Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.50 no.3 Texcoco abr./may. 2016
Articles
Growth and response to Bemista tabaci in diferent Capsicum annuum genotypes inoculated with Brevibacillus brevis CBTC1
1Instituto Tecnológico de Conkal. División de Estudios de Posgrado e Investigación. Km 16.3. Antigua Carretera Mérida-Motul. 97345. Conkal, Yucatán.
The plant growth promoting rhizobacteria are an alternative to reduce the use of chemical fertilizers as well as induce some degree of resistance to phytophagous insects. In this study the effect of the inoculation with the rizobacteria Brevibacillus brevis CBTC1 on growth and response to whitefly (Bemisia tabaci) in different genotypes of Capsicum annuum was evaluated. Experiments were established in a randomized design. In 15 repetitions (plants) the plant growth promoting variables were evaluated, and in four (composed by mixing foliage of five plants) foliar N evaluations were made. To assess the response of plants to B. tabaci, 20 repetitions (leaves) were used. The rizobacteria B. brevis CBTC1, an indole acetic acid producer, was isolated from soil from the state of Yucatan, Mexico. Inoculating the strain in four genotypes of C. annuum (Simojovel, Amaxito, Jalapeño and Х-kat ik) had significant effects on stem diameter, plant height, biomass, leaf number and root biomass in the Simojovel and Amaxito genotypes. Rizobacteria inoculation did not change the foliar N concentration or the attraction of B. tobaci adults in the evaluated genotypes. In contrast, the eggs density decreased in the Simojovel variety, and nymphal mortality increased in the Jalapeño.
Keywords: Rizobacteria; growth promoting; phytophagous insect
Las rizobacterias promotoras de crecimiento vegetal son una alternativa para disminuir el uso de fertilizantes químicos e inducir cierto grado de resistencia a insectos fitófagos. En este estudio se evaluó el efecto de la inoculación con la rizobacteria Brevibacillus brevis CBTC1 en el crecimiento y respuesta a la mosquita blanca (Bemisia tabaci) de genotipos de Capsicum annuum. Los experimentos se establecieron en un diseño completamente al azar. En 15 repeticiones (plantas) se evaluaron las variables de promoción de crecimiento vegetal y en cuatro (compuestas por la mezcla de follaje de cinco plantas) se hizo el análisis de N foliar. Para evaluar la respuesta de las plantas a B. tabaci se utilizaron 20 repeticiones (hojas). La rizobacteria B. brevis CBTC1, productora de ácido indol acético, se aisló de suelo del estado de Yucatán, México. La inoculación de la cepa en genotipos de C. annuum (Simojovel, Amaxito, Jalapeño e X-kat ik) tuvo efectos significativos en el diámetro de tallo, altura de planta, biomasa, número de hojas y biomasa de raíz en Simojovel y Amaxito. La inoculación de la rizobacteria no modificó la concentración de N foliar ni la atracción de adultos de B. tabaco en los genotipos. En contraste, la densidad de huevos disminuyó en Simojovel y aumentó la mortalidad de ninfas en Jalapeño.
Palabras clave: Rizobacteria; promoción de crecimiento; insecto fitófago
Introduction
The use of plant growth promoting rhizobacteria (RPCV) is a rational technological application in agricultural production processes (Lugtenberg and Kamilova, 2009)· The RPCV can increase the availability of nutrients in the rhizosphere and stimulate plant growth through nitrogen fixation, of nutrients solubilization and siderophores production (Loredo et al., 2004), indole acetic acid (IAA) plant hormone that promotes radical and vegetative development as well as fruit production (Martinez et al., 2013). Several RPCV species have an impact in agricultural production as they are been used as biofertilizers (Lugtenberg and Kamilova, 2009; Das et al, 2013.).
The RPCV Brevibacillus brevis belongs to the Bacilli class and there are few studies. Jha and Saraf (2011) reported that B. brevis MSI applied on Jatropha curcas caused significant increase in length (7 %) and biomass (103 %) of the roots after 60 d of inoculation. Vivas et al. (2003) and Vivas et al. (2005) observed that inoculation with B. brevis on Trifolium sp. increased aerial growth, root biomass and tolerance to heavy metals. Studies in C. annuum with RPCV from the Bacillus genus, from to the Bacilli class, showed positive results on plant growth: increased plant height (21 to 65 %), and increased root biomass (35 to 75 %) and area (15 to 37 %) (Lamsal et al, 2012; Castillo et al., 2013; Martínez et al., 2013). Furthermore, the content of some mineral elements in the foliage, particularly N, increases in response to inoculation with these rhizobacteria (Christinal and Tholkkappian, 2013; Rezvani et al, 2013). In several plant species, foliar N content can have a direct impact on the susceptibility of host plants to herbivore insects (Lu et al, 2007; Chen et al., 2008).
The RPCV from the Bacilli class can modulate the effects of phytophagous in leaves (Pineda et al, 2010). The positive effects that the RPCV inoculation has against phytophagous insects, such as the whitefly (Bemisia tabaci) in tomato (Solanum lycopersicum) are documented, with a 43 % decrease in the density of B. tabaci nymphs observed by inoculating seeds with several Bacillus species (Murphy et al, 2000). Soto et al. (2010) also reported a significant reduction (36 %) in the population of B. tabaci nymphs in s. lycopersicum plants inoculated with B. subtilis.
The aim of this study was to evaluate the effect of the rizobacteria B. brevis CBTC1 in growth as well as the leaf nitrogen content of three landraces and one commercial genotypes of C. annuum. The resistance response to B. tabaci was also evaluated in the inoculated genotypes.
Materials and Methods
Experimental site
Soil samples were collected in the Yucatan Peninsula in order to isolate its rizobacteria. Samples were taken at 10 cm depth, close to the rhizosphere, from plants at cultivated and non-cultivated areas. Samples were placed in plastic bags and taken to a laboratory where the isolation and identification of the strain was performed.
The effect of the rizobacteria inoculation in different c. annuum genotypes was evaluated in a rustic greenhouse in Conkal, Yucatan, Mexico. In the chili landraces Simojovel, Amaxito and Х-kat ik which are from Southeast Mexico and the commercial jalapeno were evaluated. These samples were collected in a germplasm enhancement study (Hernández et al., 2012).
Isolation of the bacterial strain
Two g soil samples were mixed with 10 mL of sterile distilled water in 15 mL Falcon tubes, they were vigorously stirred 30 to 40 s, heated in a water bath at 80 °С for 15 min, and then immediately immersed in ice for 15 min. One mL of the solution was mixed with 1 mL of sterile distilled water in a 2 mL microtube and then heating and cooling above described. A 50 mL aliquot was placed in 100 mm diameter Petri dishes in nutrient agar (NA). Petri dishes were incubated at 28 °С for 24 h (Travers etai, 1987).
Selection of the bacterial strain
Bacterial colonies with morphology, Gram staining and spore formation features of the Bacilli class were selected and cultured in 100 mm diameter Petri dishes with AN. After 24 h of growth, Gram staining and catalase tests were performed. Spores detection was done by smear in colonies with 5 d of growth. In that manner 36 strains with similar morphology to that of the Bacilli class were isolated. Their indoleacetic acid (IAA) production was assessed via the colorimetrie method with the Salkowski reagent (Patten and Glick, 2002). The strain rizobacteria CBTC1 was selected to study the growth promotion because it produced more IAA (26.80 μg mL-1 of culture).
Bacterial strain identification
DNA extraction was performed with the Wizard® Genomic DNA Purification Kit (Promega) commercial package. The 16s rRNA gene was amplified with Eubac27F and Eubacl49R primers as reported by Singh (2010). The integrity of the amplified fragments was visualized on 1 % agarose gels. The amplification products were sequenced at Macrogen, USA. Sequences were analyzed in NCBI Blast program.
Acquisition and inoculation of C. annuum plants
Seeds were sown in styrofoam trays with 200 cavities, in a commercial substrate (cosmopeat®); trays with 50 % of the germinated seeds were moved to a greenhouse. The transplant was performed 35 d after sowing (DAS) in 1 L foam cups with a mixture consisting on soil, commercial substrate (cosmopeat®) and gravel 50:30:20 (vol:vol:vol); the mixture was disinfected with 2 % formalin, 15 d before transplant.
Two inoculations with rizobacteria were made on the chili plants, the first 15 DAS and second at the transplant (35 DAS). Each plant was inoculated with 3 mL of a bacterial suspension containing 1x108 spores mL-1. The bacterial suspension was obtained from the colonies stablished in AN 8 d of culture.
Evaluation of variables indicating growth promotion
To evaluate the effects of rhizobacteria in promoting c. annuum growth, 10 d after transplant (45 DAS) we recorded: plant height (cm), stem diameter (mm) and number of leaves. Height was assessed with a measuring tape and the stem diameter with a digital vernier. Biomass production (g) and leaf N content was evaluated 15 d after transplant (50 DAS). For this, roots and stem were placed in separate paper bags, and then dried until constant weight in a forced air oven at 65 °С. Foliar N quantification was performed by the Kjeldahl method.
Attraction and oviposition of B. tabaci in chili plants
Brevibacillus tabaci adults were obtained from a colony previously established in chili plants, which has been in confinement for several years (Ballina-Gómez et al, 2013). For the bioassay, all the evaluated chili genotypes were inoculated with rhizobacteria in the same way described for evaluating the growth promotion.
To evaluate the attraction of adults and oviposition preference, plants 10 d form transplantation (45 DAS) were used. The plants were confined in entomological cages. An average of 30 B. tabaci adults was released per plant. The number of adults perching on two fully extended leaves at the apical third was recorded at 48 h; this assessment was made at 06:00 h in order to avoid flying high activity of B. tabaci adults. At 72 h the amount of oviposited eggs in two fully extended leaves at the apical third was evaluated, the leaf area coverage was measured to obtain the ratio of the number of eggs per cm2 (Ballina-Gómez et al, 2013).
The nymph mortality was evaluated with the method of "no choice" (Nombela et al, 2001). Thirty B. tabaci adults were confined in cages clip placed in fully expanded leaves at the apical third on plants 10 d after transplantation (45 DAS). Adults were removed 24 h after being confined. The oviposited eggs hatched and the nymphs remained within the section contained by the clip cage. After 15 d of oviposition nymphal mortality was evaluated with a stereoscopic microscope. Nymphs appeared dehydrated and their bodies were dark brown to black coloured.
Experimental design
The experimental design was completely randomized. The effects of the rhizobacteria inoculation on the growth variables of the plant and regard the response to B. tabaci were analyzed in separate plants inoculated with rhizobacteria and uninoculated (control). Treatments were established in 15 repetitions to assess growth, four for the foliar N analysis, and 20 for the response of the plants to B. tabaci. Statistical analysis (Student τ test; p≤0.05) was performed with Statgraphics Centurion (version 15.2.06).
Results and Discussion
Molecular identification of the bacterial strain
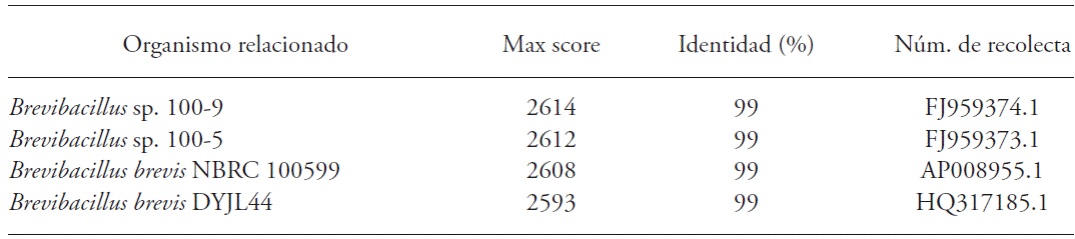
The forward and reverse sequences were obtained, were assembled with CAP3 program, and were sequenced in duplicate from two DNA extractions of the same strain and different crop. A 1423 base sequence was obtained, according to the 16s rRNA gene analysis, CTB1 strain has 99 % identity to the Brevibacillus genus (Table 1) and the B. brevis species. The selected strain was named B. brevis CTB1C1.
Growth of stem diameter and plant height
Plants of all the evaluated genotypes significantly increased their height and stem diameter because of the inoculation with B. brevis CBTC1 (Table 2). The most significant increases in stem diameter (11.3 %) and plant height (41 %) were observed in the Amaxito genotype. In contrast, the smallest increase in stem diameter (7.4 %) was recorded in the Х-kat ik genotype. The smallest increase in plant height was recorded in the Jalapeño genotype (15.8 %).
Table 2 Effect of inoculation of B. brevis CBTC1 in growth of stem diameter and plant height in Capsicum annuum.
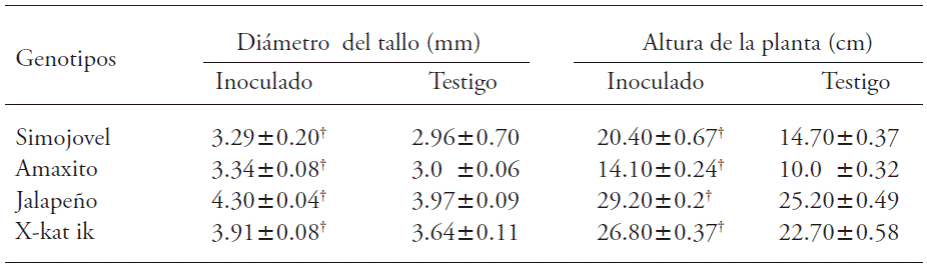
†Indicates statistical difference from control (τ -Student; p≤0.05, n=15). Mean ± mean standard error.
The plant growth promotion by inoculation rizobacteria species from the Bacilli class in C. annuum has already been evaluated. Kokalis et al. (2002) observed a 50 % increase in stem diameter in plants inoculated with a mixture of B. subtilis and B. cereus.Akgül and Mirik (2008) inoculated plants with B. megaterium and reported a 23 % increase in stem diameter. Guillen et al. (2006) reported that the inoculation of Bacillus spp. increased plant height 20 %; besides, the inoculation with B. cereus increased plant height 76 % (Damayanti et al, 2007). The 11 % increase in stem diameter reported in the present study was lower than that observed in previous studies, but not the plant height (41 %).
Leaves formation and biomass accumulation
Inoculation with B. brevis CBTC1 increased primary and axillary leaves formation in all genotypes except Х-kat ik (Table 3). In Simojovel, Amaxito and Jalapeño genotypes significantly increased the number of main leaves up to 25 %.
Table 3 Effect of В. brevis CBTC1 inoculation in primary and axillary leaves formation on Capsicum annuum plants.
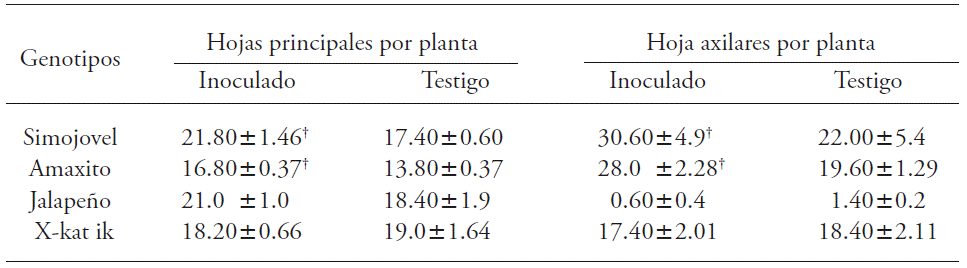
†Indicates statistical difference from control (τ -Student; p≤0.05, n=15). Mean ± mean standard error.
Inoculation with B. brevis CBTC1 increased the aerial biomass in all genotypes, and Simojovel and Amaxito root biomass (Table 4). The largest biomass gain (45-2 %) and root (44.4 %) was observed in Amaxito.
Table 4 Effect of В. brevis CBTC1 inoculation on biomass accumulation in Capsicum annuum.

†Indicates statistical difference from control (τ -Student; p≤0.05, n=15). Mean ± mean standard error.
The effect of the inoculation of rhizobacteria from the Bacili class in the aerial biomass in c. annum has already been evaluated, and there is an increase in the number and size of the leaves. According to Damayanti et al. (2007), there is a significant increase in the leaf size on plants inoculated with a B. cereus strain. In our study, plants from the Simojovel and Amaxito genotypes had up to 42 % more axillary leaves. This can improve the biological productivity of plants, and according to Ashrafuzzaman et al. (2011) a higher number of leaves in plants increase light interception, photosynthesis and accumulation of fotoasimilates, which results in better usage of the water and nutrient resources, along with increased fruit yield.
In our research, the increase in biomass (44 and 45 %) was close to the range observed in other studies. Akgül and Mirik (2008) reported a 52 % increase in leaf biomass and 48 % in root biomass in С annuum inoculated with B. megaterium. Similarly Nautiyal et al. (2013) report an increase of up to 43 % in root biomass in plants inoculated with B. amyloliquefaciens.
In our study the largest biomass in the inoculated plants resulted from the increase in length and diameter of stems and leaf number. This increase confirms that inoculation promoted root development, probably favoured water and nutrients uptake, and therefore the biomass production.
Chemical analysis of leaf nitrogen
The nitrogen content in the leaves of the inoculated plants and their controls no significant differences were observed. However, there was a significant effect for the foliar N grams in the Amaxito (33 %) and Х-kat ik (17 %) plants (Table 5).
Table 5 Effect of B. brevis CBTC1 inoculation on the nitrogen foliar concentration in Capsicum annuum plants.

†Indicates statistical difference from control (τ -Student; p≤0.05, n=15). Mean ± mean standard error.
Jones et al. (1991) classified nitrogen content into three groups: 1) high values, greater than 4.5 %; 2) optimum or sufficient values, between 3.5 to 4.5 % and; 3) low values from 3 to 3.49 %. According to this classification, the nitrogen percentage in inoculated and control plants in this study were within the best or sufficient group. It is likely that the evaluated genotypes themselves assimilate enough nitrogen, so that the B. brevis CBTC1 inoculation did not increase the N content in the foliage.
Christinal and Tholkkappian (2013) report a higher N content in leafs of C. annuum plants inoculated with B. megaterium.Rezvani et al. (2013) found that C. annuum plants inoculated with Bacillus spp. had higher foliar N content compared with the control. In our study differences were observed in the amount of foliar N (g) per plant of Х-kat ik and Amaxito. The differences are related to the aerial biomass accumulation.
Adult attraction, oviposition preference and mortality of B. tabaci on C. annuum genotypes
No significant effect of the B. brevis CBTC1 inoculation in attracting B. tabaci adults (Table 6) among the genotypes studied was detected. Only on the Simojovel genotype a significant (p ≤ 0.05) decrease (60.5 %) was observed on the oviposition (Table 6).
Table 6 Effect of В. brevis CBTC1 inoculation in attraction of adults and oviposition of Bemisia tabaci in Capsicum annuum foliage.

†Indicates statistical difference from control (τ -Student; p≤0.05, n=15). Mean ± mean standard error.
The Jalapeño genotype was the only one that showed significant (p≤0.05) effect on mortality (38.6 %) of B. tabaci nymphs by the inoculation with B. brevis CBTC1, compared to the control (Figure 1).
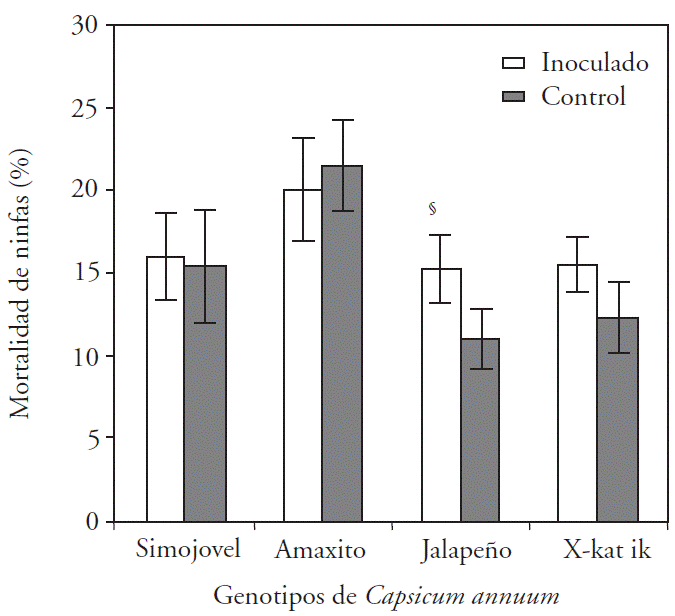
§Indicates statistical difference from control (τ -Student; p≤0.05, n=20).
Figure 1 Mortality of Bemisia tabaci nymphs on different Capsicum annuum genotypes inoculated and uninoculated with CBTC1.
Inoculation of plants with rhizobacteria induces some degree of resistance to several species of phytophagous insects. This was the case of the reduction of feeding (44 %) in a beetle (Diabrotica undecimpunctata howardi Barber) from cucumber plants inoculated with Bacillus pumilus (Zehnder et al., 1997). A significant decrease in the population of aphids (Myzus persicae Zulcer) was also observed in C. annuum plants treated with B. subtilis and B. amyloliquefaciens (Herman et al, 2008). The delay in growth and population size of a cotton aphid (Aphis gossypii) was also observed in cucumber plants treated with a Bacillus sp. strain (Stout et al, 2002).
The induction of resistance to B. tabaci by inoculation with Bacilli species has been little studied. Murphy et al. (2000) found that tomato plants from seeds inoculated with Bacillus spp. had a significant decrease (40-43 %) in the B. tabaci nymph population. Soto et al. (2010) reported that inoculation of B. subtilis in tomato (Solanum lycopersicum) significantly decreased the development of B. tabaci nymphs, from the fourth instar to adults. This effect was attributed to the induction of factors involved in induced systemic resistance (ISR) (Rojas-Solis et al, 2013). The results in our study indicated that the effect of the rhizobacteria varies among genotypes from the same species, because the oviposition and mortality of B. tabaci in plants inoculated with B. brevis CBTC1 was only detected in the Simojovel and Jalapeño genotypes. This plant resistance response produced by the inoculation of RPCV and partially attributed to RSI, varies between species or genotypes probably because of the different capacities of its roots to perceive stimuli caused by rhizobacteria (De Vleesschauwer et al., 2008).
In our study the inoculation of different genotypes of C. annuum with B. brevis CBTC1 promoted different plant growth, as it allowed increased aerial biomass, but did not alter the attraction of B. tabaci adults. However, the inoculation induced a degree of resistance to B. tabaci oviposition in the Simojovel genotype and increased nymph mortality in the Jalapeno genotype. This response from the plants inoculated with B. tabaci could be related to nutritional factors of the foliage quality, but not the N foliar content.
Conclusions
The isolated bacteria (CBTC1) showed 99 % homology with Brevibacillus brevis. Inoculation of B. brevis CBTC1 in C. annuum increased stem diameter and plant height. In the Simojovel and Amaxito genotypes leaf number and root biomass were also increased.
Inoculation of C. annuum genotypes with B. brevis CBTC1 had no effect on the attraction of B. tabaci adults. But the inoculation in the Simojovel and Jalapeño genotypes allowed some resistance due to low oviposition or increased nymph mortality.
Literatura Citada
Akgül, D. S., and M. Mirik. 2008. Biocontrol of Phytophthora Capsici on pepper plants by Bacillus megaterium strains. J. Plant Pathol. 90: 29-34. [ Links ]
Ashrafuzzaman, M., M. A. Halim, M. R. Ismail, S. M. Shahidullah, and M. A. Hossain. 2011. Effect of plastic mulch on growth and yield of chilli (Capsicum annuum L.). Braz. Arch. Biol. Technol. 54: 321-330. [ Links ]
Ballina-Gómez, H. S., E. Ruiz-Sanchez, W. Chan-Cupul, L. Latournerie, L. Alvarado, I. Islas, and J. J. Zuñiga. 2013. Response of Bemisia tabaci Genn. (Hemiptera: Aleyrodidae) Biotype B to genotypes of pepper Capsicum annuum (Solanales: Solanaceae). Neotrop. Entomol. 42: 205-210. [ Links ]
Castillo, H. F., C. F. Reyes, G. G. Morales, R. R. Herrera, and C. Aguilar. 2013. Biological control of root pathogens by plantgrowth promoting Bacillus spp. In: Weed and Pest Control Conventional and New Challenges. Chapter 4. pp: 79-103. [ Links ]
Chen, Y., J. R. Ruberson, and D. M. Olson. 2008. Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 126: 244-255. [ Links ]
Christinal, V., and P. Tholkkappian. 2013. Interaction effects of AM fungi with rhizobacteria and phosphate solubilizing bacteria on the growth and nutrient uptake of chilli. J. Appl. Chem. 2: 33-41. [ Links ]
Damayanti, A. T., H. Pardede, and N. R. Mubarik. 2007. Utilization of root-colonizing bacteria to protect hot-pepper against tobacco mosaic tobamovirus. J. Biosci. 14: 105-109. [ Links ]
Das, A. J., M. Kumar, and R. Kumar. 2013. Plant growth promoting rhizobacteria (PGPR): An alternative of chemical fertilizer for sustainable, environment friendly agriculture. Res. J. Agric. For. Sci. 1: 21-23. [ Links ]
De Vleesschauwer, D., M. Djavaheri, P. A. Bakker, and M. Höfte. 2008. Pseudomonas fluorescens WCS 374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 148: 1996-2012. [ Links ]
Guillen, C., R., H. D. Castillo F., G. Morales G., R. Herrera R., N. A. Gonzáles C., P. Corral E., y H. R. Valdés M. 2006. Bacillus spp. como biocontrol en el suelo infestado con Fusarium spp., Rhizoctonia solani Kühn y Phytophthora capsici Leonian y su efecto en el desarrollo y rendimiento del cultivo de chile (Capsicum annuum L.). Rev. Mex. Fitopatol. 24: 105-114. [ Links ]
Herman, M. A. B., B. A. Naultb, and C. D. Smart. 2008. Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Protect. 27: 996-1002. [ Links ]
Hernández, A., L. A., E. Ruiz S., L. Latournerie M., P. S. Sánchez A., y A. Pérez G. 2012. Resistencia de genotipos de Capsicum annuum a Bemisia tabaci. In: Memorias Del VI Congreso de Biotecnología y Bioingeniería del Sureste Mérida, Yucatán del 24 a 26 de octubre del 2012. [ Links ]
Jha, K. S., and M. Saraf. 2011. Effect of plant growth promoting rhizobacteria on seed germination behaviour and seedling vigor of Jatropha curcas. Int. J. Biotech. Biosci. 1: 101-113. [ Links ]
Jones, J. B., B. Wolf and H. A. Mills. 1991. Plant analysis handbook. A practical sampling, preparation, analysis, and interpretation guide. Taylor & Francis. Boca Raton, Fl. 213 p. [ Links ]
Kokalis, N. B., C. S. Vavrina, E. N. Rosskopf, and R. A. Shelby. 2002. Field evaluation of plant growth-promoting rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant Soil 238: 257-266. [ Links ]
Lamsal, K., S. W. Kim, Y. S. Kim, and Y. S. Lee. 2012. Application of rhizobacteria for plant growth promotion effect and biocontrol of anthracnose caused by Colletotrichum acutatum on pepper. Mycobiology 40: 244-251. [ Links ]
Loredo, O., L. López, y D. Espinosa. 2004. Bacterias promotoras del crecimiento vegetal asociadas con gramínea: Una revisión. Terra Latinoam. 22: 225-239. [ Links ]
Lu, Z. X., X. P. Yu, K.L. Heong, and C. Hu. 2007. Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci. 14: 56-66. [ Links ]
Lugtenberg, B. and F. Kamilova. 2009. Plant growth-promoting rhizobacteria. Ann. Rev. Microbiol. 63: 541-556. [ Links ]
Martínez, L. L., R. A. P. Martínez, M. I. Hernández, S. M. A. Medrano, y J. R. P. Aguilar. 2013. Caracterización de rizobacterias aisladas de tomate y su efecto en el crecimiento de tomate y pimiento. Rev. Fitotec. Mex. 36: 63-69. [ Links ]
Murphy, J. F., G. W. Zehnder, D. J. Schuster, E. J. Sikora, J. E. Polston, and J. W. Kloepper. 2000. Plant growth-promoting rhizobacterial mediated protection in tomato against Tomato mottle virus. Plant Dis. 84: 779-784. [ Links ]
Nautiyal, S. C., S. Suchi., C. S. Puneet., S. Karishma., M. Aradhana, and S. K. Sudhir. 2013. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol . Biochem. 66: 1-9. [ Links ]
Nombela, G., F. Beitia, and M. Muñiz. 2001. A differential study of Bemisia tabaci Q-biotype on commercial tomato varieties with or without the Mi resistance gene, and comparative host responses with the B-biotype. Entomol. Exp. Appl. 98: 333-334. [ Links ]
Patten, L. C., and B. R. Glick. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 68: 3795-3801. [ Links ]
Pineda, A., S. Zheng, J. J. A. Van Loon, C. M. J. Pieterse, and M. Dicke. 2010. Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci. 15: 507-514. [ Links ]
Rezvani, H. T., P. Moradi, and F. Soltani. 2013. The effect of nitrogen fixation and phosphorus solvent bacteria on growth physiology and vitamin C content of Capsicum annum L. Iranian J. Plant Physiol . 3: 673-682. [ Links ]
Rojas-Solís, D., M. Contreras-Pérez, y G. Santoyo. 2013. Mecanismos de estimulación del crecimiento vegetal en bacterias del género Bacillus. Biológicas 15: 36-41. [ Links ]
Singh, S. K., P. Verma, N. Ramaiah, A. A. Chandrashekar, y Y. S. Shouche. 2010. Phylogenetic diversity of archaeal 16S rRNA and ammonia monooxygenase genes from tropical estuarine sediments on the central west coast of India. Res. Microbiol. 161: 177-186. [ Links ]
Soto, V. J. H., M. G. E. Hernández, E. I. Laclette, and J. P. D. Frier. 2010. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 231: 397-410. [ Links ]
Stout, M. J., G. W. Zehnder, and M. E. Baur. 2002. Potential for the use of elicitors of plant defense in arthropod management programs. Arch. Insect. Biochem. Physiol. 51: 222-235. [ Links ]
Travers, S. R., A. W. M. Phyllis, and F. C. Reichelderfer. 1987. Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol. 53: 1263-1266. [ Links ]
Vivas, A., I. Vörös, B. Biró, E. Campos, J. M. Barea, and R., Azcón. 2003. Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus brevisisolated from cadmium polluted soil under increasing cadmium levels. Environ. Pollut. 126: 179-189. [ Links ]
Vivas, A. , J. M. Barea, and R. Azcón. 2005. Interactive effect of Brevibacillus brevis and Glomus mosseae, both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soil. Environ. Pollut. 134: 257-266. [ Links ]
Zehnder, G., J. Kloepper, S. Tuzun, C. Yao, G. Wei, O. Chambliss, and R. Shelby. 1997. Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol. Exp. Appl. 83: 81-85. [ Links ]
Received: January 01, 2015; Accepted: January 01, 2016











 texto en
texto en