Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.50 n.2 Texcoco Feb./Mar. 2016
Plant protection
Absorption velocity of glufosinate and its effects on weeds and cotton
1 Universidade Estadual Paulista “Julio de Mesquita Filho” Faculdade de Ciências Agronômicas, 1780, Zip Code 18.610-307. (14) 38807161, Botucatu SP, Brazil (carbonari@fca.unesp.br).
Glufosinate ammonium, an inhibitor of the enzyme glutamine synthetase (GS), is one of the most important herbicides for cotton cultivation and it is utilized for weed management in directed spray applications indicated for conventional cultivars. This study aimed to evaluate the absorption velocity of glufosinate ammonium and its effects on weed and cotton (Gossypium hirsutum L.) plants. The study was carried out in a greenhouse with the cotton cultivar FiberMax 910 and the weeds Brachiaria decumbens and Ipomoea grandifolia. The experimental design was completely randomized and treatments were: the herbicide glufosinate ammonium (0.4 kg a.i. ha-1); five periods before a simulated rain (times for absorption) (1, 3, 6, 24 and 48 h between herbicide application and simulated rain); a control without herbicide; and four repetitions per treatment. Plants were harvested 2 d after herbicide application to quantify their contents of ammonia, glutamine and glufosinate. Visual injury of plants was quantified at 1, 3, 6, 8 and 10 d after application (DAA) of glufosinate ammonium using a percentage scale for damages. Regression analyses were performed on the results and a standard error (by t test; p≤0.05) was established for the means. Glufosinate ammonium absorption increased up to 24 h in cotton, and 48 h in B. decumbens and I. grandifolia. Despite the increase in glufosinate levels in 48 h without rain periods, the contents of ammonia were increased and glutamine was reduced between 1 and 6 h without rains. In addition, the three species presented severe injuries starting at 3 h without rain.
Keywords: Weeds; Gossypium hirsutum; herbicide; mode of action
El glufosinato de amonio, un inhibidor de la enzima glutamina sintetasa (GS), es uno de los herbicidas más importantes para el cultivo de algodón y se utiliza para el control de malezas en aplicaciones de rociado dirigido, indicadas para cultivares convencionales. Este estudio tuvo como objetivo evaluar la velocidad de absorción del glufosinato de amonio y sus efectos sobre las malas hierbas y las plantas de algodón (Gossypium hirsutum L.). El estudio se realizó en un invernadero con el cultivar de algodón FiberMax 910 y las malezas Brachiaria decumbens e Ipomoea grandifolia. El diseño experimental fue completamente al azar y los tratamientos fueron: el herbicida glufosinato de amonio (0.4 kg a.i. ha-1); cinco períodos antes de una lluvia simulada (tiempos de absorción) (1, 3, 6, 24 y 48 h entre la aplicación de herbicidas y la lluvia simulada); un testigo sin herbicida; y cuatro repeticiones por tratamiento. Las plantas se cosecharon 2 d después de la aplicación de herbicidas para cuantificar su contenido de amoniaco, glutamina y glufosinato. La lesión visual de los vegetales se cuantificó a los 1, 3, 6, 8 y 10 d después de la aplicación (DDA) del glufosinato de amonio usando una escala de porcentaje de daños. Los análisis de regresión se realizaron con base en los resultados y se estableció un error estándar (mediante la prueba t, p≤0.05) para los promedios. La absorción de glufosinato de amonio aumentó hasta 24 h en el algodón, y 48 h en B. decumbens e I. grandifolia. A pesar del aumento en los niveles de glufosinato en 48 h sin períodos de lluvia, el contenido de amoníaco se incrementó y la glutamina se redujo entre 1 y 6 h sin lluvias. Además, las tres especies presentan lesiones graves a partir de 3 h sin lluvia.
Palabras clave: Malas hierbas; Gossypium hirsutum; herbicidas; modo de acción
Introduction
Brazil is the fifth largest producer of cotton in the world (FAO, 2012); technology and high productivity have allowed Brazil to shift from the largest importer to the third largest exporter of cotton in 12 years. In a ten-year span, Brazilian cotton cultivation was transformed from manual to total mechanization. For 2013/2014, the area under cotton cultivation in Brazil was 1,076,900 ha with a production of 1 638 100 Mg (CONAB, 2013).
According to Christoffoleti (2002), the utilization of direct-sprayed selective herbicides during initial post-emergence, along with herbicides in pre-emergence and in late post-emergence, is an important weed management system for cotton in central Brazil. Glufosinate ammonium (GA) effectively controls weeds in cotton crops and, in Brazil, it is applied during post-emergence by direct spray on areas planted with cotton genetically modified to be resistant to this herbicide (Rodrigues and Almeida, 2011). Glufosinate ammonium is a synthetic version of phosphinothricin, a degradation product of bialaphos produced by Streptomyces viridochromogenes and S. hygroscopicus (Duke et al., 2000), whose antibiotic and herbicidal properties were discovered by Droge-Laser et al. (1994). This led to the synthesis of GA as herbicide (Manderscheid and Wild, 1986) for use in several crops, different production systems and in non-agricultural areas (Krausz et al., 1999). It is a non-selective herbicide that controls a broad spectrum of weeds (Chompoo and Pornprom, 2008).
Glufosinate ammonium inhibits the activity of glutamine synthetase (EC 6.3.1.2), which converts glutamate and ammonium into glutamine (Logusch et al., 1991) and inorganic nitrogen into organic compounds. It is a key enzyme in the metabolism of nitrogen assimilating ammonia produced by nitrite reductase, and recycling ammonia produced by photorespiration and deamination reactions (Miflin and Habash, 2002). The inhibition of GA activity leads to a rapid accumulation of ammonia, which causes cell destruction (Senseman, 2007). Therefore, an increase in ammonium is used as an indicator of the performance of glufosinate (Pornprom et al., 2000; Petersen and Hurle, 2001).
The mobility of GA is low in plants and it requires good coverage at the time of application for a broad spectrum of weed control (Corbett et al., 2004; Steckel et al., 1997). Since GA is not promptly translocated within the plant, the symptoms occur principally in the leaves (Davis et al., 2013), which present chlorosis and die two to five days after treatment (Shin et al., 2011). Leaf death occurs due to a sharp elevation in ammonium levels and reduced glutamine levels in foliar tissue within a few hours (Chompoo and Pornprom, 2008).
According to Kumaratilake et al. (2002), absorption occurs in the first 24 h after GA treatment in Lolium rigidum and Avena sterilis. A 6 h period without rain after application is needed to obtain an efficient performance of this herbicide (Rodrigues and Almeida, 2011).
Given the importance of GA to control weeds in cotton crops, and the lack of data on the absorption velocity and the intensity of the effects in the metabolism on cotton and important weeds (Brachiaria decumbens and Ipomoea grandifolia), our study aimed to evaluate GA absorption (velocity and quantity) of glufosinate ammonium and its effects on the metabolism of weeds and cotton.
Materials and methods
Three experiments were carried out in a greenhouse under temperatures between 15 and 28 °C, at the São Paulo State University, UNESP, campus at Botucatu/SP/Brazil. Pots (5 L capacity) were filled with a substrate composed of turf, expanded vermiculite and pine bark, enriched with macro and micronutrients, at pH 5.8 (±0.5).
The experimental design was completely randomized with four repetitions; four plants of the cotton cultivar FiberMax 910 were utilized, and ten plants of B. decumbens Stapf and I. grandifolia Dammer per repetitions. The treatments were GA application (0.4 kg a.i. ha-1) and five periods before a simulated rain (times for absorption: 1, 3, 6, 24 and 48 h between application and simulated rain); plus a control without herbicide.
The herbicide was applied in a stationary sprayer equipped with a spray bar equipped with four Teejet XR 11002VS tips arranged 0.5 m apart and positioned at a height of 0.5 m in relation to the plants. The system was operated at a displacement velocity of 1 m s-1, with a liquid consumption of 200 L ha-1 and a constant pressure of 1.5 kgf cm-2 using compressed air. The rainfall simulation of 40 mm in each treatment was performed using the same structure previously described with an automated system allowing for constant-pressure via an hydraulic pump using a spray boom with three TK20-SS high flow spray nozzles spaced 0.5 m apart at 1.45 m above the experimental unit surface. This structure enabled simulations of rainfall of various volumes. The traveling speed of the system was 3.16 m min-1, with pressure of 79.4 kPa, enabling the formation of artificial raindrops with a median volumetric diameter of 1140 μm.
At the time of application, the cotton plants presented completely expanded second true leaves (and a third in expansion) at approximate heights of 30 cm, whereas height of B. decumbens was approximately 35 cm, and that of I. grandifolia was 18 cm.
The plants were harvested for laboratory analyses two days after the herbicide application, when the first visual symptoms injury began to appear. In these samples, the levels of ammonia, glutamine and glufosinate were analyzed.
Visual rating of cotton plant injury and weed control was rated at 3, 6, 8 and 10 d after application (DAA) of glufosinate ammonium. Ratings were based on a 0 to 100 % scale with 0 % equal to no plant response and 100 % equal to complete weed control or cotton death (Richardson et al., 2007).
Ammonia quantification
Ammonia was quantified, according to Wendler et al. (1990), utilizing colorimetry by the action of reagents and determination by spectrophotometry. For the extraction of ammonia, all leaves from each plant per replicate were placed in 300 mL polystyrene bottles. The biomass collected at 2 DAA of the herbicide was determined by weighing and then water (pH 3.5), acidified with HCl, was added to the bottles. The collected leaves were placed in an ultrasonic bath for 60 min, after which the ammonia was quantified in solution with an absorbance reading of the samples at 630 nm.
Analyses in Liquid Chromatography - Mass Spectrometry (LC-MS/MS)
All the leaves from one plant per repetition were collected, washed in distilled water and grounded in liquid nitrogen. A 200 mg aliquot of the sample was weighed in a centrifuge tube, and a 10 mL of extraction solution of water: methanol (75:25) was added to the tube and placed in an ultrasonic bath for 30 min (defined by previous tests, data not shown). After centrifugation at 3500 g for 5 min, the supernatant was filtered through a 0.2 μm membrane and the samples were analyzed in an LC-MS/ MS system.
Glutamate, glutamine and glufosinate were quantified according to Barberis (2012) in an LC-MS/MS system using a HPLC (Proeminence UFLC, Shimadzu Corporation., Kyoto, Japan), equipped with two LC-20AD pumps, an SIL-20AC autoinjector, a DGU-20A5 degasser, a CBM-20A controller system and a CTO-20AC column oven. The HPLC was coupled with a triple-quadruple type 3200 Q TRAP mass spectrometer (Applied Biosystems).
Results and discussion
In cotton plants, an increase of GA level (absorption) was observed up to 24 h without rain (Figure 1). Although the herbicide was absorbed slowly and the highest levels were found in plants after 24 h without rain, the ammonia accumulation occurred independently of herbicide absorption in greater quantities. The absorption until 2 to 5 h without rain, promoted a large accumulation of ammonia in the plants, which shows that the quantity of glufosinate absorbed in 5 h was enough to cause plant injury (Figure 2).

Figure 1 Glufosinate concentration (mg kg-1 fresh weight) in plants of cotton, B. decumbens and I. grandifolia for different periods without rain at two days after application (DAA). Vertical bars represent ± confidence interval of the mean (n=4) by the t test (p≤0.05).

Figure 2 Ammonia concentration (mg kg-1 fresh weight) in plants of cotton, B. decumbens and I. grandifolia for different without rain periods at two days after application (DAA). Vertical bars represent ± confidence interval of the mean (n=4) by the t test (p≤0.05).
The glufosinate quantity absorbed by I. grandifolia plants was superior to that by cotton plants and rose until 48 h after application without rain. For B. decumbens, the absorption was more rapid and intense in the first 6 h without rain, and there was an increase in the herbicide levels in the leaves until 48 h after application without rain simulation (Figure 1).
Everman et al. (2009a) evaluated the quantity of 14C-Glufosinate absorbed by four species, (glufosinate-resistant corn, Eleusine indica, Digitaria sanguinalis and Senna obtusifolia), and observed that S. obtusifolia absorbed 80 % of the GA applied, whereas E. indica and D. sanguinalis absorbed less than 22 % and the resistant corn absorbed only 16 %. Everman et al. (2009b) assessed absorption, translocation, and metabolism of glufosinate in transgenic and nontransgenic cotton, Amaranthus palmeri, and Ipomoea lacunosa, and point out that these species could be divided into two groups based on GA absorption: high (A. palmeri) and low (both nontransgenic and glufosinate-resistant cotton and I. lacunose).
Similarly to the cotton plants, the inside ammonia concentrations of I. grandifolia and B. decumbens stabilized in approximately 2 to 5 h without rain, despite the increase in herbicide absorbed starting from these periods. The higher accumulation of ammonia shows that the quantities of herbicide absorbed in the first hours after application were able to increase levels of this compound in the plants, leading to higher injury levels. Cotton presented the lowest ammonia accumulation, followed by I. grandifolia, and B. decumbens had the highest levels (Figure 2). Ammonium buildup is used as an indicator for glufosinate performance (Petersen and Hurle, 2001). Sellers et al. (2004) evaluated glutamine synthetase activity and ammonium accumulation as influenced by time of glufosinate application in velvetleaf, and after 160-320 g ha-1 glufosinate application, glutamine synthetase activity was reduced by at least 50 % and ammonium concentration was as least 13 times higher in treated plants, compared to control plants. This amount of accumulation is comparable to ammonium concentrations that alter chloroplast ultrastructure and inhibit ATP formation (Krogmann et al., 1959; Puritch and Barker, 1967; Barker, 1968).
According to Pline et al. (1999), the foliar absorption of glufosinate in Solanum carolinense and Asclepias syriaca was fast in the first 12 h after treatment and increased significantly after 12 h. As glufosinate ammonium presents a structure analogous to glutamate, it inhibits glutamine synthetase, which is a key enzyme for controlling nitrogen utilization inside the cells (Coetzer and Al-Khatib, 2001). This results in a rapid accumulation of ammonia content (Lea et al., 1984; Coetzer and Al-Khatib, 2001) and, subsequently, a change in the amino-acid spectrum in plant cells (Lea et al., 1984; Sauer et al., 1987). Tsai et al. (2006) reported drastic accumulation of ammonia, a biochemical marker of the inhibition of glutamine synthetase by glufosinate, in two rice lineages at 9 h after treatment with 0.26 mM glufosinate, and accumulation continued until 24 h after the treatment.
Plant cells avoid ammonium toxicity by rapidly converting the ammonium generated from nitrate assimilation or from photorespiration (Miflin and Habash, 2002; Taiz and Zeiger, 2013). The inhibition of glutamine synthetase by glufosinate delays the photosynthesis rate in C3 and C4 plants (Lacuesta et al., 1993; Wild et al., 1987), which is attributed to inhibition of photorespiration, causing the accumulation of glycolate (González-Moro et al., 1993; Wendler et al., 1990). This accumulation inhibits ribulose-1,5-bisphosphate carboxylase and, consequently, diminishes CO2 assimilation (González-Moro et al., 1997). Photorespiration functions, along with the Calvin-Benson cycle, contribute to a wide array of chloroplast processes, from bioenergy to metabolism of carbon and assimilation of nitrogen; thus, it protects the photosynthetic machinery under stress conditions (Taiz and Zeiger, 2013). This could explain the higher accumulation of ammonium by B. decumbens, as compared with cotton and I. grandifolia, because it has a C3 metabolism in the presence of photorespiration.
The exposure of B. decumbens plants to glufosinate for 1 h before rain was enough to cause the maximum reduction (approximately 80 %) in glutamine content (Figure 3). Cotton showed 60 % reduction in glutamine in 3 h without rain, whereas I. grandifolia showed the lowest glutamine levels at approximately 24 h without rain. This shows that plants must absorb a greater quantity of the herbicide to promote the most intense effects on glutamine content (Figure 3).

Figure 3 Glutamine concentration (mg kg-1 fresh weight) in plants of cotton, B. decumbens and I. grandifolia for different without rain periods at two days after application (DAA). Vertical bars represent ± confidence interval of the mean (n=4) by the t test (p≤0.05).
The inhibition of glutamine synthetase, by glufosinate, generates a glutamine decrease, amino-acid biosynthesis inhibition and ammonia accumulation in the plants (Hess, 2000). After applying 1.02 mM glufosinate, the activity of glutamine synthetase in the rice cv TNG 67, FSK and R11-2 diminished drastically within 6 h after the treatment (Tsai et al., 2006). In Amaranthus palmeri S.Wats, 80 % of the activity of glutamine synthetase was inhibited by glufosinate between 6 and 24 h after herbicide treatment (Coetzer and Al-Khatib, 2001).
Visual injuries of the species analyzed increased throughout the evaluation period (Table 1). Cotton at 3 and 10 DAA, displayed 10 % and 95 % injuries, respectively, and this verified that the quantity of herbicide absorbed in 6 h after application was sufficient to promote the highest injury levels. Symptoms of injuries are evident 3 to 5 d after application of glufosinate ammonium, beginning with chlorosis and spots of liquid saturation followed by the rapid necrosis and death of the plant (Pline et al., 1999).
Table 1 Percentage of visual injury in cotton plants (n=4) and percentage of control in B. decumbens (n=10) and I. grandifolia (n=10) plants for different without rain periods.
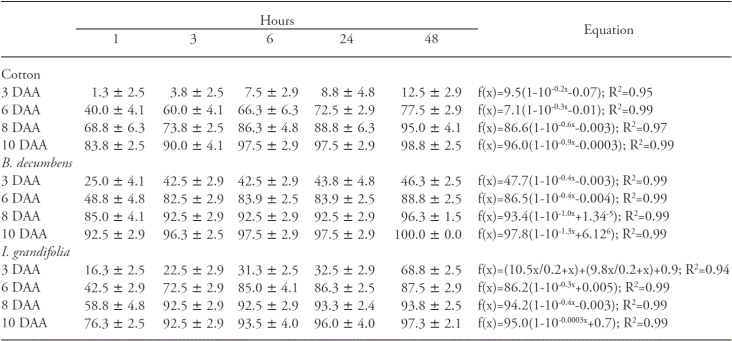
Values are the average of replications ± confidence interval of the mean (n=4) by the t test (p≤0.05). The equations models were adjusted by t test (p≤0.05
At 3 DAA, B. decumbens plants presented 40 % control, which was higher to that observed in cotton in the same period, and at 10 DAA there was 97 % control in plants treated with 3 h of herbicide absorption without rains (Table 1). In I. grandifolia at 3 DAA, plants injuries increased during the longest periods without rain, which shows that greater quantities of glufosinate absorbed in the longest periods without rain promoted more intense symptoms (Table 1). Nevertheless, in the 6, 8 and 10 DAA there were no differences starting at 3 h without rains, reaching approximately 90 % control at 10 DAA.
Anderson et al. (1993) evaluated the effects of simulated rains of 4, 9 and 22 mm on the efficacy of glufosinate ammonium on barley (Hordeum vulgare cv. Samson) and green foxtail (Setaria viridis). They observed that a period of 1 to 8 h without rain (after herbicide application) was required to achieve good control of barley, compared to less than 20 min for green foxtail. Coetzer et al. (2001) estimated glufosinate efficacy in amaranth as affected by relative humidity and temperature, and 4 d after treatment, glufosinate dose (205, 410 and 820 g ha-1) controlled all species, on average higher than 80 % for plants grown at 90 % relative humidity, but 820 g ha-1 glufosinate injured more than 80 % of plants grown at 35 % relative humidity.
Conclusions
Glufosinate ammonium absorption increased until 24 h in cotton and 48 h in B. decumbens and I. grandifolia. Despite the increment in glufosinate content in the longest periods without rain, levels of ammonia were increased and those of glutamine were reduced. Cotton leaves showed the lowest contents of glufosinate, ammonia and glutamine. The two weeds species had higher amounts of glufosinate and ammonia, which were lower for I. grandifolia as compared to B. decumbens. In the periods up to 24 h without rain, the reduction in glutamine was lower for I. grandifolia. Finally, injuries were already severe at three hours without rain for the three species.
Literature Cited
Anderson, D. M., C. J. Swanton, J. C. Hall, and B. G. Mersey. 1993. The influence of soil moisture, simulated rainfall and time of application on the efficacy of glufosinate-ammonium. Weed Res. 33:149-160. [ Links ]
Barberis, L. R. M. 2012. Metodologia para determinação de efeitos fisiológicos de metabólicos do glufosinate em soja. Thesis (Ph.D). Universidade Estadual Paulista. Botucatu. 66 p. [ Links ]
Barker, A. V. 1968. Ammonium interactions with proteins. Biochem. Biophys. Acta. 168:447-455. [ Links ]
Chompoo, J., and T. Pornprom. 2008. RT-PCR based detection of resistance conferred by an insensitive GS in glufosinate-resistant maize cell lines. Pesticide Biochem. Physiol. 90:189-195. [ Links ]
Christoffoleti, P. J. 2002. Trifloxysulfuron-sodium nos sistemas de manejo de plantas daninhas na cultura do algodão: seletividade, eficácia, custos e rendimento. In: Anais do Congresso Brasileiro da Ciência das Plantas Daninhas. Gramado, RS, Brasil. pp: 467. [ Links ]
Coetzer, E., and K. Al-khatib. 2001. Photosynthetic inhibition and ammonium accumulation in Palmer amaranth after glufosinate application. Weed Sci. 49:454-459. [ Links ]
Coetzer, E., K. Al-Khatib., and T. M. Loughin. 2001. Glufosinate efficacy, absorption, and translocation in amaranth as affected by relative humidity and temperatura. Weed Sci. 49:8-13. [ Links ]
CONAB (Companhia Nacional de Abastecimento). 2013. Acompanhamento da safra brasileira grãos, Safra 2013/2014, v.1, n.3, terceiro levantamento. http://www.conab.gov.br (Access: December 2013). [ Links ]
Corbett, J. L., S. D. Askew, W. E. Thomas, and J. W. Wilcut. 2004. Weed efficacy evaluations for bromoxynil, glufosinate, glyphosate, pyrithiobac, and sulfosate. Weed Technol. 18:443-453. [ Links ]
Davis, B., R. C Scott, and J. K. Norworthy. 2013. Response of wheat (Tritium aestivum) to low rates of glyphosate and glufosinate. Crop Prot. 54:181-184. [ Links ]
Duke, S. O., F. E. Dayan, J. G. Romagni, and A. M. Rimando. 2000. Natural products as sources of herbicides: current status and future trends. Weed Res. 40:99-111. [ Links ]
Droge-laser, W., U. Siemeling, A. Puhler, and I. Broer. 1994. The metabolites of the herbicide-phosphinothricin (glufosinate) (identification, stability, and mobility in transgenic, herbicide-resistant, and untransformed plants). Plant Physiol. 105:159-166. [ Links ]
Everman, W. J., C. R. Mayhe, J. D. Burton, A. C. York, and J. W. Wilcut. 2009a. Absorption, translocation, and metabolism of glufosinate in glufosinate-resistant corn, goosegrass, large crabgrass, and sicklepod. Weed Sci. 57:1-5. [ Links ]
Everman, W. J., W. E. Thomas, J. D. Burton, A. C. York, and J. W. Wilcut. 2009b. Absorption, translocation, and metabolism of glufosinate in transgenic and nontransgenic cotton, Palmer amaranth (Amaranthus palmeri), and Pitted morningglory (Ipomoea lacunosa). Weed Sci. 57:357-361. [ Links ]
FAO (Food and Agricultural Organization of the United Nation). 2012. Faostat. http://www.faostat.fao.org (Access: April 2014). [ Links ]
González-Moro, M. B., M. Lacuesta, R. Royuela, A. Muñoz-Rueda, and C. González-Murua.1993. Comperative study of the inhibition of photosynthesis caused by aminooxyacetic acid and phosphinothricin in Zea mays. J. Plant Physiol. 142:161-166. [ Links ]
González-Moro, M. B., M. Lacuesta, J. M. Becerril, C. González-Murua, and A. Muñoz-Rueda. 1997. Glycolate accumulation causes a decrease of photosynthesis by inhibiting RUBISCO activity in maize. J. Plant Physiol. 150:388-394. [ Links ]
Hess, F. D. 2000. Light-dependent herbicides: an overview. Weed Sci. 48:160-170. [ Links ]
Krausz, R. F., G. Kapusta, J. L. Matthews, J. L. Baldwin, and J. Maschoff. 1999. Evaluation of glufosinate-resistant corn (Zea mays) and glufosinate: efficacy on annual weeds. Weed Technol. 13:691-696. [ Links ]
Krogmann, D. W., A. T. Jagendorf, and M. Avron. 1959. Uncouplers of spinach chloroplast photosynthetic phosphorylation. Plant Physiol. 34:272-277. [ Links ]
Kumaratilake, A. R., D. F. Lorraine-Colwill, and C. Preston. 2002. A comparative study of glufosinate efficacy in rigid ryegrass (Lolium rigidum) and sterile oat (Avena sterilis), Weed Sci. 50:560-566. [ Links ]
Lacuesta, M. B., C. González-Moro, A. González-Murua, and A. Muñoz-Rueda. 1993. Time-course effect of phosphinothricin (PPT) on photosynthesis in Medicago sativa. Plant Physiol. 89:847-853. [ Links ]
Lea, P. J., K. W. Joy, J. L. Ramos, and M. G. Guerrero. 1984. The action of 2-amino-4 (methylphosphinyl)-butanoic acid (phosphinothricin) and its 2-oxo-derivative on the metabolism of cyanobacteria and higher plants. Phytochemistry. 23:1-6. [ Links ]
Logusch, E. W., D. M. Walker, J. F. McDonald, J. E. Franz. 1991. Inhibition of plant glutamine synthetases by substituted phosphinothricins. Plant Physiol. 95:1057-1062. [ Links ]
Manderscheid, R., and A. Wild. 1986. Studies on the mechanism of inhibition by phosphinothricin of glutamine synthetase isolated form Triticum aestivum L. J. Plant Physiol. 123:135-142. [ Links ]
Miflin, J. B., and D. Z. Habash. 2002. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 53:979-987. [ Links ]
Petersen, J., and K. Hurle. 2001. Influence of climatic conditions and plant physiology on glufosinate-ammonium efficacy. Weed Res. 41:31-39. [ Links ]
Pline, W. A., J. Wu, and K. K. Hatzio. 1999. Absorption, translocation, and metabolism of glufosinate in five weed species as influenced by ammonium sulfate and pelargonic acid. Weed Sci. 47:636-643. [ Links ]
Pornprom, T., S. Surawattananon, and P. Srinives. 2000. Ammonia accumulation as an index of glufosinate-tolerant soybean cell lines. Pestic. Biochem. Physiol. 68:102-106. [ Links ]
Puritch, G. S. and A. V. Barker. 1967. Structure and function of tomato leaf chloroplasts during ammonium toxicity. Plant Physiol. 42:1229-1238. [ Links ]
Richardson, R. J., H. P. Wilson, and T. E. Hines. 2007. Preemergence herbicides followed by trifloxysulfuron postemergence in cotton. Weed Technol. 21:1-6. [ Links ]
Rodrigues, B. N., and F. S. Almeida. 2011. 6a. ed. Guia de Herbicidas. Londrina. 697 p. [ Links ]
Sauer, H., A. Wild, and W. Ruhle. 1987. The effect of phosphinothricin (glufosinate) on photosynthesis. II. The causes of inhibition of photosynthesis. Z. Naturforsch. 42: 270-278. [ Links ]
Sellers, B. A., R. J. Smeda, and J. Li. 2004. Glutamine synthetase activity and ammonium accumulation in influenced by time of glufosinate application. Pest Bioch Physiol. 78: 9-20. [ Links ]
Senseman, S. A. 2007. Herbicide Handbook. Weed Science Society of America. Lawrence. 458 p. [ Links ]
Shin, J. S., K. M. Kim, D. J. Lee, S. B. Lee, N. R. Burgose, and Y. I. Kuka. 2011. Resistance levels and fitness of glufosinateresistant transgenic sweet potato in field experiments. Field Crops Res. 121:324-332. [ Links ]
Steckel, G. J., S. E. Hart, and L. M. Wax. 1997. Absorption and translocation of glufosinate on four weed species. Weed Sci. 45:378-38. [ Links ]
Taiz, L., and E. Zeiger. 2013. Fisiologia Vegetal. 5a. ed. Artmed. Porto Alegre. 918 p. [ Links ]
Tsai C. J., C. S. Wang, and C. Y. Wang. 2006. Physiological characteristics of glufosinate resistance in rice. Weed Sci. 54:634-640. [ Links ]
Wendler, C., M. Barniske, and A. Wild. 1990. Effect of phosphinothricin (glufosinate) on photosynthesis and photorespiration of C3 and C4 plants. Photosynth. Res. 24:55-61. [ Links ]
Wild, A., H. Sauer, and W. Ruhle. 1987. The effect of phosphinothricin (glufosinate) on photosynthesis. I. Inhibition of photosynthesis and accumulation of ammonia in Sinapis alba. Z. Naturforsch 42: 263-269. [ Links ]
Received: November 2014; Accepted: January 2016











 text in
text in 


