Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.49 no.6 Texcoco ago./sep. 2015
Fitociencia
Effects of water deficit on gas exchange of different age leaves and both stem and leaves solute content of sunflowers
Efectos del déficit hídrico en el intercambio de gases en hojas de diferentes edades y contenido de solutos en tallo y hojas de girasol
Öner Canavar
Adnan Menderes University, Faculty of Agriculture, Crop Science Department. Aydin 09100 Turkey. * Author for correspondence (oner.canavar@hotmail.com).
Received: March, 2015.
Approved: June, 2015.
Abstract
Water deficit alters biochemical and physiological processes ranging from photosynthesis to protein synthesis and solute accumulation in the different organs of the plant. The objectives of this study were to determine the effect of water deficit on photosynthetic responses of leaves at different ages and the accumulation of soluble sugar and starch in the stems and leaves of sunflowers (Helianthus annuus L.). Two sunflower cultivars (Sanbro and TR-3080) were used under two water regimes, well watered (70 % irrigation) and water deficit (20 % irrigation) according to the field capacity of soil under greenhouse conditions. The experimental design was randomized complete block with five replicates. The transpiration rate (E), the ratio of intercellular air space and atmospheric CO2 molar fractions (Ci/Ca), the stomatal conductance (gs), and the net CO2 assimilation rate (A) were used to determine leaf gas-exchange parameters. These data were used to evaluate stomatal limitations to photosynthesis in relation to water deficit. It was found that water deficit (WD) reduced the rate of photosynthesis to a different level in young, fully developed and old leaves in both sunflower cultivars. Under water deficit condition, the sunflower leaves of varying ages showed different responses to water deficit in terms of their resistanance to dehydration. Especially, as the net CO2 assimilation rate was decreased among old leaves, transpiration rate was increased in mature leaves under water deficit. The percentage of soluble sugar was approximately 2-fold higher in the stem of water deficit plants as compared to well watered ones. This study suggests that the cv. Sanbro is likely to be more tolerant due to its high photosynthetic activity, high soluble sugar and starch accumulation and low relative membrane permability (RMP) as compared to the cv. TR-3080.
Key words: Helianthus annuus L., photosynthesis, stomatal limitation, water deficit.
Resumen
El déficit hídrico altera los procesos bioquímicos y fisiológicos, abarcando desde la fotosíntesis hasta la síntesis de proteínas y la acumulación de solutos en los distintos órganos de la planta. Los objetivos de este estudio fueron determinar el efecto del déficit hídrico en las respuestas fotosintéticas de las hojas a distintas edades y la acumulación de azúcar soluble y almidón en los tallos y hojas de girasol (Helianthus annuus L.). Dos cultivares de girasol (Sanbro y TR-3080) se usaron bajo dos regímenes hídricos, buena irrigación (70 % de irrigación) y déficit hídrico (20 % de irrigación), con base en la capacidad de campo del suelo en condiciones de invernadero. El diseño experimental fue en bloques completos al azar con cinco repeticiones. La tasa de transpiración (E), la proporción de espacio aéreo intercelular y CO2 atmosféricos en fracciones molares (Ci/Ca), la conductancia estomática (gs) y la tasa neta de asimilación de CO2 (A) se usaron para determinar los parámetros de intercambio gaseoso. Estos datos se usaron para evaluar las limitaciones estomáticas para la fotosíntesis en relación con el déficit hídrico. El déficit hídrico (DH) redujo la tasa de fotosíntesis a un nivel diferente en las hojas jóvenes, completamente desarrolladas y viejas en ambos cultivares de girasol. Bajo condiciones de déficit hídrico, las hojas de girasol de distintas edades mostraron respuestas diferentes al déficit hídrico en términos de su resistencia a la deshidratación. En especial, al reducir la tasa neta de asimilación del CO2 entre las hojas viejas, la tasa de transpiración aumentó en las hojas maduras bajo déficit hídrico. El porcentaje de azúcar soluble fue cercano al el doble en el tallo de las plantas con déficit hídrico comparado con las de buena irrigación. Este estudio sugiere que el cv. Sanbro puede ser más tolerante debido a su actividad fotosintética alta, alta azúcar soluble y acumulación de almidón, y baja permeabilidad relativa de la membrana (PRM) en comparación con el cv. TR-3080.
Palabras clave: Helianthus annuus L., fotosíntesis, limitación estomática, déficit hídrico.
INTRODUCTION
Water deficit is probably the most important factor controlling crop yield worldwide due to the fact that yield reduction is managed through lower photosynthetic activity (Pejić et al., 2009) and a subsequent reduced leaf growth (Canavar et al., 2014). Water deficit experiments have focused on improving crop genotypes for drought-prone areas. Mechanisms behind drought resistance because of water deficit alter a variety of biochemical and physiological processes ranging from photosynthesis to protein synthesis and solute accumulation (Hu and Schmidhalter, 1998). The reduction of osmotic potential in response to water stress is a well-established mechanism whereby many plants adjust to low soil water availability (Krizmanic et al., 2003). According to Evans et al. (1994), the change in leaf anatomical characteristics can alter the CO2 conductance diffusion components from the substomatal cavities to the sites of carboxylation and thus contribute to maintaining particular photosynthetic rates, despite the low stomatal conductance under drought stress. Similarly, measurements of leaf photosynthesis at high CO2 concentration in several C3 plants at saturating light showed that the photosynthetic capacity had not significantly decreased until leaf water deficit reached a critical value. Thus, the decrease of leaf net CO2 uptake during mild drought stress is mainly attributed to stomatal closure (Morgan, 1984).
With the closure of the stomata, the plants reduce not only water loss by transpiration, but also CO2 supply to the leaves (Baker, 1993; Chaves et al., 2009). Reductions in net assimilation of CO2 (ACO2) in leaves, stomatal conductance (gs) and transpiration rate (E) are often used as indicators of drought stress (Baker and Rosenqvist, 2004). Cechin et al. (2010) pointed out that water stress reduced photosynthesis (Pn, gs, and E in both young and mature leaves. However, the extent of the reduction depended on leaf age. The intercellular CO2 concentration (Ci) increased in mature leaves but did not change in young leaves. Instantaneous water use efficiency (IWUE) in mature stressed leaves was reduced when compared to control leaves, whereas in young stressed leaves it maintained the same level as the control. The acclimatization of photosynthesis to low leaf water potentials in Helianthus appears to involve changes primarily at the chloroplast level (Sayed, 2003).
Inorganic cations, organic acids, amino acids, and sugars, which are the primary osmotica, bring about osmotic adjustment through either internal synthesis or the uptake of osmotically active substances (Rhodes et al., 1986; Pritchard et al., 2000). There is little doubt that soluble sugars can contribute to osmotic adjustment. In agronomic crops there way an increament in soluble sugar concentration in response to water stress in roots and leaves (Premachandra et al., 1992; Shahbaz et al., 2011). Wingler et al. (2005) stated that by signaling a high availability of carbon relative to nitrogen in the old leaves, sugar accumulation could trigger leaf senescence. Sugar-induced senescence is therefore particularly important under low nitrogen availability and may also play a role in light signaling. Drought resistance and its components (stomatal and nonstomatal) are redefined to express the outstanding inventive capacity for terminology. But in the literature reviewed, the investigation of soluble sugar and starch in both leaf and stem of sunflowers and the photosynthetic response to water deficit of leaves of three different ages, are limited under water deficit conditions. Therefore, the objectives of this study were: 1) to determine the accumulation of soluble sugar and starch in leaves and stem of sunflowers under water deficit conditions; and 2) to compare differences of chlorophyll content and the response of photosynthesis among different age leaves (young, fully developed and old leaves) under water deficit conditions.
MATERIALS AND METHODS
Plant material and experiment establishment
This experiment was carried out at the research greenhouse of the Crop Science Department, Faculty of Agriculture, Adnan Menderes University, Turkey, and in collaboration with the Horticulture Faculty, Humboldt University, Germany, in 2013. Two sunflower cultivars were tested: Sanbro, breeded by Syngentha ©, and TR-3080 (Directorate Trakya Agricultural Research Institute, Turkey). Sunflower cultivars were used to determine variation under two water regimes (water deficit and well-watered) and environmental conditions of approximately 12/12 h light/dark, 25/15±3 °C and 40-50 % RHin a greenhouse experiment. The sunflower cultivars were planted in Mitschelin pots (30 cm deep 20 cm in diameter) in the greenhouse and exposed to the natural sunlight of the summer months. We used clay loam soil to fill pots. The cultivars were set up in a completely randomized block design with five replications. We applied chemical fertilizers following the manufacturer's instructions of 1 g nitrogen from 3.70 g KAS (Kalkammonsalpeter) fertilizer according to field conditions, and then six seed were sowed. After sowing and once the seedlings had emerged, thinning was carried out and the plant populations were maintained (3 plants in a pot).
Determination of water holding capacity of soil
To determine the soil field capacity, soil samples with a pH 6.3, low carbon level 0.7 mg 100 g-1 and sandy soil 73 %, was obtained from the experimental field area, they were air-dried and ground to pass through a 5 mm sieve at room temperature. Each pod was filled with 6 kg of ground soil. Water holding capacity was determined using a gravimetric method with five replicates and mean moisture was estimated (percentage). Firstly the bottoms of five 100 cm3 cylindrical tubes were covered with paper and a plastic strap for the filter and they were weighed without soil and then filled completely with soil (by compression). Each cylindrical tube with soil was weighed (g) and settled in a tray, which was approximately as deep as the height of the cylindrical tube. The tray was fully filled with water up to the top of the cylindrical tube for 3 h in order to allow soil water saturation. Further, all cylindrical tubes were left on the quartz soil for 2 h (for drainage and filtering). Ultimately, all the saturated cylindrical tubes were cleaned and weighed again (wet weight, g) while all the tubes were oven-dried at 105 °C for 24 h and the weight of the oven-dry soil samples was measured (dry weight, g). The field capacity of undisturbed soil was calculated with the following formula; F.C (%) = wet soil weight — dry weight / dry weight x 100.
Water deficit treatment
The variation of soil water content of each pot were measured and checked daily by weighing each pot at the emergence of two cotyleydon and end of the removed plant. The soil water factor included two irrigation regimes: irrigation at 20 % (water deficit) and 70 % (well-watered) of field capacity. The treatments began after cotyledon leaves appeared Plants were harvested 45 d after sowing when they were at the R1 stage (bud visible).
Chlorophyll content
Leaves chlorophyll content (in SPAD unit) was assessed using a chlorophyll meter (SPAD-502, Minolta) and measurements were taken at six points on the both sides of six young leaves, six young fully-developed leaves and six old leaves on just a cultivar in one replicate under two water regimes before harvest. One hundred eight readings were averaged for a plant in a replicate (36 readings for young, 36 readings for young fully developed and 36 readings for old leaves). Total readings were 2160 on three different age leaves of two sunflower cultivars within five replicates.
Leaf-gas exchange measurements
Leaf-gas exchange was measured on young leaves (top of the plant), mature fully developed young leaves (upper part of the plant) and old leaves (lower part of the plant) in both cultivars and treatments at 40 d after sowing using a portable infrared gas exchange analyzer-fluorescence system (WALZ GFS-3000, Effeltrich, Germany). Assimilation rates (A), stomatal conductance to water vapour (gsw), transpiration rate (E), and intercellular CO2 molar fractions (Ci) were calculated using the equations of von Caemmerer and Farquhar (1981). Measurements were made inside the greenhouse before the middle of the day and under atmospheric CO2 concentration (360 μmol mol-1) on each leaf. A leaf chamber with 4 cm2 surface area was used. The flow rate was set to 750 μmol m-2 s-1. Chamber temperature (Tcuv °C) was set to ambient temperature (24 °C). Photosynthetic active radiation (PAR) of 1700 μmol m-2 s-1 was supplied by a light unit containing blue LED's at 470 nm and red ones at 660 nm mounted on the top of the leaf chamber. Instantaneous water use efficiency (IWUE) was calculated by dividing A by E. Measurements were carried out on two young leaves, two young fully developed leaves, and two old leaves, with two measures per leaf in five replications for each treatment. Forty readings were averaged by readings of two leaves per plant for five replicates per genotype by one leaf group in both conditions.
Determination of leaf and stem soluble sugar
The young fully developed leaves from the shoot apex and stem were freeze-dried for 24 h and ground finely with a mortar and pestle. The frozen leaves were directly dried using the method of lyophilization (CHRIST Lyophilizer GAMMA 1-16 LSC model, London, England). Soluble sugars were extracted from 0.1 g dry matter with 80 % ethanol (v/v) at 70 °C for 1 h. The extracts (centrifuge tube 10 mL) were mixed with a vibra mixer (Bender 8 Hobein AG, Zurich, Switzerland). All extracts were shaken (Julabo SW20 shaker) in a water bath at 70 °C for 30 min. All tubes were centrifuged at 4000 rpm at 20 °C for 7 min in a Hettich centrifuge (Rotanta 460 R model). Retained supernatant was transferred to a 25 mL flask. This procedure was repeated three times. The extract was filtered through Whatman no. 1 filter paper and the ethanol evaporated. The residue was redissolved in 25 mL of deionized water and an aliquot of the resultant solution was filtered through a 0.45 μm Millex-HA filter unit (Millipore; Bedford, Mass.). Then, 0.1 % anthrone solution (Alfa-Aesar Gmbh & Co KG, Karlsruhe, Germany) in 1000 mL 95 % H2SO4 (Merck KGaA, Darmstadt, Germany) was prepared in a dark bottle. 1 mL of sample extract was transferred to a test tube and 2.5 mL of anthrone solution was added. All test tubes were mixed by a vibra mixer and put in boiling water for 15 min. Soluble sugar in leaf and stem was measured against a glucose standard spectrophotometrically at 620 nm (Analytikjena Specord model, Jena, Germany). Two zero standards were used as a reference. Standard solution: 500 mg L-1 glucose in 48 % ethanol (50 mg glucose in 100 mL 48 % ethanol). Soluble sugar of leaf and stem was determined from a standard curve calculated as μg g-1 according to the following formula:
Sugar concentration (mg L-1) = glucose mg L-1 x 0.9.
Eight standard curves were constructed from a dilution series of mg L-1 glucose (0, 12.5, 25, 50, 75, 100, 125 mg L-1) of soluble sugar, from a stock solution in 25 mL flask (0, 0.625, 1.25, 2.5, 3.75, 5, 6.25, 7.5 mg L-1), respectively.
Determination of leaf and stem starch
The sample remaining from sugar analysis in the centrifuge tube was added with 2 mL of distilled water and mixed with a vibra mixer (Bender 8 Hobein AG, Zurich, Switzerland). Afterwards all tubes were covered with aluminum foil and put in boiling water for 15 min. All tubes had 2 mL of perchloric acid (9.2 mol L- 1) and were mixed by a vibra mixer. After 15 min, 4 mL of distilled water was added to all tubes. The tubes were centrifuged for 10 min at 4000 rpm in a Hettich centrifuge (Rotanta 460 R model). The remains in the tube were transferred to 50 mL flask. This procedure was repeated three times with perchloric acid. 1 mL of sample was extracted and transferred to a test tube and 2.5 mL of anthrone solution was added. All test tubes were mixed with a vibra mixer and put in boiling water for 15 min. Starch in leaf and stem was measured spectrophotometrically against a glucose standard at 620 nm (Analytikjena, Specord model, Jena, Germany). Two zero standards were used as reference. Standard solution: 500 mg L-1 glucose (55 mg glucose-monohydrate in 100 mL distilled water). Starch of leaf and stem was determined from a standard curve calculated as mg L-1. Eight standard curves were constructed from a dilution series of mg L-1 glucose (0, 12.5, 25, 50, 75, 100, 125 mg L-1) of soluble sugar in mL stock solution in 25 mL flask (0, 0.625, 1.25, 2.5, 3.75, 5, 6.25, 7.5 mg L-1), respectively. Calculations were made by standard curves and the measured extinction coefficient was used to read concentration directly in the program to obtain soluble starch concentration (mg g-1 dry weight) = Reading of standard × sample reading × dilution factor.
Relative membrane permeability (RMP)
The RMP of the leaf cells was determined by the method described by Yang et al. (1996), about the extent of ion leakage. The fully expanded young leaves from each plant were cut into three discs (1.0 cm diameter) and these discs (0.5 g) were placed into test tubes containing 20 mL distilled water. After vortexing the samples for 3 s, initial electrical conductivity (EC0) of each sample was measured. Then the samples were incubated at 4 °C for 24 h and electrical conductivity (EC1) was measured again. The samples were then autoclaved at 120 °C for 15 min, cooled to room temperature and (EC2) was measured for the third time (Akram and Ashraf, 2008; Heideri et al., 2011). The RMP was calculated using the following formula:
RMP (%) = (EC1 — EC0) / (EC2 — EC0) x 100.
Statistical analysis
To analyze the variance of water deficit in the two sunflower cultivars and the interaction of water deficit, cultivars and different organs an ANOVA was performed. The ANOVA and LSD values for each variable were analyzed by the variance of water regime, cultivars and their interactions. The analysis was performed using SPSS and Tarist (Açikgöz et al., 1994) statistical computer programs. Significant differences between the mean of replications were tested using Fisher's LSD method.
RESULTS AND DISCUSSIONS
There were statistically significant differences between E, the ratio Ci/Ca, gs, A and IWUE between the two cultivars (Table 1 and Figure 1). Water deficit had statistically affected on A, E, C/Ca, gs and IWUE except for Chl of sunflower plant (Table 1). There were also statistically significant differences in terms of A, E, Ci/Ca, gs, IWUE and Chl among the young, mature (young fully-developed) and old leaves of both cultivars (Table 1). Cultivars x Leaves interaction was statistically significant in terms of A, Ci/Ca, gs, IWUE and Chl except for E (Table 1).
Different leaves aged young, mature and old showed statistically remarkable response under both water regimes (Table 1). Two sunflower cultivars and young, mature and old leaves and between interactions, respond differently with regard to A, E, Ci/Ca, gs, IWUE under the two water regimes (Table 1).
The mean A rates of both cultivars in young, mature and old leaves were 9.45, 8.05 and 7.2 μmol m-2 s-1, respectively, and they were significantly decreased by water deficit. The mean A rates of young, mature and old leaves of TR-3080 were 9.9, 6.5 and 7.0 μmol m-2 s- 1, respectively, and they were reduced by water deficit condition, as compared to well watered condition. Sanbro cultivar showed higher A than that of TR-3080 in terms of age of leaves, both under well watered and water deficit conditions (Figure 1). However, the highest reduction of A was recorded by Sanbro and TR-3080 with a mean value of 8.8 and 9.9 μmol m-2 s-1, respectively, in young conditions (Figure 1). The highest reduction in E under water deficit was also observed in young leaves of both cultivars (Figure 1). There were significant differences of E in the mature and old leaves between Sanbro and TR-3080 treatments (Figure 1). However, the reduction of E in young leaves of both cultivars under water deficit was lowest by 2.15 μmol m- 2 s- 1. Also, the old leaves in both cultivars had higher E than the young and mature leaves under water deficit (Figure 1). In our study, the E of young, mature and old leaves of Sanbro were higher by 0.1mmol m-2 s-1 than that of TR-3080 under water deficit condition (Figure 1). It is considered that A in old leaves (senescent leaves) is associated with the increase in E, gs and low chlorophyll content, low efficiency of leaf photochemistry and photosynthesis, low capacity for both electron transport through photosystem II (Johnson and Maxwell, 2000) and low CO2 fixation under stress conditions. Flexas and Medrano (2002) also explained that the metabolism is impaired as a consequence of decreased synthesis of ribulose bisphosphate, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, photochemistry and ATP synthesis in the early phases of water stress. Thus, old leaves lose more water by transpiration through high depletion energy. Also carbohydrate metabolism in the leaves changes greatly during leaf development. Young leaves are heterotrophic, which depends in part on carbohydrate imported from other regions of the plant, while mature leaves are the major source of transported sugars and minerals (Rennie and Turgeon, 2009). This result also corresponds with that of explained by Rawson and Hackett (1974), who found that the leaf position on the stem of tobacco affected gas exchange and transpiration rate, with intermediate position leaves (not young) having higher net photosynthesis and transpiration. Thereby, the surplus of carbohydrate is used to maintain young fully developed mature leaves.
Although young leaves of both cultivars had higher stomatal conductance (gs) by a mean of 0.31 mol m- 2 s-1 than the other leaves, mature and old leaves under well watered conditions, it was lower in younger leaves (0.11 mol m-2 s-1) than that of the mature and old leaves under water deficit conditions (Figure 1). The lowest percentage reduction was recorded in mature and old leaves, compared with the percentage reduction in young leaves under water deficit condition (Figure 1). Sanbro cultivar had greater gs than that of TR-3080 in all age leaves and under both water conditions (Figure 1). The reason why we consider an increase in abscisic acid (ABA) concentration within tissue of young leaves under water-deficit condition is because this stimulates efflux from guard cells, which leads to stomatal movement and closure (Raschke and Hedrich, 1985). Also, it may be due to the challenge of the old leaves of sunflowers in the complete and rapid recovery of photosynthesis from the adverse effects of water stress on both stomatal and nonstomatal factors (Raschke and Hedrich 1985).
IWUE in all leaves of both cultivars was significantly decreased by water deficit conditions (Figure 1). According to Raschke and Hedrich (1985) this effect appeared in order to close stoma under drought stress, limiting CO2 availability and ultimately reducing photosynthesis. The IWUE of young leaves under both conditions was higher than that of mature and old leaves. However, the reduction percentage of old leaves (47 %) was higher than that of the other leaves, with an IWUE 1.69 and 1.50 for TR-3080 and Sanbro (data not shown). Especially, the results show that the young leaves of Sanbro contained higher IWUE than that of TR-3080 under water deficit conditions (Figure 1). According to Uzunova and Zlatev (2013) this is a molar ratio of assimilation to transpiration. In others words, they keep their stomata relatively open allowing to compensate for water losses or for a loss of stomatal control, the water use efficiency could remain unchanged or insignificantly reduced. The transpiration rates for Sanbro leaves across ageing were higher than those for TR-3080 (Figure 1) and also stomatal conductance of Sanbro was higher than TR-3080. Therefore, high transpiration losses of young, mature and old leaves of Sanbro can be attributed to more open stomata, which caused more water lost.
The results of our study showed that in the old leaves of sunflower plants, Ci/Ca decreased due to water deficit. Also Ci/Ca of young, mature and old leaves of Sanbro were lower than that of TR-3080 under water deficit conditions. There were markedly differences in terms of Ci/Ca among the leaves of different ages. The highest change ratio of intercellular air space and atmospheric CO2 molar fractions, in response to water deficit conditions was observed in old leaves. Reduction in photosynthetic rate as leaves age is attributed to a reduction in concentrations of enzymes involved in the photosynthetic reactions (Lemonie et al., 2013) and chloroplast membrane composition (Bruce, 2000).
The results show that there were significant negative effects of water deficit on the chlorophyll content or greening of old leaves in both genotypes (Figure 1). However, statistical analyses revealed that the chlorophyll in young and mature leaves of TR-3080 increased under the water deficit conditions. On average, the increase of chlorophyll in Sanbro, under water deficit conditions, was not statistically different (Figure 1). The increase in young and fully expanded leaves is attributed to biochemical changes in the production of fully developed chloroplasts, including synthesis of a variety of molecules and increases in the total number of chloroplasts (Leech and Baker 1983; Lieth and Pasian 1990; Burke, 2007).
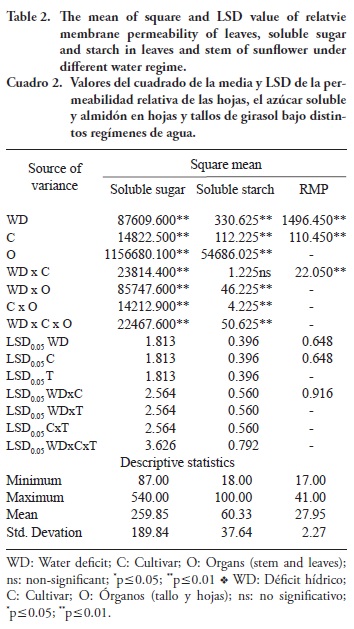
There were statistically significant differences in soluble sugar, starch and relative membrane permeability (RMP) between the two sunflower cultivars (Table 2). Water deficit had statistically significant effect on soluble sugar, starch and RMP of sunflower plants (Table 2). There were also statistically significant differences results with regard to soluble sugar and starch between the stem and leaves of both sunflower cultivars (Table 2). Cultivars x organs (stem and leaves) interactions were statistically significant for soluble sugar and starch (Table 2). It was observed that different water regime x organs interaction was markedly significant for soluble sugar and starch of the sunflower plant (Table 2). In addition, WD x C x O interaction was statistically significant for soluble sugar and starch (Table 2). The RMP value of both sunflower cultivars responded statistically differently against the water deficit condition (Table 2).
Imposition of water deficit resulted in a significant increase in total soluble sugar content in both leaves and stem (Figure 2). TR-3080 cultivar had a higher soluble sugar content (172 mg g-1) in its stem, as compared to Sanbro under well watered condition; however, Sanbro had higher soluble sugar content (24 mg g-1) than TR-3080 under water deficit condition (Figure 2). These results agree with those of Prado et al. (2000) who indicated that water stress induce accumulation of soluble sugars, and Conroy et al. (1988) pointed out that the concentration of ethanol, soluble sugars and potassium increased during drought conditions. The solutes accumulation may allow plants to maintain a positive pressure potential which is required to keep stomata open and to sustain gas exchange and growth (White etal., 2000). In particular, the stem of the sunflower had higher total soluble sugar than that of leaves under both conditions. It was revealed that the percentage increase of the soluble sugar in the stem was higher than that of leaves under water deficit conditions. The rate of soluble sugar increment in Sanbro stem was higher (294 mg g-1) than that of TR-3080, under water deficit conditions. Singh (2004) showed that a greater accumulation of sugar lowers the osmotic potential of cells and reduces turgidity loss in plants. Another possible role of sugar is indicated as a readily available energy source. Water-stress significantly enhanced the absolute amount and the percentage of carbon in the cell wall, glucose and sucrose fractions in both upper and middle canopy leaf positions relative to the control.
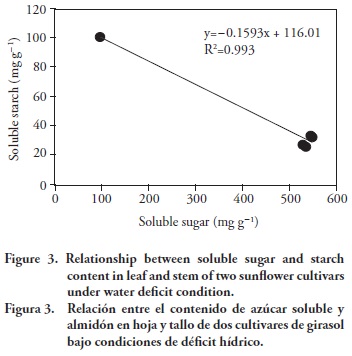
In contrast, accumulation of soluble starch in leaves and stems are shown to be the inverse of soluble sugar under water deficit condition (R2=0.993) (Figure 3). The increase in sugar levels accompanied by a decrease in starch content in seedlings was directly linked to the activity of α and β-amylases, which is in agreement with reports by Sauer (2007). Koshkin and Tretiakov (1990) and Hummels et al. (2010) also emphasized that measurements of soluble sugars and starch contents in cotton leaves indicated that a significant portion of the carbon fixed during the daytime is stored as starch, which appeared due to gradually hydrolyzed and actively transported to other plant parts during the night. This result was performed due to carbon structure from starch in chloroplast and conversion to sucrose. Accumulation of soluble starch in leaves was higher than that of stems in both conditions (Figure 2). Sanbro cultivar had higher soluble starch in leaves and stems than that of TR-3080 under water deficit conditions (Figure 2). It seemed that Sanbro successed to show tolerance in water deficit conditions compared to TR-3080, in terms of having a higher accumulation of soluble sugar and starch in leaves and stems than that of TR-3080. Kerepesi and Galiba (2000) pointed out that tolerant genotypes accumulated more soluble carbohydrate than sensitive ones did.
In our study, water deficit stress caused an increase in cell membrane permeability of leaves of sunflowers (Figure 4). TR-3080 had statistically higher percent values (8 %) for cell membrane permeability than that of Sanbro, because there was more electrolyte leakage compared to Sanbro under water deficit conditions (Figure 4). This was speculated due to membrane damage by abiotic stresses owing to ROS (reactive oxygen species) production (Mansour and Salama, 2004). Thus, it can be assumed from the consistency in data that Sanbro, which had lower RMP than that of TR-3080, may be more water deficit tolerant because of protecting its membrane from damage by ROS.

CONCLUSIONS
The effects of water deficits on photosynthesis in the different leaf age of sunflower plants are very complex by the observed change in net assimilation rate, stomatal conductance and Ci/Ca ratio in drought-stressed sunflower plants. The effect of leaf age on photosynthetic capacity per unit leaf area was similar, but differed slightly in the direction expected by cost-benefit theory. Photosynthesis and photochemical efficiency depended on node position, which on each cultivar has a differently photosynthesis response against water deficit. In a photosynthesis study with infrared gas exchange analyzer system, all leaves of any cultivar cannot be used to determine photosynthetic capacity of a cultivar because of long analysis time for each leaf, oversampling, excessive time, the difference of measuring time from the first leaf to the last leaf in all treatments. Therefore, continued sampling of young fully expanded leaves from a plant over an extended period may likely to be suitable to determine photosynthetic activity throughout an experiment because there is a more reliable measurement of A, Ci/Ca, gs and chlorophyll under water stress condition.
The increase of soluble sugar and starch in stem was higher than that of leaves under water stress conditions. Water deficit tolerance index showed that Sanbro could be relatively water deficit tolerant as compared to TR-3080 because of its high soluble sugar starch accumulation and photosynthetic activity.
LITERATURE CITED
Açikgöz, N., M. E, Akba, A, Moghaddam, and K, Özcan. 1994. Turkish data based statistics programmer for PC. Turkey Field Crops Congress, Ege University Press pp: 264-267. [ Links ]
Akram, M. S., and A. M. Ashraf. 2008. Alleviation of adverse effects of salt stress on sunflower (Heliantus annuus L.) by exogenous applicaiton of potassium nitrate. J. Appl. Bot. Food Qual. 83: 19-27. [ Links ]
Baker, N. R. 1993. Light-use efficiency and photoinhibition of photosynthesis in plants under environmental stress. In: Smith, J. A. C., and H. Griffiths (eds). Water Deficits Plant Responses from Cell Community. Oxford: Bios Scientific Publisher. pp: 221-235. [ Links ]
Baker, N. R., and E. Rosenqvist. 2004. Review article: Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 55: 1607-1621. [ Links ]
Bruce, D. B. 2000. Chloroplast transit peptides: structure, function and evolution. Trends Cell Biol. 10: 440-447. [ Links ]
Burke, J. J. 2007. Evaluation of source leaf responses to water-deficit stresses in cotton using a novel stress bioassay. Plant Physiol. 143: 108-121. [ Links ]
Von Caemmerer, S., and G. D, Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 153: 376-387. [ Links ]
Canavar, Ö., P. K, Götz., F, Ellmer, M. F, Chmielewski, and M. A, Kaynak. 2014. Determination of the relationship between water use efficiency, carbon isotope discrimination and proline in sunflower genotypes under drought stress. Austra. J. Crop Sci. 8: 232-242. [ Links ]
Cechin, I., N, Corniani, T. F, Fumis, and A. C. Cataneo. 2010. Differential responses between mature and young leaves of sunflower plants to oxidative stress caused by water deficit. Cienc. Rural Santa Maria. 40: 1290-1294 [ Links ]
Chaves, M. M., J, Flexas, and C. Pinheiro. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103: 551-560. [ Links ]
Conroy, J. P., J. M, Virgona, R. M, Smillie, and E. W, Barlow. 1988. Influence of drought acclimation and CO2 enrichment on osmotic adjustment and chlorophyll a fluorescence of sunflower during drought. Plant Physiol. 4: 1108-1115. [ Links ]
Evans, J. R., S, von Caemmerer, B. A, Setchell, and G. S. Hudson. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust. J. Plant Physiol. 21: 475-495. [ Links ]
Flexas, J., and H. Medrano. 2002. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann. Bot-London. 89: 183-189. [ Links ]
Hu, Y., and U. Schmidhalter. 1998. Spatial distributions and net deposition rates of mineral elements in the elongating wheat (Triticum aestivum L.) leaf under saline soil conditions. Planta. 204: 212-219. [ Links ]
Hummels, I., F. Pantin, R. Sulpice, M. Piques, G. Rolland, and M. Dauzat. 2010. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 154: 357-72. [ Links ]
Johnson, N. G., and K. Maxwell. 2000. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51: 659-668. [ Links ]
Kerepesi, I., and G. Galiba. 2000. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2: 482-487. [ Links ]
Koshkin, E., and N. Tretiakov. 1990. Gas exchange measurements required to predict the newly fixed carbon pool size in a source leaf of corn. Plant Cell Environ. 13: 147-153. [ Links ]
Krizmanic, M., I. Liovic, A. Mijic, M. Bilandzic, and G. Krizmanic. 2003. Genetic potential of OS sunflower hybrids in different agroecological conditions. Sjemenarstvo 20: 237-245. [ Links ]
Leech, R. M., and N. R. Baker. 1983. The development of photosynthetic capacity in leaves. In: Dale, J. E. and F. L. Milthorpe (eds). The Growth and Functioning of Leaves. Cambridge University Press, Cambridge, England. pp: 271-307. [ Links ]
Lieth,J. H., and C.C. Pasian. 1990. A model for net photosynthesis of rose leaves as a function of photosynthetically active radiation, leaf temperature, and leaf age. J. Am. Soc. Hort. Sci. 115: 486-491. [ Links ]
Mansour, M. M. F., and K. H. A. Salama. 2004. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 52: 113-122. [ Links ]
Morgan, J. M. 1984. Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol. 35: 299-319. [ Links ]
Pejić, B., L, Maksimović, D. Škorić, S. Milić., R. Stričević, and B. Ćupina. 2009. Effect of water stress on yield and evapotranspiration of sunwlower. Helia 32: 19-32. [ Links ]
Prado, F. E., C. Boero, M. Gallardo, and J. A. Gonzalez. 2000. Effect of NaCl on germination, growth and soluble sugar content in Chenopodium quinoa wild seeds. Bot. Bull. Acad. Sinica 41: 27-34. [ Links ]
Premachandra, G., H. Saneoka, K. Fujita, and S. Ogata. 1992. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in sorghum. J. Exp. Bot. 43: 1569-1576. [ Links ]
Pritchard, S. G., Z. Ju, E.van Santen, J. Qiu, D. B. Weaver, S. A. Prior, and H. H. Rogers. 2000. The influence of elevated CO2 on the activities of antioxidative enzymes in two soybean genotypes. Aust. J. Plant Physiol. 27: 1061-1068. [ Links ]
Raschke, K., and R. Hedrich. 1985. Simultaneous and independent effects of abscisic acid on stomata and the photosynthetic apparatus in whole leaves. Planta 163: 105-118. [ Links ]
Rawson, H. M, and C. Hackett. 1974. An Exploration of the carbon economy of the tobacco plant. III. Gas exchange of leaves in relation to position on the stem, ontogeny and nitrogen content. Aust. J. Plant Physiol. 1: 551-560. [ Links ]
Rennie, E. A., and R. Turgeon. 2009. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. U.S.A. 106 14162-14167.10.1073/pnas.0902279106. [ Links ]
Rhodes, D., S. Handa, and R. A. Bressan. 1986. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 82: 890-903. [ Links ]
Sauer, N. 2007. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 581 2309-2317.10.1016/j. febslet.2007.03.048. [ Links ]
Sayed, O. H. 2003. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41: 321-330. [ Links ]
Shahbaz, M., M. Ashraf, N. A. Akram, A, Hanif, S. Hameed, S. Joham, and R. Rehman. 2011. Salt-induced modulation in growth, photosynthetic capacity, proline content and ion accumulation in sunflower (Helianthus annuus L.). Acta. Physiol. Plant. 33: 1113-1122. [ Links ]
Singh, A. K. 2004. The physiology of salt tolerance in four genotypes ofchickpea during germination. J. Agric. Sci. Techn. 6: 87-93. [ Links ]
Uzunova, K., and Z. Zlatev. 2013. Drought-induced changes in photosynthesis of young cowpea plants. Agric. Sci. Techn. 5: 32-34. [ Links ]
Wingler, A., E. Brownhill, and N. Pourtau. 2005. Mechanisms of the light-dependent induction of cell death in tobacco plants with delayed senescence. J. Exp. Bot. 56: 2897-2905. [ Links ]
White, D. A., N. C. Turner, and J. H. Galbraith. 2000. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol. 20: 1157-1165. [ Links ]














