Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.48 no.7 Texcoco oct./nov. 2014
Protección vegetal
Multiplex PCR assay to identify Trichogramma parasitoids (Hymenoptera, trichogrammatidae) reared from mexican insectaries
PCR múltiple para identificar especies de Trichogramma (Hymenoptera, trichogrammatidae) parasitoides criadas en insectarios mexicanos
Jaime González-Cabrera1, Hugo C. Arredondo-Bernal1* , Richard Stouthamer2
1 Centro Nacional de Referencia de Control Biológico. Km 1.5 Carretera Tecomán-Estación FFCC. 28110. Colonia Tepeyac. Tecomán, Colima, México. (jgonz017@student.ucr.edu) *Author for correspondence (hugo.arredondo@senasica.gob.mx).
2 Department of Entomology, University of California, Riverside, CA 92521, USA. (richard.stouthamer@ucr.edu).
Received: June, 2013.
Approved: July, 2014.
Abstract
Accurate identification of species as biological candidates is the first step in establishing successful biological control programs. In Mexico, Trichogramma parasitoids are identified using morphological characters, which is a laborious procedure made only by experts; in addition, some morphological structures are susceptible to changes due to differences in diet or environment, and consequently there is a latent risk of misidentification. Moreover, morphological identification requires the presence of males, leaving female-only populations unidentifiable. As an alternative to morphological identification, the purpose of this work was to develop a molecular method for the identification of Trichogramma parasitoids from Mexican insectaries. Trichogramma species reared in six insectaries were DNA-identified and subsequently, based on differences in ITS2 sequences, a DNA-multiplex PCR assay was designed to distinguish among those reared species. Thus, discrepancies were found between the reported and DNA-determined species identity, whereas the sample of all the insectaries together was supposed to contain three species of Trichogramma (T. pretiosum, T. exiguum and T. platneri), only two species (T. pretiosum and T. fUentesi) were present. In addition, it was found the presence of unnoticed species replacement. Fortunately, an accurate taxonomic identification of Trichogramma species can be made by using the DNA-multiplex PCR assay that was generated in this work. Additionally, this methodology may identify the native Trichogramma species, whose use in mass rearing projects is uncommon.
Key words: Trichogramma pretiosum, T. exiguum, T platneri, T. fuentesi, DNA-identification, ITS2 sequences.
Resumen
La identificación precisa de insectos benéficos es el primer paso en el establecimiento de programas exitosos de control biológico. En México, los parasitoides Trichogramma se identifican mediante caracteres morfológicos, lo cual es un procedimiento laborioso realizado solo por expertos; además, algunas estructuras morfológicas son susceptibles a cambios debido a las diferencias en la dieta o las condiciones ambientales, y por tanto existe el riesgo latente de una identificación taxonómica errónea. Más aún, la identificación morfológica requiere la presencia de machos, dejando a las poblaciones conformadas por solo hembras (partenogenéticas) sin identificar. Como alternativa a la identificación morfológica, el propósito de este estudio fue desarrollar un método molecular para la identificación de parasitoides Trichogramma de insectarios mexicanos. Las especies de Trichogramma reproducidas en seis insectarios se identificaron por ADN y después, con base en diferencias de las secuencias de ITS2, se diseñó una prueba PCR-múltiple para distinguir entre las especies reproducidas. Así, se encontraron discrepancias entre la identidad reportada y la determinada por ADN: se suponía que las muestras de todos los insectarios contenían tres especies de Trichogramma (T. pretiosum, T. exiguum y T. platneri), pero solo hubo dos especies (T. pretiosum y T. fuentesi) presentes. Además, se encontró la presencia de especies de reemplazo. Afortunadamente, utilizando la prueba PCR ADN-múltiple generada en este estudio puede hacerse una identificación taxonómica exacta de las especies de Trichogramma. Además, esta metodología puede identificar las especies nativas de Trichogramma, cuyo uso en proyectos de reproducción masiva es rara.
Palabras clave: Trichogramma pretiosum, T. exiguum, T. platneri, T. fuentesi, Identificación-DNA, secuencias de ADN ITS2.
INTRODUCTION
In México, Trichogramma parasitoids are important agents of biological control against lepidoptera pests (Williams et al., 2013). These parasitoids are released to protect crops such as apples, sugarcane or tobacco, in approximately 1.5 million ha -1 (Domínguez, 1996). Worldwide, Mexico occupies the third place in area treated with Trichogramma (van-Lenteren and Bueno, 2003; González-Cabrera et al., 2014). Althoug the efficacy of Trichogramma parasitoids is known, there are reports that mass-reared Trichogramma, as compared to wild wasps, have a low field performance with respect to survival (Mansfield and Mills, 2002), parasitization (Ashley et al., 1973; Lundgren and Heimpel, 2002; Vejar-Cota et al., 2005) and host acceptance (Bergeijk et al., 1989). Inadequate field performance of Trichogramma parasitoids can be caused by cold temperatures, heavy rain, high parasitoid dispersion or a mismatch between the intended and released specie. Some Trichogramma species are habitat (Thorpe, 1985; Hassan, 1994; Romeis et al., 2005) and host specific (Curl and Burbutis, 1978; Yu et al., 1984; Stevens, 1995); consequently, mass rearing and releasing the wrong Trichogramma specie could result in failure to control the target pest.
Trichogramma parasitoids are identified using morphological characters, a method with the following drawbacks: 1) it is a laborious procedure carried out only by experts (Pinto, 1998; Platner et al., 1999); 2) some morphological structures are susceptible to changes due to differences in diet or environment (Pinto et al., 1989); consecuently, some of these characters can be misinterpreted by the observer; 3) morphological identification requires the presence of males (Pinto, 1998), leaving female-only populations unidentifiable. Using molecular biology techniques it is possible to generate an accurate multiplex PCR key to identify Trichogramma species in a geographical area (Stouthamer et al., 1999). Misidentification of Trichogramma parasitoids using morphological characters was reported in Mexico (García-González et al., 2005; España-Luna et al., 2006) and delivering wrong species does not contribute to the field success of Trichogramma parasitoids, and should be prevented. Therefore, the objective of this study was to develop a molecular method for the identification of those Trichogramma parasitoids that are reared in Mexican insectaries.
MATERIALS AND METHODS
In early 2010, an e-mail letter stating the objectives of this study was sent to 27 Trichogramma producers (privates, it included the centers for the study and production of beneficial insects, hereafter called CREROB), which were listed in the "Directorio-2010 de laboratorios reproductores y comercializadores de agentes de control biológico, Dirección General de Sanidad Vegetal y del Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria/Centro Nacional de Referencia de Control Biológico". By the end of 2010, six insectaries sent three separate samples of their Trichogramma rearings (individual colonies), which were a total of 10. In this study, to maintain anonymity of the collaborating insectaries, it is mentioned only their state location: Baja California Sur, Coahuila, Colima, Durango and Sonora.
Obtention of Trichogramma samples
On site, personnel of each insectary put live host egg cards on enclosed containers and let the wasp to emerge. Upon the natural death of the emerged adults these containers were sent to San Luis Rio Colorado, Sonora, México. One week after the arrival of each shipment, these containers were sent to the Entomology Department of the University of California, Riverside, USA, for its analysis.
Sequencing of DNA, aligning and comparing of ITS2 sequences
The reared species from the 10 Trichogramma spp. colonies were DNA-identified, as follows: per rearing sample the DNA of four random individuals was obtained using the Chelex DNA extraction method (Walsh et al., 1991). Each wasp was ground in 60 μL 5 % Chelex-100 (Bio-Rad laboratories, Hercules, California, USA) and 2 μL proteinase K (20 mg mL -1); the mixture was incubated for 60 min at 55 °C, followed by 10 min at 99 °C. Using the lTS2-forward (5'-TGTGAACTGCAGGACACATG-3') and lTS2-reverse (5'-GTCTTGCCTGCTCTGAG-3') primers the entire ITS2 region of rDNA (Stouthamer et al., 1999) was amplified. PCR was performed in 25 μL reactions containing 2 μL DNA template, 1X PCR-buffer (New England Biolabs. Ipswich Massachusetts, USA), 0.2 μmol L -1 each dATP, dCTP, dGTP 0.4 μM dUTP 1 μmol L-1 MgCl2, 0.2 μM forward and reverse primer, 1 U Taq polymerase enzyme (NEB), and 13.3 μL sterile distilled water. PCR was performed using a thermocycler Ep gradient S (Eppendorf AG. Hamburg, Germany). The PCR cycling program was 3 min at 95 °C, followed by 37 cycles of 45 s at 92 °C, 45 s at 53 °C and 1 min at 72 °C with 3 min at 72 °C after the last cycle. PCR products were separated on a 1 % agarose gel and stained with ethidium bromide; size ladders (1 kb bp, Fermentas®, Thermo Fisher Scientific, Vilnius Lithuania) were run along with the samples for reference. PCR products and ladders were photographed with a Carestream Molecular Imaging V.5.0.2.3.0 (Carestream Health, Inc. Rochester New York, USA). PCR products were purified using the Wizard PCR Preps DNA Purification System (Promega Corporation. Madison Wisconsin, USA) and direct-sequenced in both directions at the University of California, Riverside, Genomics Institute, Core Instrumentation Facility using an Applied Biosytems 3730 DNA analyzer with a Big-Dye® V3.1 kit (Applied Biosystems. Foster City California, USA). The resultant ITS-2 sequences were imported and manually aligned and edited in BioEdit version 7.0.5.3 (Hall, 1999). The identity of specimens was determined by comparing their DNA sequence with those present in GenBank® (Benson etal., 2008) (accession numbers 37730880AY182765 and 37778494AY187261).
Multiplex-PCR design
A multiplex PCR assay was designed to identify the species (as indicated by the ITS-2 sequences) in the samples, as follows (Gariepy et al. 2005, for an overview of the principles of multiplex PCR): based on the alignment of ITS2 sequences, three PCR primers were designed using Primer3 v.0.4.0 (Rozen and Skaletsky, 2000): a common forward primer T.pf-uni-F (5'-TCAAACGAAACGCAAGAGAA-3'); two species-specific reverse primers, T.sps1-R (5'-GAGCCTGATCGTGTGCTAAA-3') and T.sp2-R (5'-GAGCTAGCCAGGCGCTATAA-3'). T.sp1-R and T.sp2-R primers resulted in PCR products of 173 bp and 250 bp, respectively.
Multiplex-PCR conditions
To identify Trichogramma species that were reared in the insectaries, this multiplex PCR assay was used on 20 random wasps per sample. The described DNA extraction method, PCR master mix, PCR cycling program, agarose gel and UV-photograph equipment were used with the following modifications: PCR master mix, 12.8 μL sterile distilled water, 0.2 μmol L -1 forward and two reverse primer; PCR program, 30 s in the first and second step of the 37 cycles, the first step at 94 °C and the second step at 59 °C; and the product was run in a 1.5 % agarose gel. In each gel, negative and positive controls were included; such DNA belonged to the material that previously was sent to sequencing.
RESULTS AND DISCUSSION
Intraspecific variation in the ITS2 sequences allowed the design of a common forward primer T.pf-uni-F and two species-specific reverse primers, T.sps1-R and T.sp2-R; those primers resulted in PCR product size consistent with either T. pretiosum or T. fuentesi. The utility of the DNA-multiplex PCR assay to identify Trichogramma species that were reared in six Mexican insectaries was demonstrated using 20 random wasps per sample, in agarose gel each specimen matched one of the positive controls (Figure 1), i.e., it identified T. pretiosum and T. fuentesi. Along with an accurate identification of the mass-reared Trichogramma, this DNA-Multiplex may open a path for using native or local species; in México 23 especies were reported (Pinto, 1998; García-González et al., 2005; España-Luna et al., 2008). The local Mexican especies can be identified accurately using the DNA-based method that was described in this study, and after linking the ITS2 DNA sequence for those species with their names based on morphology, these local parasitoids can be tested for their fecundity or host specificity. To develop a multiplex-PCR for identifying these 23 local species, first, it is necessary to determine the DNA sequences of the ITS2 region for each species. Second, based in the size of each ITS2, roughly subdivide the species in three species-specific multiplex-PCR groups, grouping together the species with similar ITS2 sizes. Third, based on the need for identifying specific species, species-specific primers can be developed for each group based on the areas of maximal difference of the species specific DNA fragments (as it was done in this study). In Portugal and España, a similar approach of molecular identification was developed to distinguish five local species of Trichogramma (Silva et al., 1999; Del-Pino et al., 2013); and in Mexico, España-Luna et al. (2008) developed a dichotomous molecular key to identify five Trichogramma species of agricultural importance. These latter authors, reported along with T. fuentesi and T. pretiosum, also T. atopovirilia, T. exiguum and T. pintoi. Twenty three Trichogramma species are present in Mexico; however, as shown in this (Table 1) and others studies (García-González et al., 2005; España-Luna et al., 2008), the Mexican insectaries rear almost exclusively T. pretiosum. In addition to identifying accurately mass reared and local Trichogramma, this DNA-multiplex can identify female-only populations because only one specimen is required to make its taxonomic identification. In Mexico, female-only populations of Trichogramma were reported (Stouthamer and Luck, 1993; Stouthamer and Werren, 1993).
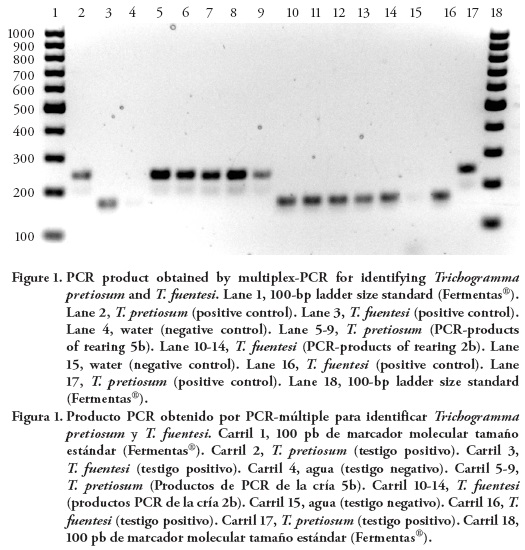
Mexican insectary personnel reported that in the ten rearings (individual colonies) three species of Trichogramma (T. pretiosum, T. exiguum and T. platneri) were reared; however, the multiplex PCR assay identified either T. pretiosum or T. fuentesi (Table 1, Figure 1). This homogeneity (only two species were reared) is explained because the initial stock in five cases originated from other Mexican insectaries and because in two cases the insectaries initiated their rearing stocks from field collected Trichogramma. For the latter situation, Pinto (1998) and Kuske et al. (2003) mentioned that collecting in the field raises the possibility of capturing species that were previously released, and in México field release of Trichogramma occurred since 1920s (Williams et al., 2013).
From the ten rearings, the insectary personnel correctly identified T. pretiosum in four cases, but in two cases T. fuentesi were mistaken as T. exiguum and also in two cases T. pretiosum were thought as T. exiguum; in a similar vein, in one case T. pretiosum was misidentified as T. platneri (Table 1). Misidentification of Trichogramma parasitoids using morphological characters were reported in México (García-González et al., 2005; España-Luna et al., 2006). Slight morphological variation in the broad and short ventral ridge of T. fuentesi can be interpreted as narrow and long; if this mistake occurs the identified species would be T. exiguum (Pinto, 1998). For the same reason, i.e., phenotypic plasticity of the morphological characters, T. pretiosum can be identified either as T. platneri or T. exiguum (Pinto, 1998).
Throughout the study, unnoticed species replacement was found. In the first shipment, T. fuentesi was produced in two rearings; however, in one rearing during the second and third shipments T. fuentesi was replaced by T. pretiosum (Table 1). Unnoticed replacement of Trichogramma species was reported in México (García-González et al., 2005) and USA (Lundgren and Heimpel, 2002; Lundgren and Heimpel, 2003).
The laborious training required to identify Trichogramma parasitoids using morphological characters, which is a procedure made only by experts (Pinto, 1998; Platner et al., 1999), along with the latent risk of misidentification, remains an obstacle for using native or local Trichogramma species in biological control projects (Pinto, 1998). Then, to avoid unnoticed replacement, taxonomic misidentification (both cases were found in this study) and foment the use of local Trichogramma species, it is recommended to identify Trichogramma species using a DNA-Multiplex PCR assay. The biggest obstacle to adopt molecular techniques is the cost of the equipment. Molecular identification is accurate (Stouthamer et al., 1999), and possibly in the long run the benefits of molecular identification will outweigh the high initial cost of molecular equipment.
CONCLUSIONS
As a results of implementing DNA-multiplex on the Trichogramma samples from the Mexican insectaries there were found discrepancies between the reported and DNA-determined species identity, while the sample of all the insectaries together was supposed to contain three species of Trichogramma (T. pretiosum, T. exiguum and T. platneri), only two species were present (T. pretiosum and T. fuentesi). Also there was found unnoticed species replacement: throughout the study, in one colony T. fuentesi was replaced by T. pretiosum.
ACKNOWLEDGEMENTS
Thanks to Paul Rugman-Jones for guidance in the molecular analysis and for proof reading the article, Anabel Valencia Villalobos and Marco Antonio Mellin Rosas for their coordination on the delivery of the Trichogramma samples, and special thanks to the personnel of the collaborating insectaries. This research was supported in whole or in part by UC MEXUS—CONACYT agreement in higher education and research, Robert and Peggy van den Bosch Memorial Scholarship, and the University of California, campus Riverside, Entomology department.
LITERATURE CITED
Ashley, T. R., D. González, and T. F. Leigh. 1973. Reduction in effectiveness of laboratory-reared Trichogramma. Environ. Entomol. 2: 1069-1073. [ Links ]
Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and D. L. Wheeler. 2008. GenBank. Nucl. Acids Res. 36: 25-30. [ Links ]
Bergeijk, K. E. V., F. Bigler, N. K. Kaashoek, and G. A. Pak. 1989. Changes in host acceptance and host suitability as an effect of rearing Trichogramma maidis on a factitious host. Entomol. Exp. Appl. 52: 229-238. [ Links ]
Curl, G. D., and P. P. Burbutis. 1978. Host preference studies with Trichogramma nubilale (Hymenoptera- Trichogrammatidae). Environ. Entomol. 7: 541-543. [ Links ]
Del-Pino, M., P. F. Rugman-Jones, E. Hernández-Suárez, A. Polaszek, and R. Stouthamer. 2013. Rapid molecular identification of five species of Trichogramma occurring in the Canary Islands with notes on their distribution in banana groves. Biocontrol 58: 515-524. [ Links ]
Domínguez, E. R. 1996. Control biológico de plagas agrícolas en México. In: Zapatero, M. C. (ed). El Control Biológico en América Latina. IOBC. Buenos Aires, Argentina. pp: 55-62. [ Links ]
España-Luna, M. P., O. C. Alvarado-Gómez, A. González-Hernández, S. Favela-Lara, J. Lozano-Gutiérrez, and F. García- González. 2006. Diferenciación genética de especies crípticas de Trichogramma Westwood (Hymenoptera: Trichogrammatidae). Folia Entomol. Mex. 45: 283-290. [ Links ]
España-Luna, M. P., A. González-Hernández, O. G. Alvarado-Gómez, and J. Lozano-Gutiérrez. 2008. Identificación molecular de especies crípticas de Trichogramma Westwood (Hymenoptera: Trichogrammatidae) de importancia agrícola en México. Acta Zool. Mex. (n.s.) 24:1-14. [ Links ]
García-González, F., A. González-Hernández, and M. P España-Luna. 2005. Especies de Trichogramma Westwood (Hymenoptera: Trichogrammatidae) presentes en centros reproductores de México. Acta Zool. Mex. (n.s.). 21(3): 125-135. [ Links ]
Gariepy, T. D., U. Kuhlmann, T. Haye, C. Gillott, and M. Erlandson, M. 2005. A single-step multiplex PCR assay for the detection of European Peristenus spp., parasitoids of Lygus spp. Biocontrol Sci. Technol. 15: 481-495. [ Links ]
González-Cabrera, J., H. C. Arredondo-Bernal, and R. Stouthamer. 2014. Quality assessment of trichogramma parasitoids (Trichogramma spp.) from six Mexican insectaries. Agrociencia 48: 321-329. [ Links ]
Hassan, S. A. 1994. Strategies to select Trichogramma species for use in biological control. In: E. Wajnberg and S. A. Hassan (eds). Biological Control with Egg Parasitoids. CAB International, Wallingford, pp: 55-72. [ Links ]
Kuske, S., F. Widmer, P. J. Edwards, T. C. J. Turlings, D. Babendreier, and F. Bigler. 2003. Dispersal and persistence of mass released Trichogramma brassicae (Hymenoptera: Trichogrammatidae) in non-target habitats. Biol. Control 27: 181-193. [ Links ]
Lundgren, J. G., and G. E. Heimpel. 2002. Augmentation of Trichogramma brassicae for control of cruciferous lepidoptera. In: Proc. 1st Int. Sympo. on Biological Control of Arthropods. Honolulu, Hawaii, USA. pp: 160-166. [ Links ]
Lundgren, J. G., and G. E. Heimpel. 2003. Quality assessment of three species of commercially produced Trichogramma and the first report of thelytoky in commercially produced Trichogramma. Biol. Control 26: 68-73. [ Links ]
Mansfield, S., and N. J. Mills. 2002. Direct estimation of the survival time of commercially produced adult Trichogramma platneri Nagarkatti (Hymenoptera: Trichogrammatidae) under field conditions. Biol. Control 25: 41-48. [ Links ]
Pinto, J. D. 1998. Systematics of the North American species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae). Memoirs of the Entomological Society of Washington. Num. 22. The Entomological Society of Washington, Washington, D.C. [ Links ]
Pinto, J. D., R. K. Velten, G. R. Platner, and E. R. Oatman. 1989. Phenotypic plasticity and taxonomic characters in Trichogramma (Hymenoptera, Trichogrammatidae). Ann. Entomol. Soc. Am. 82: 414-425. [ Links ]
Platner, G. R., R. K. Velten, M. Planoutene, and J. D. Pinto. 1999. Slide-mounting techniques for Trichogramma (Trichogrammatidae) and other minute parasitic Hymenoptera. Entomol. News 110: 56-64. [ Links ]
Romeis, J., D. Babendreier, F. L. Wackers, and T. G. Shanower. 2005. Habitat and plant specificity of Trichogramma egg parasitoids: underlying mechanisms and implications. Basic Appl. Ecol. 6: 215-236. [ Links ]
Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz, S. and S. Misener (eds). Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press. Totowa, NJ, USA, pp: 365-386. [ Links ]
Silva, I. M. M. S., J. Honda, F. V. Kan, J. G. Hu, L. Neto, B. Pintureau, and R. Stouthamer. 1999. Molecular differentiation of five Trichogramma species occurring in Portugal. Biol. Control 16: 177-184. [ Links ]
Stevens, P. S. 1995. Host preferences of Trichogrammatoidea Bactrae fumata (Hym.: Trichogrammatidae) an egg parasitoid of leafrollers (Lep.: Tortricidae). Entomophaga 40: 379-385. [ Links ]
Stouthamer, R., and J. H. Werren. 1993. Microbes associated with parthenogenesis in wasps of the genus Trichogramma. J. Invertebr. Pathol. 61: 6-9. [ Links ]
Stouthamer, R., and R. F. Luck. 1993. Influence of microbe-associated parthenogenesis on the fecundity of Trichogramma deion and Trichogramma pretiosum. Entomol. Exp. Appl. 67: 183-192. [ Links ]
Stouthamer, R., J. G. Hu, F. V. Kan, G. R. Platner, and J. D. Pinto. 1999. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. Biocontrol 43: 421-440. [ Links ]
Thorpe, K. W. 1985. Effects of height and habitat type on egg parasitism by Trichogramma minutum and Trichogramma pretiosum (Hymenoptera, Trichogrammatidae). Agric. Ecosyst. Environ. 12: 117-126. [ Links ]
van-Lenteren, J. C., and V. Bueno. 2003. Augmentative biological control of arthropods in Latin America. Biocontrol 48: 123-139. [ Links ]
Vejar-Cota, G., A. Caro, L.A. Rodríguez-del-Bosque, and D. Sahagún. 2005. Inundative releases of hymenopterous parasitoids against Diatraea considerata (Lepidoptera: Crambidae) on sugarcane in Northwestern México. J. Entomol. Sci. 40: 231-233. [ Links ]
Walsh, P. S., D. A. Metzger, and R. Higuchi. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506-513. [ Links ]
Williams, T., H. C. Arredondo-Bernal, and L. A. Rodríguez-del-Bosque. 2013. Biological pest control in México. Annu. Rev. Entomol. 58: 119-40. [ Links ]
Yu, D. S. K., E. A. C. Hagley, and J. E. Laing. 1984. Biology of Trichogramma minutum Riley collected from apples in Southern Ontario. Environ. Entomol. 13: 1324-1329. [ Links ]














