds was measured with the Folin-Ciocalteu reactive test using chlorogenic acid as the standard. A sample of 150-200 mg of fresh frond was macerated with 1 mL methanol (80 %) at 4 °C and centrifuged 15 min at 17 000 g. The mixture of 30 mL extract+90 mL Na2CO3 and 150 mL of Folin-Ciocalteau reagent was placed on microplates and 30 min later, absorbance was read at 725 nm with a spectrophotometer UV/vis (Synergy 2, Biotek Instruments™, USA). The results were expressed in equivalent micrograms of chlorogenic acid per gram of fresh tissue (mg g-1 FW).
Nitrogenase activity and chlorophyll fluorescence
Nitrogenase activity was assessed indirectly by determining the concentration of ethylene produced by 1 g of fresh A. filiculoides, floating on 200 mL nutritive solution (of the four NP and SMP treatments) in a l-L recipient hermetically sealed with teflon tape. From the recipient, 10 % of the air was extracted and substituted with an equivalent volume of acetylene gas. After 2 h, 5 mL gas was extracted and put into vacutainer tubes for gas chromatography analysis (HP 5890 Series II PLUS GC with FID and TCD, Agilent Technologies), using an ethylene standard of 20 ppm N2 balance (INFRA de México, S. A. de C. V.). Based on the area under the curve, concentrations of problem samples were adjusted. The results were expressed in micromoles of ethylene per gram of fresh tissue (mmol ethylene g-1 FW).
Chlorophyll fluorescence was evaluated with a portable fluorometer (OS-30p, Chlorophyll Fluorometer, USA). The fronds were preconditioned in darkness for 5 min before measuring the maximum efficiency of the PSII (Fv/Fm) system photochemical potential following the procedure of Küpper et al. (2002).
Zn microanalysis with EDS-ESM
Energy dispersive X ray spectroscopy (EDS) coupled with scanning electron microscopy (SEM), allow to identify and quantify element composition in specific areas of a sample. After 6 d of treatment, unwashed A. filiculoides fronds were dried at 60 °C until constant weight. The dried fronds were mounted on Cu sample holder, with double-sided adhesive copper tape and coated with gold/palladium (80/20) with a metal evaporator (Fine Coat, Jeol, Japan). The abaxial and adaxial surfaces of the frond were analyzed with EDS (INCA x-ACT, Oxford Instruments, UK) attached to a SEM (Jeol JSM-6390, Japan) operating at 20 Kv. The relative concentration of Zn element was expressed in percentages of weight and mass.
Experimental design and statistical analysis
The experimental design was completely randomized, the treatments were two sizes of particles, small (NPs 26.7±1 nm) and large (SMPs 238±30.7 nm), three ZnO particle concentrations (100, 200 and 400 mg L-1) and one control without particles; each treatment was replicated three times, and the experiment was repeated three times (n=9). The data were analyzed with an ANOVA and treatment means were compared with the Tukey test (p≤0.05), using SAS (Statistical Analysis System Institute Inc., Cary NC, USA, 2002). Values of each variable were expressed as averages ± standard error of the mean (SE).
Results and discussion
Characterization of ZnO particles
Analytical grade NPs and observed with TEM, had an average size of 26.7±1.0 nm, roundness index of 1.09±0.058, isodiametric shape and smooth texture. The most abundant NP diameter was 21 to 30 nm (Figure 1 A and C). Pharmaceutical grade SMPs had an average diameter of 238±30.7 nm and a roundness index of 3.2±0.54; the most abundant submicrometric size was between 201-300 nm (Figures 1 B and D). The SMPs were nine times larger and three times longer than the NPs.
Effect of ZnO NPs and SMPs on growth
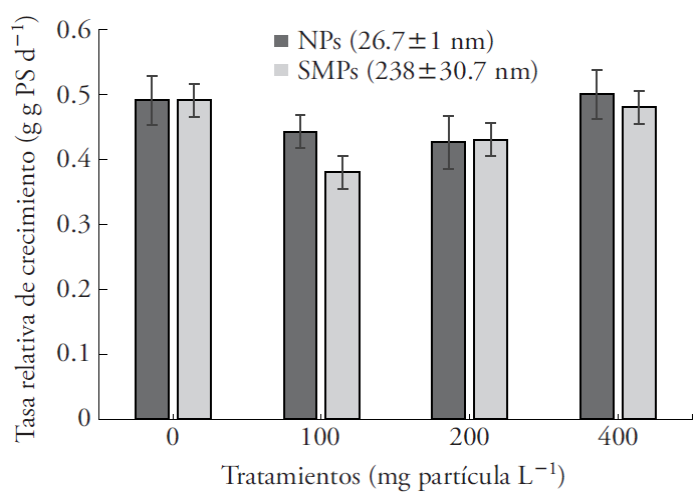
The relative growth rate of A. filiculoides was not affected by the presence of the particles, regardless of the size or concentration (Figure 2). This contrasts with Arabidopsis thaliana, whose growth decreased with 100 mg L-1 ZnO NPs (Landa et al., 2012).
Photosynthetic pigments
The NPs significantly reduced levels of Chla, Chlb and total Chl by 33.3 %, 71.8 % and 49.1 %, relative to the SMPs (Table 1), whereas application of 400 mg NPs decreased the pigments by 47.3 %, 10.4 % and 20.8 %, relative to the control (Table 1). These results are similar to those observed in Salvinia natans exposed to 1, 10, 20, 50 mg L-1 25 nm ZnO NPs (Hu et al., 2013). NPs can produce peroxidation of the chloroplast membrane, altering accumulation of pigments and of photosynthesis (Ma et al., 2013; Hu et al., 2013). The ZnO NPs reduced total concentration of carotenoids and xanthophylls (c+x) by 29.1 % in the fern fronds; application of 400 mg of NPs reduced the content of c+x by 24.3 %, relative to the control (Table 1). The c+x are important molecules that participate in detoxification of several forms of activated oxygen produced in photosynthetic complexes. Thus b-carotene quenchs the state of excitation of the chlorophyll triplet, which reduces the formation of singlet oxygen (Foyer and Shigeok, 2011). In this sense, the small particles (ZnO NPs) are more toxic than SMPs because they reduce the levels of x+c in A. filiculoides. This response is similar to that observed when Oryza sativa L. was exposed to 25 nm Ag NPs, which reduced the levels of photosynthetic pigments and caused greater toxicity than larger particles (Prakash et al., 2014; Yin et al., 2013). Toxicity of small NPs was observed in in vitro cultures of bacteria, yeasts, algae, crustaceans and mammal cells (Ivask et al., 2014). NP toxicity is associated with their high surface/volume ratio ability to enter the cells and chloroplasts, destabilizing plant photosynthesis and antioxidant system. The effect of concentration, of both particle sizes, was significant at 400 mg L-1 , which reduced the level of all the photosynthetic pigments studied (Table 1).
Table 1 Effect of the concentration and size of ZnO particles on photosynthetic pigments of Azolla filiculoides after 6 d of exposure.
| Concentración (mg partículas -1 ) | Chla (mg g -1 PF) | Chlb (mg g -1 PF) | C+x (mg g -1 PF) | Chl total (mg g -1 PF) |
|---|---|---|---|---|
| 0 | 0.2056 ± 0.0062 a | 0.0774 ± 0.0074 ab | 0.0745 ± 0.0024 a | 0.2831 ± 0.0092 a |
| 100 | 0.1958 ± 0.0133 a | 0.0788 ± 0.0118 ab | 0.0709 ± .0044 a | 0.2756 ± 0.0252 a |
| 200 | 0.1953 ± 0.019 a | 0.0851 ± 0.0134 a | 0.069 ± 0.0056 a | 0.279 ± 0.0319 a |
| 400 | 0.1086 ± 0.0017 b | 0.0688 ± 0.0103 b | 0.0564 ± 0.0038 b | 0.2243 ± 0.0236 b |
| Tamaño de partícula (nm) | ||||
| NPs (26.7 ± 1 ) | 0.1451 ± 0.0078 b | 0.0341 ± 0.0020 b | 0.0561 ± 0.0026 b | 0.1792 ±0.0095 b |
| SMPs (238 ± 30.7) | 0.2067 ± 0.0108 a | 0.1209 ± 0.0027 a | 0.0793 ±0.0023 a | 0.3518 ±0.0087 a |
Data are the mean ± standard error; n=9. Different letters in a column indicate significant differences (Tukey, p≤0.001).
Total antioxidant activity, total phenolic compounds, nitrogenase activity and chlorophyll fluorescence
Total antioxidant activity and total soluble phenolic compound contents in A. filiculoides significantly decreased in the presence of NPs, but increased in the presence of SMPs (Figure 3). Greater toxicity of the small particles (NPs) may be explained by its small size that favors rapid absorption, particle solubility and liberation of Zn+ ions (Reed et al., 2012). Stress caused by heavy metals raises the number of reactive oxygen species (ROS) and, in response, the plant produces more antioxidant compounds to inhibit oxidative degradation of biological molecules (Foyer and Noctor, 2005b; Sheng et al., 2008; Hernández et al., 2012). An increase in the antioxidant catalase (CAT) and glutathione (GSH) was observed in Fagopyrum esculentum in presence of ZnO NPs (Lee et al., 2013). Our study is the first of its kind to address the effect of two ZnO particle sizes on physiological variables in aquatic plants, particularly A. filiculoides, and its relationship to tolerance and attenuation of oxidative stress. Some phenolic compounds can sequester free radicles (Gulcin et al., 2003) and act as electron donors as a detoxification mechanism (Sánchez et al., 2010; Forni et al., 2012). The decrease in antioxidant activity and total phenolic compound content in the presence of NPs (Figure 3) was similar to the effect produced by cadmium and uranium in Azolla and Lemna minor (Sela et al., 1988; Forni et al., 2012). The effect of exposure to 100 mg L-1 ZnO NPs on A. thaliana gene expression was studied with micro-arrays; after 7 d, the induced genes were associated with oxidative stress, the response to lesions and electron transport (Landa et al., 2012). Azolla filiculoides forms symbiosis with Anabaena azollae cyanobacteria, which fixes atmospheric nitrogen by means of the activity of the enzyme nitrogenase, which facilitates N assimilation and fern growth (Reddy, 1987; Vessey, 1994).
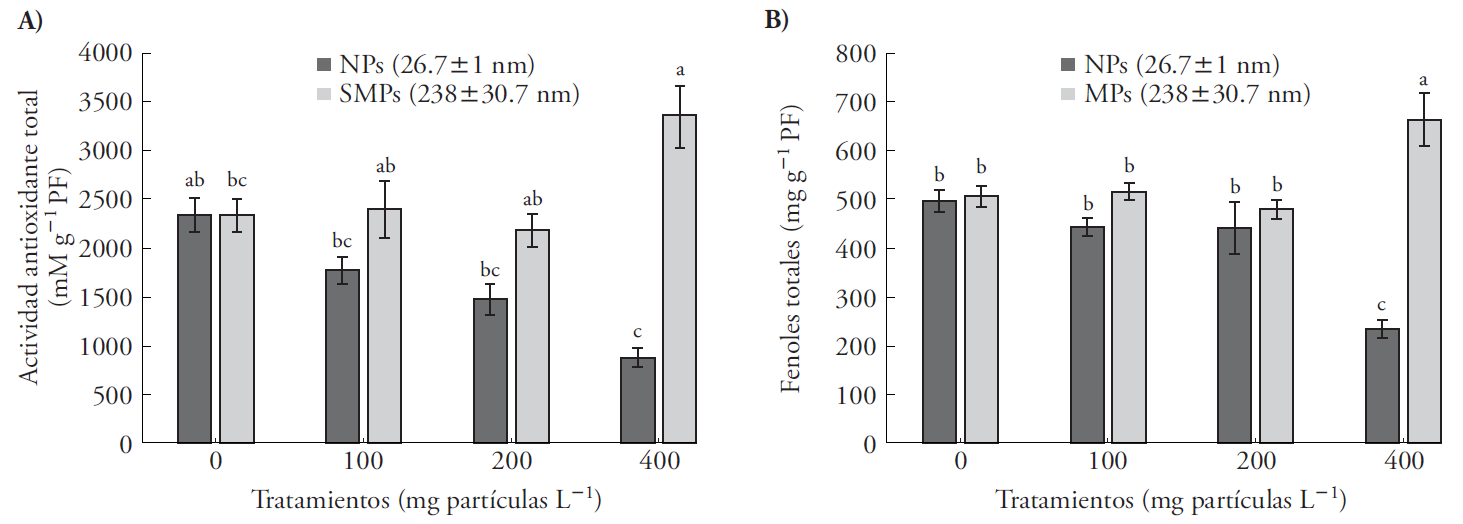
Figure 3 Total antioxidant activity and total phenols in Azolla filiculoides exposed to NPs (26.6±1 nm) and SMPs (268±30 nm) at three concentrations (100, 200 and 400 mg particles L-1) for 6 d. Mean ± standard error; n=9. Different letters indicate significant differences between treatments (Tukey, p≤0.05); PF: fresh weight.
Our results are the first to reveal the effect of ZnO NPs on nitrogenase activity in the symbiosystem Azolla-Anabaena and show the response of the symbiont to the particles. Nitrogenase activity in the A. filiculoides-Anabaena symbiosystem decreased significantly (82 %) as of the lowest concentration (100 mg L-1) (Figure 4). NPs reduced this enzymatic activity by 23.5 %, relative to SMPs (Table 2). The reduction was 79 % and 83 % in concentrations of 100-400 mg L-1, relative to the control (Figure 4). These results indicate a clear alteration in N metabolism of cyanobacteria and, consequently, a reduction in assimilable N for the plant. Sood et al. (2012) compared the phytoremediating potential of aquatic macrophytes (Elodea, Eichhornia, Lemna, Pistia, Salvinia, Ceratophyllum and Azolla) and underlined the phytoremediating capacity of Azolla because it grows rapidly, fixes nitrogen and hyper-accumulates metals.
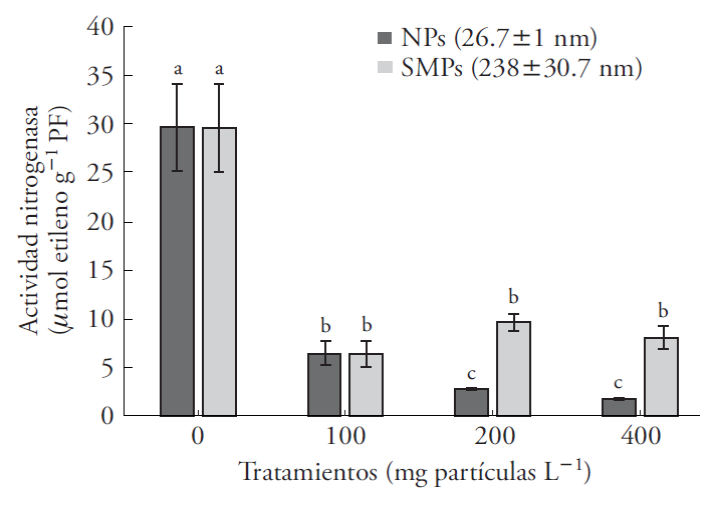
Figure 4 Nitrogenase activity in Azolla filiculoides exposed to nanoparticles (NPs) and submicrometric particles (SMPs) of ZnO for 6 d. Mean ± standard error; (n=6). Different letters indicate significant differences between treatments of the same particle size (Tukey, p≤0.05); PF: fresh weight.
Table 2 Microanalysis of Zn using EDS-SEM in Azolla filiculoides exposed to three concentrations of ZnO nanoparticles (NPs) and ZnO submicrometric particles (SMPs) for 6 d.
| Tamaño de las partículas (nm) | Tratamiento (mg partícula L -1 ) | Elemento Zn (% peso) |
|---|---|---|
| testigo | 0 | |
| NPs (26.7 ± 1) | 100 | 19.9 ± 1.8 d |
| 200 | 41.3 ± 4.1 bc | |
| 400 | 72.3 ± 6.6 a | |
| SMPs (238 ± 30.7) | 100 | 65.3 ± 6 ab |
| 200 | 48.3 ± 3.3 bc | |
| 400 | 20.3 ± 4.6 d |
Different letters indicate significant differences between concentrations (Tukey, p≤0.05), (n=3).
The particles did not affect fern growth for 6 d, possibly suggesting remediation potential, but the toxicity observed for the symbiont compromises growth in the medium term.
Chlorophyll fluorescence decreased when exposed to both particle sizes of ZnO and in all of the concentrations tested (Figure 5). This reduction was 15.3 % on average, relative to the control (Table 2). Chlorophyll fluorescence was used to assess toxicity of CuO NPs in Lemna gibba (Perrault et al., 2010). Moreover, heavy metals have a toxic effect for most plants (Viehweger, 2014). Thus, exposure of A. filiculoides and A. caroliniana to copper ions (2 mM Cu2+) significantly depress Fv/Fm levels after 35 h of exposure (Sánchez et al., 2010). In our study, exposure of A. filiculoides to 100 mg NPs L-1 reduced Fv/Fm by 22 %, compared with the SMPs (238±30.7 nm), which reduced fluorescence by only 15 % (Figure 5). Yield of PSII (Fv/Fm) measures photochemical potential and electron transport efficiency of PSII to PSI.
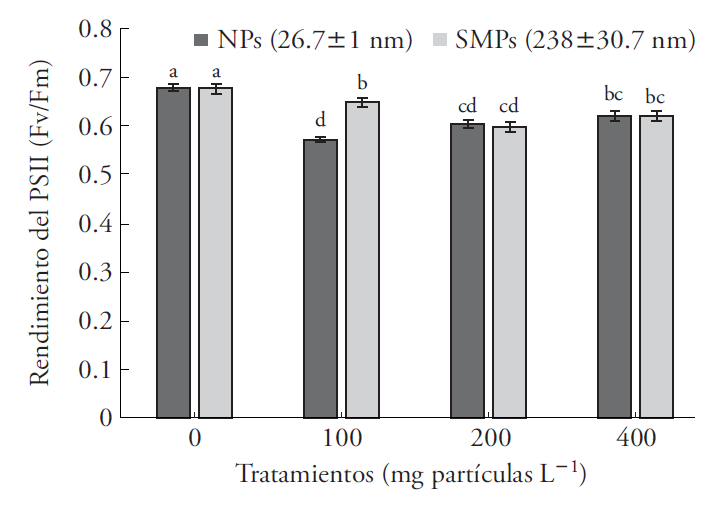
Figure 5 Chlorophyll fluorescence, PSII yield of Azolla filiculoides exposed to nanoparticles (NPs) and submicrometric particles (SMPs) of ZnO for 6 d. Mean ± standard error; n=9. Different letters indicate significant differences between treatments of the same particle size (Tukey, p≤0.05).
This variable is used as an indicator of stress in plants and allows characterization of the effects and mechanisms of different types of environmental stress by heavy metals, herbicides and detergents in aquatic systems (Ralph et al., 2007).
Maximum Fv/Fm values measured in healthy plants are above 0.7 (Butler and Kitajima, 1975; Sánchez-Viveros et al., 2010). Although there are no reports on the effect of ZnO NPs on PSII photochemical efficiency, inhibition of this variable in diverse plants and species of Azolla is associated with the presence of heavy metals, for example, application of 2 mM Cu2+ (Michalak, 2006; Sánchez-Viveros et al., 2010; Viehweger, 2014). In our study, at 100 mg L-1 NPs reduced Fv/Fm by 22 %, relative to the control, and 15 %, compared with the SMPs (Figure 5).
Reduction in chlorophyll fluorescence indicates lower yield of the reaction centers of plastocyanin to catalyze electron transfer efficiently between PS II (cytochrome b6f) and PS I (Küpper et al., 2002; Letelier et al., 2010). A reduction in Fv/Fm caused by ZnO NPs may be partially explained by the toxic effect of the Zn2+ ion on the PSII photochemical reaction, which blocks electron transport (Padua et al., 2010). Expression of genes associated to electron transport was suppressed in A. thaliana exposed to 100 mg L-1 ZnO NPs (Landa et al., 2012). According to Ivask et al. (2014), there is an effect of Ag NP size on toxicity for bacteria, algae, crustaceans and mammal cells. Our study contributes new information on the response of the photosynthetic reaction centers to two particle sizes of ZnO, nanometric and submicrometric, in A. filiculoides.
Microanalysis of Zn with EDS-ESM
The X ray signal of Zn element was produced from agglomerates and crystals adhered to the abaxial epidermis of the frond. In the treatments with NPs, Zn accumulation increased proportionally with concentration. These observations were similar to those from a study on Fagopyrum sculentum, in which ZnO NPs accumulated mostly on the root surface and accumulation increased in function of NP concentration (Lee et al., 2013). In contrast, in treatments with SMPs, the higher the concentration of SMPs in the solution, the lower the accumulation of Zn on the frond. The appearance of precipitates in the nutritive solution at 200 and 400 mg L-1 suggests that, because of their size, these particles precipitated and the Zn did not accumulate on the leaf. In order to better understand Zn toxicity, further studies are required to understand mobility of this element in plants.
Conclusions
ZnO NPs did not affect growth of the fern Azolla filiculoides during the 6 d. However, the reduction in photosynthetic pigments, antioxidants, nitrogenase activity and chlorophyll fluorescence indicate their toxicity for the symbiosystema AzollaAnabaena. The ZnO SMPs did not affect growth or photosynthetic pigment content, but they did reduce chlorophyll fluorescence and nitrogenase activity, which indicates stress in the plant. Azolla filiculoides was better able to deal with SMPs, which promoted an increase in antioxidants and total soluble phenols in order to compensate the free radicals produced by stress.
Summarizing, small particles (NPs) were more toxic than large particles (SMPs), indicating that size of ZnO particles is more important than concentration in determining toxicity for the fern A. filiculoides.
Literatura citada
Boxall, A. B. A., Q. Chaudhry, C. Sinclair, A. D. Jones, R. Aitken, B. Jefferson, and C. Watts. 2007. Current and future predicted environmental exposure to engineered nanoparticles. Report by the Central Science Laboratory, Sand Hutton, UK. 89 p. [ Links ]
Butler, W. L., and M. Kitajima. 1975. Fluorescence quenching in photosystem II of chloroplasts. Biochim. Bioph. Acta 376: 116-125. [ Links ]
Dhawan, A., V. Sharma, and D. Parman. 2009. Nano materials a challenge for toxicologists. Nanotoxicology 3: 1-9. [ Links ]
Forni, C., R. Braglia, F. J. Harren M., and S. M. Cristescu. 2012. Stress responses of duckweed (Lemna minor L.) and water velvet (Azolla filiculoides Lam.) to anionic surfactant sodiumdodecyl-sulphate (SDS). Aquat. Toxicol. 110-111: 107-113. [ Links ]
Foyer, C. H., and S. Shigeoka. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155: 93-100. [ Links ]
Foyer, C. H. , and G. Noctor. 2005a. Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28: 1056-1071. [ Links ]
Foyer, C. H. , and G. Noctor. 2005b. Redox Homeostasis and Antioxidant Signalling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866-1875. [ Links ]
Gottschalk, F., T. Sonderer, R. W. Scholz, and B. Nowack. 2009. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 43: 9216-9222. [ Links ]
Handy, R. D., R. Owen, and E. Valsami-Jones. 2008. The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17: 315-325. [ Links ]
Hernández, V., J. A., M. H. Castillo, A. D. Servin, J. R. Peralta V., and J. L. Gardea T. 2011. Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina (velvet mesquite) treated with ZnO nanoparticles. Chem. Eng. J. 170: 346-352. [ Links ]
Hernández-Ortega, H. A., A. Alarcón, R. Ferrera-Cerrato, H. A. Zavaleta-Mancera., H. A. López-Delgado, and M. R. Mendoza-López. 2012. Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a dieselcontaminated substrate. J. Environ. Manage. 95: 5319-5324. [ Links ]
Hernando-Grande, A. 2007. Nanotecnología y nanopartículas magnéticas: la Física actual en lucha contra la enfermedad. Rev. Royal Acad. Cienc. Exact. Fis. Nat. 101: 321-327. [ Links ]
Hu, C., X. Liu, X. Li, and Y. Zhao 2013. Evaluation of growth and biochemical indicant of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ. Sci. Pollut. Res. Doi 10.107/s11356-013-1970.9 [ Links ]
Huang, Y., S. C. Lenaghan, L. Xia, J. N. Burris, C. N. Stewart, and M. Zhang. 2013, Characterization of physicochemical properties of nanoparticles for cosmetic application. J. Nanobiotech. 1; 11:3. doi: 10.1186/1477-3155-11-3. [ Links ]
Hossain, Z., M. Ghazala, and S. Komatsu. 2015. Plant responses to nanoparticles stress. Int. J. Mol. Sci. 16: 26644-26653. [ Links ]
Ivask, A., I. Kurvet, K. Kasemets, I. Blinova, V. Aruoja, S. Suppi, H. Vija, A. Käkinen, T. Titma, M. Heinlaan, M. Visnapuu, D. Koller, V. Kisand, and A. Kahru. 2014. Sizedependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One. 9(7): e102-108. doi: 10.1371/journal.pone.0102108 [ Links ]
Jiang, H-S., M. Li, F. Y. Chang, W. Li, and L. Y. Yin. 2012. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 31: 1880-1886. [ Links ]
Küpper, H., I. Šetlík, M. Spiller, F. C. Küpper and O. Prášil 2002. Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J. Phycology 38: 429-441. [ Links ]
Landa, P., R. Vankova., J. Andrlova, J. Hodek, P. Marsik, H. Storchova, J. C. White, and T. Vanek. 2012. Nanoparticlespecific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2 and fullerene soot. J. Hazard. Mater. 241-242: 52-62. [ Links ]
Lee, S., S. Kim, S. Kim, and I. Lee. 2013. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 20: 848-854. [ Links ]
Letelier, M. E, S. Sánchez-Jofré, L. Peredo-Silva, J. Cortés-Troncoso, and P. Aracena-Parks. 2010. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem. Biol. Interact. 188: 220-227. [ Links ]
Lichtenthaler, H. K., and A. R. Wellburn. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Bioch. Soc. Tran. 11: 591-592. [ Links ]
Lin, D., and B. Xing. 2007. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 20: 1-8. [ Links ]
López-Moreno M.L., G. de la Rosa, J. A Hernández-Viezcas, H. Castillo-Michel, C. E. Botez, J. R. Peralta-Videa, and J. L. Gardea-Torresdey. 2010. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 44: 7315-7320. [ Links ]
Love, S. A., M. A. Maurer-Jones, J. W. Thompson, Y. S. Lin, and C. L. Haynes. 2012. Assessing nanoparticle toxicity. Ann. Rev. Analyt. Chem. 5: 181-205. [ Links ]
Ma, H., P. L. Williams, and S. A. Diamond 2013. Ecotoxicity of manufactured ZnO nanoparticles: A review. Environ. Pollut. 172: 76-85. [ Links ]
Matthäus, M. 2002. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agri. Food Chem. 50: 3444-3452. [ Links ]
Maynard, A. D., R. J. Aitken, T. Butz, V. Colvin, K. Donaldson, G. Oberdörster, M. A. Philbert, J. Ryan, A. Seaton, V. Stone, S. S. Tinkle, L. Tran, N. J. Walke, and D. B. Warheit. 2006. Safe handling of nanotechnology. Nature 444: 267-269. [ Links ]
Michalak, A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 15: 523-530. [ Links ]
Padua, M., A.M. Cavaco, S. Aubert, R. Bligny, and A. Casimiro. 2010. Effects of copper on the photosynthesis of intact chloroplasts: interaction with manganese. Physiol. Plant 138: 301-11. [ Links ]
Panyala, N. R., E. M. Peña M., and J. IIavel. 2008. Silver or silver nanoparticles: a hazardous threat to the environmental and human health? J. Appl. Biomed. 6: 117-129. [ Links ]
Perrault, F., A. Oukarroum, L. Pirastru, L. Sirois, W. Gerson M., and R. Popovic. 2010. Evaluation of cooper oxide nanoparticles toxicity using chlorophyll a fluorescence imaging in Lemna gibba. J. Bot. doi: 10.1155/2010/763142. [ Links ]
Pokhrel, L. R., and B. Dubey. 2013. Evaluation of developmental responses of two crop plants exposed to silver on zinc oxide nanoparticles. Sci. Total Environ. 1:321-332. doi: 10.1016/j. scitotenv.2013.02.059. [ Links ]
Ralph, P. J., R. A. Smith, C. M. O. Macinnis-Ng, and C. R. Seery. 2007. Use of fluorescence-base ecotoxicological bioassay in monitoring toxicants and pollution in aquatic systems: Review. Toxicol. Environ. Chem. 89: 589-607. [ Links ]
Reddy, K. R. 1987. Nitrogen fixation by Azolla cultured in nutrient enriched waters. J. Aquat. Plant Manage. 25: 43-48. [ Links ]
Reed, R. B., D. A. Ladner, C. P. Higgins, P. Westerhoff, and J. F. Ranville. 2012. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 31: 93-99. [ Links ]
Rico, C. M., S. Majumdar, M. Duarte-Gardea, J. R. Peralta Videa, and J. L. Gardea-Torresday 2011. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 59: 3485-3498. [ Links ]
Sánchez-Viveros, G., D. González-Mendoza, A. Alarcón, and R. Ferrera-Cerrato, C. R. 2010. Copper effects on photosynthetic activity and membrane leakage of Azolla filiculoides and A. caroliniana. Int. J. Agric. Biol. 12: 365-368. [ Links ]
Sánchez-Viveros, G. , R. Ferrera-Cerrato, and A. Alarcón. 2011. Short-term effects of arsenate-induced toxicity on growth, chlorophyll and carotenoid contents, and total content of phenolic compounds of Azolla filiculoides. Water Air Soil Pollut. 217: 455-462. [ Links ]
Sela M., E. Tel-Or, E. Fritz, and A. Huttermann. 1988. Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol. 88: 30-36. [ Links ]
Sheng, M., M. Tang, H. Chen, B. Yang, F. Zhang, and Y. Huang. 2008. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salts stress. Miycorrhiza 18: 287-296. [ Links ]
Sood, A., L. U. Perm, R. Prasana, and A. S. Ahluwalia. 2012. Phytoremediation potential of aquatic macrophyte Azolla. AMBIO 41: 122-137. [ Links ]
Jiyan S., A. B Abid, I. M. Kennedy, K. R. Hristova, and W. K. Silk. 2011. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut. 159: 1277-1282. [ Links ]
Vessey J. K., 1994. Measurement of nitrogenase activity in legume roots nodules: in defense of the acetylene reduction assay. Plant and Soil 158: 151-162. [ Links ]
Viehweger K. 2014. How plants cope with heavy metals. Bot. Studies 55:35. doi: 10.1186/1999-3110-55-35. [ Links ]
Yoshida S., D. Forno, J. Cock, and K. Gomez. 1981. Laboratory Manual for Physiological Studies of Rice, 3rd ed. Manila Philippines: International Rice Research Institute. 269 p. [ Links ]
Received: August 01, 2015; Accepted: May 01, 2016











 texto en
texto en