Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.49 no.2 Texcoco feb./mar. 2015
Biotecnología
Association of IGF-1 content with whole, reduced-fat, and low-fat milk in México
Asociación del contenido del factor de crecimiento insulínico tipo 1 con leche entera, semi-descremada y descremada en México
Arlette Marín-Quiroga1, Ignacio Villanueva-Fierro1*, M. Alberto Rodríguez-Pérez2, I. Antonio Lares-Asseff1, Isaías Cháirez-Hernández1, J. Bernardo Proal-Nájera1
1 Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Instituto Politécnico Nacional, Unidad Durango, Sigma 119 Fraccionamiento 20 de Noviembre II, 34220, Durango, Durango. * Author for correspondence (ifierro62@yahoo.com).
2 Centro de Biotecnología Genómica, Instituto Politécnico Nacional, Boulevard del Maestro S/N esquina Elías Piña, Colonia Narciso Mendoza, 88710, Ciudad Reynosa, Tamaulipas.
Received: Agust, 2014.
Approved: February, 2015.
Abstract
The content of insulin-like growth factor 1 (IGF-1) in bovine milk in México is unknown, and high levels could increase the health risk for adult people who ingest milk. The aim of this study was to determine the level of IGF-1 and its relationship with fat content using liquid chromatography with ion trap as a mass detector in milk marketed in Durango, México. During 18 months we analyzed, three times, six lots chosen randomly of whole, reduced-fat, and low-fat milk of three brands. Each sample was pre-treated using cut-off membranes, digested with trypsin, and subjected to liquid chromatography-mass spectrometry. There was a significant difference in the mean concentration of IGF-1 between the brands and types of milk analyzed (Chi square=34.66; p≤0.005). The correlation analysis revealed a positive exponential association between IGF-1 concentration and the fat content of milk with determination coefficients of 0.950 for brand 1, 0.974 for brand 2, and 0.984 for recombinant bovine growth hormone-free. To the best of our knowledge, this is the first study documenting an association between IGF-1 levels and fat content in milk. The decision to consume a brand with or without fat depends on the milk taste and IGF-1 requirements.
Keywords: Cut-off filtration, fat, insulin-like growth factor 1, milk analysis, somatomedin.
Resumen
El contenido del factor de crecimiento insulínico tipo 1 (IGF-1) en leche bovina se desconoce y los niveles altos podrían incrementar el riesgo para la salud de los adultos que ingieren leche. El objetivo de este estudio fue determinar el nivel de IGF-1 en leche comercializada en Durango, México, y su relación con el contenido de grasa, mediante cromatografía líquida, con una trampa de iones que actúa como detector de masas. Durante 18 meses se analizaron seis lotes elegidos al azar de leche entera, semi-descremada y descremada de tres marcas, en tres ocasiones. Cada muestra fue pre tratada con membranas que restringen el peso molecular, digerida con tripsina, y se sometió a cromatografía líquida de alta resolución acoplada a espectrometría de masas. Hubo una diferencia significativa en la concentración media de IGF-1 entre las marcas y los tipos de leche analizados (Ji cuadrada = 34.66; p≤0.005). El análisis de correlación reveló una asociación exponencial positiva entre la concentración de IGF-1 y el contenido de grasa de la leche, con coeficientes de determinación de 0.950 para la marca 1, 0.974 para la marca 2 y 0.984 para la leche libre de hormona de crecimiento bovino recombinada. Por lo que sabemos, este es el primer estudio que documenta una asociación entre los niveles de IGF-1 y el contenido de grasa en la leche. La decisión de consumir una marca con o sin grasa depende del sabor de la leche y los requerimientos de IGF-1.
Palabras clave: Filtración de restricción de peso molecular, grasa, factor de crecimiento insulínico tipo 1, análisis de leche, somatomedina.
INTRODUCTION
According to official estimates, the state of Durango in northern México is the third largest producer of cow's milk in the country (Lactodata, 2014). In México and other countries, including USA, some dairy producers use recombinant bovine growth hormone (rBGH) to boost cows' milk production (Machlin, 1973; Daxenberger et al., 1998). However, in Australia, Canada, Japan, and the EU, the sale of rBGH to dairy farmers and its use are prohibited (Cummins, 1999).
The use of rBGH stimulates liver production of insulin-like growth factor 1 (IGF-1) (Purup et al., 1993; Epstein, 1996). The IGF-1 is a peptide of 70 amino acids with a molecular weight of 7650 Da (Clemmons, 1997) and its structure is identical in bovines and humans (Honegger and Humbel, 1986), which accounts for the recognition and utilization of the hormone by the human body. The natural concentration of IGF-1 in milk is 1.27-8.10 ng mL-1 (Collier et al., 1991), but IGF-1 concentration in 5777 random milk samples ranges from 1.0 to 83 ng mL-1 (Daxenberger et al., 1998) and 420 ng IGF-1 mL-1 in colostrum (Piot et al., 2004) ranging from 32 to 2,000 ng mL-1 in some cases (Guauthier et al., 2006).
Cattle that receive rBGH supplements may suffer mastitis due to an increase of up to 30 % in milk production and udders get swollen, coupled with endocrine effects that cause excessive hormones in the body and the development of diseases such as infertility and problems in the hooves that reduce the cow's life expectancy due to exhaustion and weakness (Resnicoff et al., 1995). The increment of IGF-1 levels in blood and milk (Daxenberger et al., 1998) and the increased influx of immunoreactive cells from blood into milk, induce a large increase of milk somatic cell counts (Bruckmaier et al., 2004); besides, IGF-1 enhanced mammary cancer progression in rats (Hadsell and Bonnette, 2000; Thordarson et al., 2004).
A meta-analysis confirmed a moderately elevated risk of Parkinson disease among people with high dairy consumption (Chen et al., 2007). High quantities of IGF-1 are associated with the appearance of breast, colon, and prostate cancers, due to activity on the pituitary to induce powerful endocrine and metabolic effects, including cell growth and replication (Ashdown, 1996). Consumption of milk increases IGF-1 concentration in serum levels of infants and it might explain the positive effect in growth (Hoppe et al., 2004); the higher levels of IGF-1 in milk could also favor obesity (Ejlerskov et al., 2014). Rzehak et al. (2013) analyzed 1090 neonates fed milk formula with two protein concentrations (Low = 1.25 and 1.6 g 100 mL milk; High = 2.05 and 3.2 g 100 mL-1 milk) and a control group (588 breastfed children); they found three single nucleotide polymorphisms (SNPs; rs1520220, rs978458 and 2 195 239), that the IGF-1 gene influenced positively the circulating levels IGF-1 and the molar concentration of IGF-1 / IGFBP-3 serum, which show that a higher intake of protein metabolic programming contributes to growth. The IGF-1 helps to regenerate damaged muscle mass (Mourkioti et al., 2005), inhibits apoptosis (Dunn et al. , 1997), and enhances stem cell recruitment to injured skeletal muscle (Schulze and Spate, 2005). Aging appears to be associated with lower amounts of the growth hormone (GH) and IGF-1, and low calorie intake (Anderson et al., 2009; Bartke, 2011). Milk consumption is associated with high levels of plasma IGF-1 (Morimoto et al., 2005; Rich-Edwards et al. , 2007) and fetal weight increment with maternal milk (Heppe et al., 2011); besides, the levels of IGF-1 and insulin are diminished if milk is substituted with cola beberages (Hoppe et al., 2009), confirming that milk intake increases IGF-1 levels.
Gauthier et al. (2006) used radioimmunoassay (RIA) and report that the IGF-1 content ranged from 0.6 to 150 ng mL-1 for milk and 32 to 2000 ng mL-1 for colostrum. The RIA test is not allowed in our research facilities, because a government permit is needed to handle radioactive substances (DOF, 2012). IGF-1 is also analyzed through an enzyme-linked immunoabsorbent assay (ELISA) of serum (Ibrahim et al., 2013). For milk, Castigliego et al. (2011), and Ollikainen and Muuronen (2013) used ELISA, due to its simplicity and reliability provided by a sample pre-treatment. Analysis of IGF-1 can also be performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) (Sparbier et al., 2005), and by MALDI-TOF with confirmation of metabolites by Western blotting (King et al., 2009). Hence, we developed an analytical approach using liquid chromatography and mass spectrometry (LC-MS), with a cut-off pre-treatment of the sample, to quantify IGF-1 levels in milk commercially available in Durango, northern México. The pre-treatment method was based on that used by Bobe et al. (1998) and Ollikainen and Muuronen (2013) with LC-MS, to determine the concentration of IGF-1 in whole, reduced-fat, and low-fat milk of three brands sold in Durango. Milk fat was analyzed using the 969.16 method (AOAC, 1997). So far, a safe level of IGF-1 in milk has not been established; in childhood it is associated with healthy growth and helps muscle regeneration, but it is not known whether it is healthy for adults to consume due to potential health risks associated with milk ingestion.
MATERIALS AND METHODS
Chemicals
Acetonitrile, formic acid, trypsin type I (10 000 units mg protein-1) from bovine pancreas, IGF-1, guanidine hydrochloride (GdnHCl), bis-tris, dithiothreitol (DTT), sodium citrate, hydrochloric acid (HCl) and Amicon™ ultra centrifugal filter units, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Milk sampling
Three brands of cow's milk marketed in Durango were used to carry out the study from September 2011 to February 2013. All the samples were packed in Tetra Pak and taken randomly from the main grocery stores. Two out of the three brands cover more than 80 % of the milk consumed in Durango (Brands 1 and 2); the third brand was chosen as a control, labeled as rBGH-free, because the producers feed their cows with their own products free of pesticides and herbicides and they do not inject cows with hormones. In México, it is not forbidden to inject cattle with rBGH. For each brand, whole, reduced-fat, and low-fat milk were tested. The samples were replicated six times (3x3x6=54 samples), as recommended by the power test (Montgomery, 2002), taking the average of three analysis from each sample.
Chemical analysis
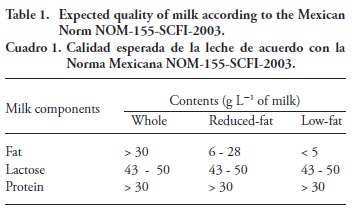
Besides the analysis of milk fat (method 969.16), the milk parameters required by the Mexican Norm (NOM, 2003) were analyzed: water (method 948.1), proteins (method 991.23) and lactose (930.28), which were determined according to AOAC, (1997), in order to determine whether the national specifications of milk are fulfilled (Table 1).
Pre-treatment of the samples
Two mL of milk were dissolved in 2 mL of a pH 6.8 buffer composed of 6 M GndHCl, 0.1 M bis-tris, 19.5 mM DTT, and 5.37 mM sodium citrate (Bobe et al., 1998). The buffer was used to allowing protein denaturation, breaking the sulfur-sulfur bonds and decoupling them from the binding receptors. The mixture was transferred to a 10 kDa Amicon™ cut-off filter, centrifuged at 4000 x g for 30 min, and rinsed with 2 mL of buffer. The retentate, containing αs1-casein, αs2-casein, β-casein, κ-casein, α-lactoglobulin and β-lactoglobulin, because of their molecular weights higher than 10 000, was discarded. The flow-through or eluate was then transferred to a 3-kDa Amicon™ cutoff filter, centrifuged at 20 000 x g for 20 min, and rinsed with 2 mL of the buffer to eliminate lactose, salts, and low molecular weight components. The Amicon™ filter was inverted to retrieve the retained solution, which was reconstituted with 200 μL of 1 mg mL-1 trypsin solution in 1 mM HCl, and digested for 18 h at 37 °C. The digested sample was filtered at 20 000 x g through a 3000 Mw Amicon™ cut-off filter, rinsed with a solution of acetonitrile:water:formic acid (100:900:1) to a pH of 4.3, and brought to a final volume of 200 μL (10 times pre-concentrated). The retentate was discarded.
LC-MS analysis
A Varian 500-MS LC™ ion trap mass spectrometer with electrospray ionization (ESI), (Agilent Technologies, México), was used to perform the analysis of IGF-1. Firstly, the conditions were optimized to generate fragments from intact IGF-1, including needle voltage, spray shield, nebulizer gas (nitrogen), drying gas pressure rate (air), and drying gas temperature. Once the parameters were optimized, 20 μL of each individually digested sample were injected using a Rehodyne™ 6 port injection manual valve (Agilent Technologies, México). A flow rate of 0.05 mL min-1 with a mobile phase of 70 % of solvent A and 30 % of solvent B, were used; solvent A was a 100:900:1 mixture of acetonitrile:water:formic acid and solvent B was a 900:100:1 mixture of acetonitrile:water:formic acid. The separation was performed using a Poroshell 300SB-C18 column (2.1x75 mm; 5 μm particle size) (Agilent Technologies, México).
Statistical analysis
A total of 54 samples were analyzed during 18 months and for the statistical analysis the Kruskal-Wallis test with the Statistica software version 7.0 was used because the data were not normalized. A calibration curve was constructed plotting each standard of known concentration versus the area of each standard. Linear regression was considered if the Pearson correlation coefficient (r) was > 0.99. The fat content of each type of milk for the three brands was plotted vs. IGF-1 content, and the curve that had the best fit according to the r2 coefficients of determination was found.
RESULTS AND DISCUSSION
The sequences of the peptide fragments of IGF-1 and those resulting from digesting IGF-1 with trypsin were retrieved and blasted from the webpage of the Universal Protein Resource3 (Table 2), and they were organized by position of the fragment. Only fragment 5 had a molecular weight above 3000 Da, and it was retained in the 3000 Da cut-off filter.
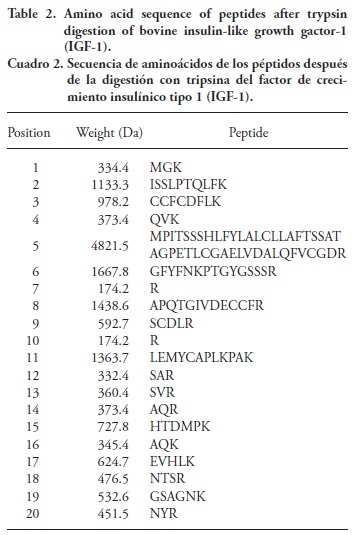
The ESI chamber and standard optimized parameters were: needle voltage of 5000 V; spray shield of 600 V; nebulizer gas (nitrogen) pressure rate of 241 kPa; drying gas pressure rate (air) of 69 kPa; and drying gas temperature of 350 °C. The peptide fragments at positions 6 (GFYFNKPTGYGSSSR), 8 (APQTGIVDECCFR), and 19 (GSAGNK) gave the highest signals during optimization of the LC-MS system for undigested IGF-1. Optimization parameters such as capillary voltage, radiofrequency, and excitation voltage for the highest signals of the most abundant peptides found by LC-MS are summarized in Table 3. The optimal capillary voltage ranged from 150 to 250 V, the frequency from 118 to 183 Hz, and the excitation voltage from 0.94 to 2.55 V.

Since fragment 6 was the most abundant, analyses were based on the fragment of molecular weight of 1667.8 Da. Linearity was between 0.1 and 50 ng mL-1 (low concentrations), and between 100 and 1000 ng mL-1 (high concentrations) of the IGF-1 standard; a linear regression with low and high concentrations indicated that both Pearson correlation coefficients were >0.99. The IGF-1 recovery of spiked milk samples of low and high IGF-1 concentrations was between 82 and 86 %. Based on a signal to noise ratio of 3:1, the detection limit of fragment 6 was 0.2 ng mL-1 of milk (Table 4). The concentrations of IGF-1 for rBGH-free and low-fat milk were left for visual purposes, even though they were below the limit of quantification. Whole milk had the highest IGF-1 content, with 79.7±23.1, 38.8±10.4 and 5.9±2.0 ng mL-1 of milk for Brand 1, Brand 2, and rBGH-free, respectively. These concentrations are within the range reported by Daxenberger et al. (1998), of 1 to 83 ng mL-1 of milk, from the RIA analysis of 5777 samples of natural milk, and within the range of 0.6 to 150 ng mL- 1 of milk described by Guauthier et al. (2006). However, they are lower than the values reported by Ollikainen and Muuronen (2013), who used pre-treatment and ELISA analysis.
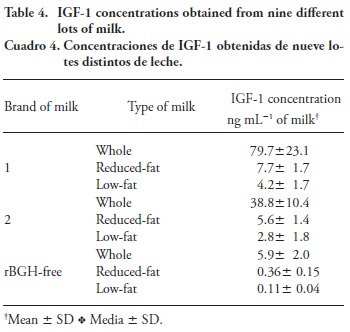
The IGF-1 concentrations in milk are similar to those reported by Daxenberger et al. (1998) and Guauthier et al. (2006), which confirms that using the pre-treatment buffer reported by Bobe et al. (1998) followed by cut-off filtration generates reliable data. Analysis with LC-MS using the fragment of 1667.8 Mw has a shorter analysis time than the ELISA method, but LC-MS is less accurate than ELISA (De Dios et al., 2013). The IGF-1 in the whole-milk rBGH-free brand had a low concentration (5.9±2.1 ng mL-1) and within the range reported by Colliers et al. (1991) of 1.27 to 8.10 ng mL-1, confirming that milk labeled as rBGH-free had lower amounts of IGF-1. The high standard deviations of the samples suggest poor quality control by milk producers. Whole milk for each brand had the highest concentration of IGF-1, followed by reduced-fat and low-fat milk.
Protein contents were 32.7±0.6, 34.4±0.5, and 33.8±0.5 g L-1 of milk for whole, reduced-fat, and low-fat milk, respectively; they all fulfill the limit (>30 g L-1 of milk) established by the Mexican Norm. The fat contents were 36.8±1.2, 18.3± 1.5, and 5.5 ±1.0 g L-1 of milk, for whole, reduced-fat, and low-fat milk, respectively. The fat content of low-fat milk was slightly above the limits set by the Mexican Norm (<5 g L-1 milk), but the fat contents of the other milk, were within the limits (Table 1 and Table 5). Lactose contents were 34.7± 1.5, 32.7±1.5, and 31.3 ±0.9 g L-1 of milk for whole, reduced-fat, and low-fat milk, respectively. None of them fulfill the Mexican Norm for lactose (43-50 g L-1 of milk), probably because water was added.
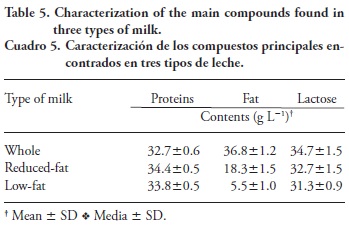
Since higher contents of IGF-1 were observed for whole milk, followed by reduced-fat and low-fat milk, the average fat content for each kind of milk was plotted against the average IGF-1 content for each brand of milk (Figure 1). Correlation analysis revealed a positive association between IGF-1 concentration and milk fat content that increased exponentially with the increase of fat content and depended on the brand: the coefficients of determination (r2) were 0.950, 0.974 and 0.984 for Brands 1, 2, and rBGH-free, respectively. The association between IGF-1 and fat contents in milk has not been reported before. However, it seems that Ollikainen and Muuronen (2013) found this correlation, but they were not aware of it. Single samples of each brand were sent to a National Laboratory to be analyzed by MALDI-TOF. These results also showed that IGF-1 concentration was related to fat content (data not shown), but their limits of detection are above ours.
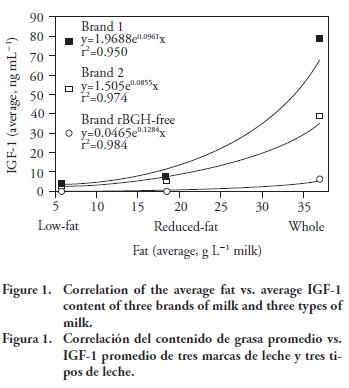
According to the Kruskal-Wallis test, there were significant differences between the brands and types of milk analyzed (Chi squared=34.66; p≤0.005). There were also significant differences in IGF-1 levels between Brands 1 and 3 (p≤0.005) and between kinds 1 and 3 (p≤0.005). The differences between milk samples of Brands 1 and 2 were not significant, and they are similar to those reported by Daxenberger et al. (1998) and Gauthier et al. (2006). These observations plausibly suggest that rBGH is probably not administered to cattle in the study area. However, given that IGF-1 levels may be influenced by many factors and vary considerably, it would be reasonable to expand this study to include a larger sample of different brands and types of milk.
CONCLUSIONS
An exponential correlation of fat in relation to the IGF-1 concentration and type of milk was found. Consumption of the type of milk depends on the taste or amount of fat in milk desired by the consumer and, with this study, on its IGF-1 content. If these studies are continued, consumers could choose milk according to age and body mass index to avoid obesity. This study will be expanded to the other Mexican states to increase the number of samples and corroborate the association of fat and IGF-1, and the ELISA analysis will be implemented to compare the accuracy, sensibility and cost versus the LC-MS test.
ACKNOWLEDGMENTS
We thank M.G. Reyes for lab assistance in preparing and testing the samples. We appreciate the support of Secretaría de Investigación y Posgrado of the Instituto Politécnico Nacional for grants 20120967, 20110855, and FOMIX DGO-2008-C04-94500. All authors were supported by the Operation and Development Committee of Academic Activities (COFAA).
LITERATURE CITED
Anderson, R. M., D. Shanmuganayagam, and R. Weindruch. 2009. Caloric restriction and aging: studies in mice and monkeys. Toxicol. Pathol. 37: 47-51. [ Links ]
AOAC. 1997. Official Methods of Analysis. Association Official Analytical Chemist Inc. (16th ed. Vol. II, Chapter 33). Gaithersburg, Maryland, USA. [ Links ]
Ashdown, K. 1996. New Study Warns of Breast and Colon Cancer Risks from rBGH Milk, Press Release, Press Conference, National Press Club Washington D. C., p. 3. [ Links ]
Bartke, A. 2011. Single-gene mutations and healthy ageing in mammals. Phil. Trans. R. Soc. B. 366: 28-34. [ Links ]
Bobe, G., D. C. Beitz, A. E. Freeman, and G. L. Lindberg. 1998. Separation and quantification of bovine milk proteins by reversed-phase high-performance liquid chromatography. J. Agric. Food Chem. 46: 458-463. [ Links ]
Bruckmaier, R. M., C. E. Ontsouka, and J. W. Blum. 2004. Fractionized milk composition in dairy dows with subclinical mastitis. Vet. Med. -Czech. 8: 283-290. [ Links ]
Castigliego, L., X. Li, Armani, Andrea, M. Mazzi, and A. Guidi. 2011. An immunoenzymatic assay to measure insulin-like growth factor 1 (IGF-1) in buffalo milk with an IGF binding protein blocking pre-treatment of the sample. Int. Dairy J. 21: 421-426. [ Links ]
Chen, H., E. O'reilly, M. L. Mccullough, C. Rodriguez, M. A. Schwarzschild, E. E. Calle, et al. 2007. Dairy products and risk of Parkinson's disease. Am. J. Epidemiol. 165: 998-1006. [ Links ]
Clemmons, D. R. 1997. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth F. R. 8: 45 -62. [ Links ]
Collier, R. J., M. A. Miller, J. R. Hildebrandt, A. R. Torkelson, T. C. White, K. S. Madsen, et al. 1991. Factors affecting insulin-like growth factor-1 concentration in bovine milk. J. Dairy Sci. 74(9) 2905-2911. [ Links ]
Cummins, R. 1999. Hazards of Genetically Engineered Foods and Crops. In Motion Magazine. Little Marais, Minnesota, USA. pp: 1-4. [ Links ]
Daxenberger, A., B. H. Breier, and H. Sauerwein. 1998. Increased milk levels of insulin-like growth factor 1 (IGF-1) for the identification of bovine somatotropin (bST) treated cows. Analyst 123: 2429-2435. [ Links ]
De Dios, K., A. Manibusan, R. Marsden, and J. Pinkstaff. 2013. Comparison of bioanalytical methods for the quantitation of PEGylated human insulin. J. Immunol. Methods. 396: 1-7. [ Links ]
DOF 2012. Official Journal od the Federation. Agreement establishing the clasification and codification of goods whose import and export is subject to authorization by the Ministry of Energy. http://www.normateca.gob.mx/Archivos/54_D_3244_12-10-2012.pdf (Accesed: December 2014). [ Links ]
Dunn, S. E., R. A. Hardman, F. W. Kari, and J. C. Barrett. 1997. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 57: 2687-2693. [ Links ]
Ejlerskov K. T., A. Larnkjaer, D. Pedersen, C. Ritz, C. Mølgaard, and K.F. Michaelsen. 2014. IGF-I at 9 and 36 months of age - relations with body composition and diet at 3 years - the SKOT cohort. Growth Horm. IGF Res. 24: 239-244. [ Links ]
Epstein, S. S. 1996. Unlabeled milk from cows treated with biosynthetic growth hormones: a case of regulatory abdication. Int. J. Health Serv. 26: 173-185. [ Links ]
Gauthier, S. F., Y. Pouliot, and J. L. Maubois. 2006. Growth factors from bovine milk and colostrum: composition, extraction and+ biological activities. Lait 86: 99-125. [ Links ]
Hadsell, D. L., and S. G. Bonnette. 2000. IGF and insulin action in the mammary gland: lessons from transgenic and knockout models. J. Mammary Gland Biol. Neoplasia 5: 19-30. [ Links ]
Heppe, D. H., R. M. Van Dam, S. P. Willemsen, H. Den Breeijen, H. Raat, A. Hofman, E. A. P. Steegers, V. W. V. Jaddoe. 2011. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the Generation R Study. Am. J. Clin. Nutr. 94: 501-509. [ Links ]
Honegger, A., and R. E. Humbel. 1986. Insulin-like growth factors I and II in fetal and adult bovine serum. Purification, primary structures, and immunological cross-reactivities. J. Biol. Chem. 261: 569-575. [ Links ]
Hoppe, C., C. Mølgaard, A. Juul, and K. F. Michalsen. 2004. High intakes of skimmed milk, but not meat, increase serum IGF-1 and IGFBP-3 in eight-year-old boys. Eur. J. Clin. Nutr. 58: 1211-1216. [ Links ]
Hoppe, C., M. Kristensen, M. Boiesen, J. Kudsk, K. Fleischer Michaelsen, and C. Mølgaard. 2009. Short-term effects of replacing milk with cola beverages on insulin-like growth factor-I and insulin-glucose metabolism: a 10 d interventional study in young men. Br. J. Nutr. 102: 1047-1051. [ Links ]
Ibrahim, A. S., H. A. Attia, A. M. Rabea, and A. M. El-Gayar. 2013. Serum levels of glycosaminoglycans (GAGs) and insulin like growth factor-1 (IGF-1) as diagnostic markers for early hepatocellular carcinoma in cirrhotic patients with or without diabetes. J. Med. Lab. Diagn. 4: 8-20. [ Links ]
King, C. C., K. Bouic, and T. Friedmann. 2009. A fractionation method to identify qauntitative changes in protein expression mediated by IGF-1 on the proteome of murine C2C12 myoblasts. Proteome Sci. 7: 28. [ Links ]
Lactodata. 2014. Information about the Dairy Industry, in Spanish, http://www.lactodata.com/lactodata/docs/ind/lacto_ind_prod.pdf (Accesed: March 2014). [ Links ]
Machlin, L. J. 1973. Effect of growth hormone on milk production and feed utilization in dairy cows. J. Dairy Sci. 56: 575-580. [ Links ]
Montgomery, D. C. 2002. Diseño y Análisis de Experimentos (2ª. ed.). México, DF: Editorial Limusa, SA de CV. pp: 40-41. [ Links ]
Morimoto, L. M., P. A. Newcomb, E. White, J. Bigler, and J. D. Potter. 2005. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States). Cancer Causes Control 16: 917-927. [ Links ]
Mourkioti, F. and N. Rosenthal. 2005. IGF-1 inflammation and stem cells: interaction during muscle regeneration. Trends Immunol. 26 (10): 535-542. [ Links ]
NOM 2003. Milk, milk formula and combined milk product-names, physical and chemical specifications, commercial information and test methods, http://www.ordenjuridico.gob.mx/Publicaciones/CDs2007/CDAgropecuaria/pdf/83NOM.pdf (Accesed: March 2014). [ Links ]
Ollikainen, P., and K. Muuronen. 2013. Determination of insulin-likegrowth factor-1 and bovine insulin in raw milk and its casein and whey fractions after microfiltration and ultrafiltration. Int. Dairy J. 28: 83-87. [ Links ]
Piot, M., J. Fauquant, M.-N. L. Madec, and J.-L. Maubois. 2004. Preparation of serocolostrum by membrane microfiltration. Le Lait 84: 333-341. [ Links ]
Purup, S., K. Sejrsen, J. Foldager, and R. M. Akers. 1993. Effect of exogenous bovine growth hormone and ovariectomy on prepubertal mammary growth, serum hormones and acute in-vitro proliferative response of mammary explants from Holstein heifers. J. Endocrinol. 139: 19-26. [ Links ]
Resnicoff, M., D. Abraham, W. Yutanawiboonchai, H. L. Rotman, J. Kajstura, R. Rubin, P. Zoltick, R. Baserga. 1995. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 55: 2463-2469. [ Links ]
Rich-Edwards, J. W., D. Ganmaa, M. N. Pollak, E. K. Nakamoto, K. Kleinman, U. Tserendolgor, W. C. Willett, A. L. Frazier 2007. Milk consumption and the prepubertal somatotropic axis. Nutr. J. 6: 28. [ Links ]
Rzehak P., V. Grote, E. Lattka, M. Weber, D. Gruszfeld, P. Socha, R. Closa-Monasterolo, J. Escribano, M. Giovannini, E. Verduci, P. Goyens, F. Martin, J. P. Langhendries, H. Demmelmair, N. Klopp, T. Illig, B. Koletzko. 2013. Association of IGF-1 gene variants and milk protein intake with IGF-1 concentrations in infants at age 6 months - Results from randomized clinical trial. Growth Horm. IGF Res. 23: 149-158. [ Links ]
Schulze, P. C., and U. Spate. 2005. Insulin-like growth factor-1 and muscle wasting in chronic heart failure. Int. J. Biochem. Cell Biol. 37: 2023-2035. [ Links ]
Sparbier, K., S. Koch, I. Kessler, T. Wenzel, and M. Kostrzewa. 2005. Selective isolation of glycoproteins and glycopeptides for MALDI-TOF MS detection supported by magnetic particles. J. Biomol. Tech. 16: 407-413. [ Links ]
Thordarson, G., N. Slusher, H. Leong, D. Ochoa, L. Rajkumar, R. Guzman, et al. 2004. Insulin-like growth factor (IGF)-I obliterates the pregnancy-associated protection against mammary carcinogenesis in rats: evidence that IGF-I enhances cancer progression through estrogen receptor-alpha activation via the mitogen-activated protein kinase pathway. Breast Cancer Res. 6: R423-436. [ Links ]














