Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.48 n.5 Texcoco Jul./Aug. 2014
Fitociencia
Development and initial growth of castor bean (Ricinus communis) crop submitted to different soil water tensions
Desarrollo y crecimiento inicial de la higuerilla (Ricinus communis) sometida a diferentes tensiones de agua en el suelo
Marília A. Brito-Pinto1*, Carlos Reisser-Júnior2, L. Carlos-Timm3, Gabriel Franke-Brixner4, S. Delmar dos Anjos e Silva4, Letiane Helwig-Penning5
1 Postgraduate Program in Agronomy, Faculty of Agronomy Eliseu Maciel, Federal University of Pelotas, Campus Universitário s/n, Caixa Postal 354, CEP: 96010- 900, Pelotas, Rio Grande do Sul, Brazil. * Author for correspondence (ma.agro@gmail.com).
2 Embrapa Clima Temperado, Pelotas, Rio Grande do Sul, Brazil.
3 Department of Rural Engineering, Faculty of Agronomy, Federal University of Pelotas, Rio Grande do Sul, Brazil.
4 Postgraduate Program in Agronomy, Federal University of Santa Maria, Rio Grande do Sul, Brazil.
5 Postgraduate Program in Soil and Water Management and Conservation, Federal University of Pelotas, Rio Grande do Sul, Brazil.
Received: January, 2014.
Approved: July, 2014.
Abstract
With the biodiesel production incentive grant program in Brazil, castor bean (Ricinus communis) crop has gained greater importance in the agricultural scenario. Therefore, the objective of this study was to evaluate the castor bean response to different levels of soil water tension. A greenhouse study was carried out with castor bean plants, cv. Al Guarany 2002, cultivated in pots, and a tensiometer was installed at 0.15 m deep to monitor soil water tension. The experimental design was completely randomized with three treatments (T) and four replicates: T1, water tension maintained at 0.01 MPa; T2, when soil water tension reached 0.03 MPa, water was added to restore it to 0.01 MPa; T3, when soil water tension reached 0.06 MPa, water was added to restore it to 0.01 MPa. Between 30 and 105 d after plant emergence, the leaf area, plant height, stem diameter and transpiration rates were measured every two weeks. The time of initial flowering and height of insertion of the first raceme were also determined. Results show that the castor bean is sensitive to the increase in water soil tension, and higher stress conditions lead to decreased growth, and lower transpiration rates and vegetative period.
Keywords: Ricinus communis L., transpiration, water stress.
Resumen
Con el programa de becas de incentivo de producción de biodiesel en Brasil, el cultivo de la higuerilla (Ricinus communis) adquirió mayor importancia en el escenario agrícola. Por lo tanto, el objetivo de este estudio fue evaluar la respuesta de la higuerilla a diferentes tensiones de agua en el suelo. El estudio se realizó en invernadero con plantas de higuerilla, cv. Al Guarany 2002, cultivadas en macetas, y se instaló un tensió-metro a 0.15 m de profundidad para controlar la tensión de agua en el suelo. El diseño experimental fue completamente al azar con tres tratamientos (T) y cuatro repeticiones: T1, tensión de agua mantenida a 0.01 MPa; T2, cuando la tensión de agua en el suelo alcanzó 0.03 MPa, se agregó agua para restaurarlo a 0.01 MPa; T3, cuando la tensión de agua en el suelo alcanzó 0.06 MPa, se agregó agua para restaurarlo a 0.01 MPa. Entre 30 y 105 d después de emerger la planta, se midieron el área foliar, altura de la planta, diámetro del tallo y los índices de transpiración cada dos semanas. También se determinó el tiempo de la floración inicial y la altura de la inserción del primer racimo. Los resultados muestran que la higuerilla es sensible al aumento de la tensión de agua en el suelo y las condiciones de mayor estrés conducen a un crecimiento reducido, e índices de transpiración y período vegetativo menores.
Palabras clave: Ricinus communis L., transpiración, estrés hídrico.
INTRODUCTION
Brazil is the third largest producer of castor bean Ricinus communis, behind China and India; however, considering the present government program on biofuels, the country may, in the coming years, regain the first position held in 1980's. The average production of castor in the last three seasons was 61 000 t (IBGE, 2014).
In addition to the high potential of castor bean for oil production, which has a wide range of applications in the chemical and pharmaceutical industries, and the possibility of its usage as raw material for biofuel production (Savy Filho, 2005), castor bean by-products can also be used. Castor presscake, for instance, obtained from the crushing of seeds, can be employed as an organic fertilizer since it has nematicidal and fungicidal properties, presents all macro and micro nutrients, and is rich in organic matter and nitrogen (Silva et al., 2007).
The cultivated area of castor beans in the state of Rio Grande do Sul, Brazil, has increased driven by the Brazilian biofuel policy that promoted many oil and biofuel industries in the state. This generated a great demand for technical and scientific information about this crop (Silva et al., 2007), among which is the effect of water deficit on castor bean development.
According to Beltrão et al. (2003), despite being drought resistant, the castor bean is very sensitive to changes in soil moisture in its initial phase, which may compromise its production of assimilates. By assessing the response of castor bean cv. BRS Energy to different irrigation levels, Silva et al. (2009) observed that as the water availability increased, the plant height, leaf area, and yield of castor bean also did so. Lacerda et al. (2009) studied castor bean cv. BRS 188-Paraguassu, and found that increasing soil water levels caused higher rates of growth and development, biomass production and photosynthetic efficiency of this cultivar.
As the soil dries, it becomes more difficult for plants to absorb water because the energy retention by the soil matrix (soil water tension) increases, thus decreasing its availability to plants. However, the greater the evaporative demand of the atmosphere, the higher the need for water flow in the Soil-Plant-Atmosphere system will be (Santos and Carlesso, 1998).
Knowledge of the castor bean water demand allows either to adapt the seeding to regions where rainfall is favorable to the crop or to use irrigation in order to meet the crop water needs when it is economically viable. The objective of this study was to evaluate the growth and development of the castor bean cv. Al Guarany 2002 submitted to three levels of soil water tension.
MATERIAL AND METHODS
The experiment was conducted in a greenhouse at Embrapa Clima Temperado, located in Pelotas, Rio Grande do Sul (32° 45' S and 52° 30' W), Brazil, from February to May 2010. The castor bean plants cv. Al Guarany 2002 were cultivated in pots of flexible polyethylene (30 dm3 volume), filling each pot with a substrate of 40 % soil, 40 % sand and 20 % cattle manure. Sowing was carried out in February 2010, soil fertilization (4.5 g NPK; 10-20-20) was applied in each plot, equivalent to 300 kg ha-1, which was the recommendation based on the chemical analysis of the substrate and in accordance with the technical instructions described by (Silva et al., 2007). To monitor the soil water tension in the substrate, a tensiometer with mechanic vacuum gauge was installed in each pot at 0.15 m deep. Tensiometer readings were performed every day early in the morning, during 75 d.
Solar radiation data was obtained from Embrapa weather station. Since it is a controlled environment, the values of solar radiation were reduced by 30 % due to losses caused by the covering material of the greenhouse. The value of 30 % was adopted in line with data reported by Farias et al. (1993), Camacho et al. (1995), and Beckmann et al. (2006).
The experimental design was completely randomized with three treatments (T) and four replicates: T1, soil water tension in the substrate maintained at 0.01 MPa; T2, when soil water tension in the substrate reached 0.03 MPa, water was added to restore it to 0.01 MPa; T3, when soil water tension in the substrate reached 0.06 MPa, water was added to restore it to 0.01 MPa.
The amount of water to be added in the substrate to restore the soil water tension to 0.01 MPa was calculated as proposed by Libardi (2005): ∆h=Z(θf -θi where ∆h is the amount of water to be added (mm), Z is the depth of the pot (mm), θf is the soil water content retained at the soil water tension of 0.01 MPa (m3m-3), and θi is the soil water content at the moment of the tensiometer reading (m3 m-3). The θ values were obtained through the substrate water retention curve.
The evapotranspiration values were calculated every fortnight based on the water balance equation (Reichardt and Timm,2012): P+I-DP-ET=∆ARM, where P is the precipitation (mm), I is the depth of irrigation (mm), DP is the depth of deep drainage (mm), ET is the actual evapotranspiration (mm), and ∆ARM is the soil water storage changes (mm) in each period. Precipitation values were disregarded since the study was carried out in a greenhouse. Irrigation depth was quantified by using graduated cylinders and corresponded to the total amount of water added fortnightly; to measure deep drainage depth, after irrigating, the accumulated water collected in the trays installed under the pots was quantified by using a graduated cylinder. Due to the fact that the pots were covered with aluminum foil, soil water evaporation (E) was considered null and plant transpiration (T) calculated according to the water balance equation, as described above.
Measurements of leaf area (LA), plant height, and stem diameter were taken once every two weeks in a period from 30 to 105 d after plant emergence (DAE). To estimate LA a mathematical model was used (Severino et al., 2005): LA=0.2439 (NP+NL)2.0598, where LA is the leaf area (m2), NP is the length of the main rib (m), and NL is the average length of the side ribs (m). Plant height was measured from the substrate surface to the youngest leaf point of insertion, and also the stem diameter using a caliper ruler at 0.10 m above the substrate surface. The time of initial flowering and the height of insertion of the first cluster were also determined.
The growth curves for LA, plant height and stem diameter were adjusted based on the following logistic model: y=a/1+(x/b)c, where y= is the response variable; x= is the number of days after plant emergence; and a, b and c= are adjusted parameters of the logistic model, and a is the difference between the maximum and minimum point of the growth curve, b is the time consumed for reaching 50 % of the variable response, and c is the slope of the growth curve (Regazzi, 2003). The adjusted parameters of the logistic function for each replicate were analyzed using ANOVA and the Tukey test (p≤0.05) was used to compare treatment means. A correlation analysis between plant transpiration and radiation, LA and soil water tension was performed. Statistical analyses were carried out using SAS (SAS Institute, 1985).
RESULTS AND DISCUSSION
The LA growth curve was higher in T1, followed by T2 and T3 (Figure 1A), indicating that water availability in the substrate (T1 average tension was 0.01 MPa) affected the castor bean Al Guarany 2002 LA growth.
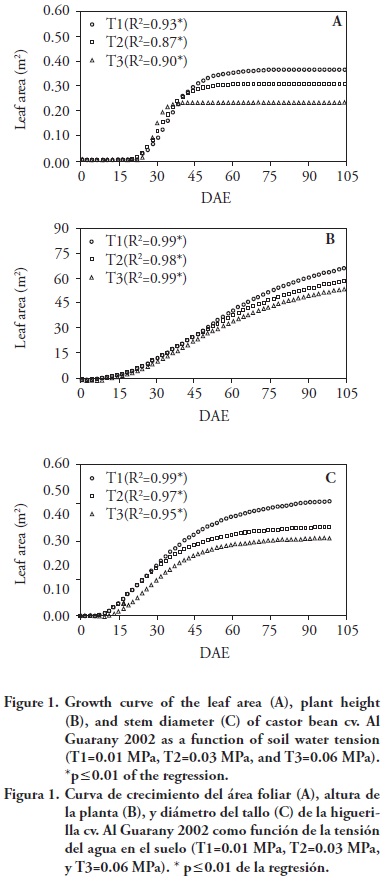
Besides, the LA growth curve in T1 stabilized after 75 DAE; however, with the increase in water average tension in the substrate to 0.03 MPa (T2) and 0.06 MPa (T3), the plants LA growth curve became stable after 30 DAE and 45 DAE, respectively (Figure 1A). The reduction in LA growth curve of plants in T2 and T3 might be the plants response in order to avoid water loss. In case of water deficit, most vegetables seek for alternatives to decrease evaporation, mainly reducing transpiration. Fageria (1989) reported a reduction in leaf area, thereby decreasing water loss through transpiration. Rodrigues et al. (2009) also observed that in castor beans the LA was the most affected growth variable regarding the reduction of soil water content.
In all treatments the castor bean plant height growth curve increased up to 105 DAE. However, from 40 DAE as the substrate water tension, the plant height decreased (Figure 1B). The water tension in the substrate also influenced the castor bean plant stem diameter (SD) growth curve (Figure 1C). In T1, with more water availability in the substrate, SD growth curve increased up to 105 DAE, whereas in T2 and T3 SD growth curve became stable from 75 DAE as water average tension increased in the substrate.
Adjustment parameter values (a, b and c) of logistic models for LA growth curves, plant height and stem diameter in each treatment, are presented in Table 1. LA growth curve b parameter, which is 50 % of the time the plant takes to reach the maximum LA value, was influenced by the increase in water tension in the substrate (p≤0.05). The a parameter, which is the LA maximum value in the castor bean plant growth curve, was significantly higher in T1 followed by T2 and T3, highlighting, therefore, reduction in the castor bean LA maximum value as a function of the increase in substrate water tension. Silva et al. (2009) observed reduction in the maximum peak values (a parameter in this study) of the LA growth curve of the castor bean cv. BRS Energy, as a function of the decrease of the total depth irrigation. The castor bean plant LA growth rate is expressed by the c parameter, in which the higher the c value the faster the plant reaches the maximum LA, and the higher c value was observed in T3.
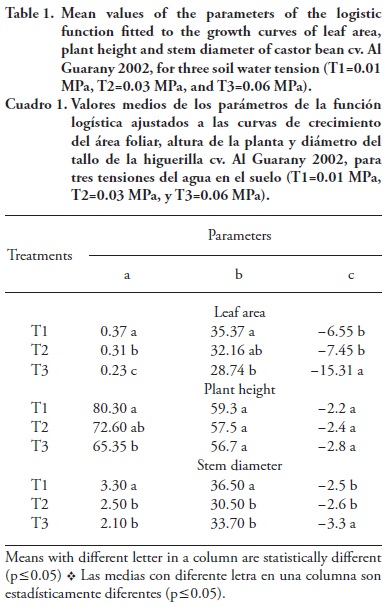
Based on the tree parameters of logistic function, it was observed that as the soil water tension increased, the LA development was decreases. This may be due to the fact that cell growth is the most sensitive process to low water availability in the soil (Kráme and Boyer, 1995); both cell division and expansion are directly inhibited by water stress (Zhu, 2002). In a study with castor bean plants under water stress, Heckenberger et al. (1998) observed that cell division and expansion were affected, causing maturation of the leaves into smaller sizes.
Parameters b and c of the castor bean plant height growth curve were not influenced by the increase in water tension in the substrate, whereas, parameter a was affected (Table 1).
Parameters a and b of stem diameter growth curve were not statistically different between T2 and T3, but they were significantly lower than those in T1 (Table 1). Parameter c values were not different between T1 and T2, indicating that increasing substrate water tension from 0.01 MPa to 0.03 MPa did not affect the castor bean cv. Al Guarany 2002 stem diameter growth curve slope. These results agree with those reported by Xavier et al. (2009), who observed higher castor bean BRS Nordestina stem diameter growth with 80 and 100 % available water levels, as compared to 70 % available water level.
Castor bean plant transpiration rate, as a function of time, was reduced significantly in the three treatments (Figure 2). The effect of time on the castor bean plant transpiration may be due its strong relation with solar radiation. During the experiment from March to May 2010, days become shorter and there was a reduction in the radiation rates, decreasing the availability of daily energy, and, consequently, the plant transpiration rate. Besides, in T1, plants presented larger LA leaf area and higher water availability, causing the highest transpiration rate values (Figure 2).
The correlation between plant transpiration and radiation, and the LA and soil water tension, shows that the solar radiation is the variable with the highest correlation with transpiration (r=0.746, p≤0.001), followed by the soil water tension (r=-0.512, p≤0.001) and leaf area (r=0.485, p≤0.001). Thus, the castor bean plant transpiration rate increases when soil water tension is reduced, and also when LA and incidence of solar radiation are increased, being, therefore, a result of the interaction soil-plant-atmosphere system.
The time for flowering onset and the primary raceme insertion height were significantly influenced by the soil water tension (Table 2). The castor beans plant in T1 presented higher primary raceme insertion height (66.75 cm) and flowered later (75.25 d). The opposite was seen in plants of T3, which presented lower primary raceme insertion height (31.75 cm) and earlier flowering onset (51 d). Aires et al. (2011) found a positive and significant correlation between precipitation and length of the castor bean cv. BRS Energy vegetative period, indicating that the greater the water availability, the longest this cycle period will be. According to Zuchi et al. (2010), in the season with more water availability, the height of insertion of the primary raceme was 20 cm higher compared to castor bean plants growing in drier seasons.
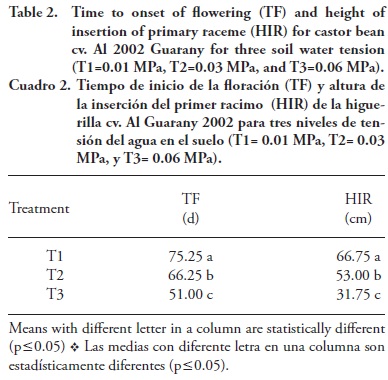
In our study there was a close relation between decrease in castor beans vegetative growth and flowering time, once the lowest values of LA, plant height and stem diameter were found in treatment T3, where plants flowered earlier (Figure 1, Tables 1 and 2). Larcher (2000) pointed out that interruption in the vegetative growth as a function of production acceleration occur due to the energy canalization and materials destined to flowering and fruit production, which in turn are originated in the photosynthetic process, incorporation of mineral substances and mobilization of reserves to produce fruits.
CONCLUSION
Initial growth and development of castor beans cv. Al Guarany 2002 were reduced by the water stress caused by the increase in soil water tension.
The increase in soil water tension reduces the vegetative period of castor beans cv. Al Guarany 2002, indicated by the lower time necessary to its reproductive phase onset and lower primary raceme insertion height.
Castor bean is a species sensitive to drought whose values, vegetative growth behavior, and flowering are altered by lack of water. Maintaining soil moisture at the initial stage of its growth is essential, as restrictions at this stage will possibly impact the plant development.
ACKNOWLEDGMENTS
To CNPq for the scholarship, to Embrapa Clima Temperado for the experimental infrastructure needed for this work and to FAPERGS for the financial support.
LITERATURE CITED
Aires, R. F., D. A. Silva S., D. Eicholz E. 2011. Análise de crescimento de mamona semeada em diferentes épocas. Ciência Rural 41:1347-1353. [ Links ]
Beckmann, M. Z., G. R. Duarte B., V. De Paula A., M. E. Mendez G., R. M. Peil N. 2006. Radiação solar em ambiente protegido cultivado com tomateiro nas estações verão-outono do Rio Grande do Sul. Ciência Rural 36:86-92. [ Links ]
Beltrão, N. E. M., G. Souza J., W. Santos J., F. Jerônimo J., X. Costa F., M. A. Lucena A., C., Queiroz, U. 2003. Fisiologia da mamoneira, cultivar BRS 149 nordestina, na fase inicial de crescimento, submetida a estresse hídrico. Rev. Bras. Ol. Fibros. 7: 659-664. [ Links ]
Camacho, M. J., F. Assis N., S. Martins R., M. E. Mendez G. 1995. Avaliação de elementos meteorológicos em estufa plástica em Pelotas, RS. Rev. Bras. Agromet. 3: 19-24. [ Links ]
Fageria, N. K. 1989. Solos Tropicais e Aspectos Fisiológicos das Culturas. Embrapa/DPU. Brasília. 425 p. [ Links ]
Farias, J. R., B. Bergamaschi H., S. Martins, R., M. Berlato A. 1993. Efeito da cobertura plástica de estufa sobre a radiação solar. Rev. Bras. Agromet. 1:31-36. [ Links ]
Heckenberger, U., U. Roggatz, and U. Schurr. 1998. Effect of drought stress on the cytological status in Ricinus communis. J. Exp. Bot. 49:181-189. [ Links ]
IBGE - Instituto Brasileiro de Geografia e Estatística. 2014 <http://www.ibge.gov.br/home/estatistica/indicadores/agropecuaria/lspa/lspa.htm> (Acesso: mai 2014). [ Links ]
Kramer, P. J., and J. Boyer S. 1995. Water Relations of Plants and Soils. Academic Press, San Diego. 495 p. [ Links ]
Lacerda, R. D., H. O. Guerra C., B. Júnior. G. 2009. Influência do déficit hídrico e da matéria orgânica do solo no crescimento e desenvolvimento da mamoneira BRS 188-Paraguaçu. Rev. Bras. Ciencia Agrar. 4: 440-447. [ Links ]
Larcher, W. 2000. Ecofisiologia Vegetal. Rima. São Carlos. 531 p. [ Links ]
Libardi, P. L. 2005. Dinâmica da Água no Solo. Edusp. Piracicaba. 497 p. [ Links ]
Regazzi, A. J. 2003. Teste para verificar a igualdade de parâmetros e a identidade de modelos de regressão não-linear. Ceres 50: 9-26. [ Links ]
Reichardt, K., C. Timm L. 2012. Solo, Planta e Atmosfera: Conceitos, Processos e Aplicações. 2. ed. Manole, Barueri. 500 p. [ Links ]
Rodrigues, L. N., R. Nery A., D. Fernandes P., E. M. Beltrão N., R. Gheyi H. 2009. Crescimento e produção de bagas da mamoneira irrigada com água residuária doméstica. Rev. Bras. Eng. Agric. Amb. 13: 825-835. [ Links ]
Santos, R. F., R. Carlesso. 1998. Déficit hídrico e os processos morfológico e fisiológico das plantas. Rev. Bras. Eng. Agric. Amb. 2: 287-294. [ Links ]
SAS-Statistical Analysis System. 1985. User's Guide. 5th ed. Cary, N. C.: SAS Institute Inc. [ Links ]
Savy Filho, A. 2005. Mamona Tecnologia Agrícola. EMOPI. Campinas. 105 p. [ Links ]
Severino, L. S., D. Cardoso G., S. Vale L., W. Santos J. 2005. Método para determinação da área foliar da mamoneira. Embrapa Algodão. Campina Grande. 20 p. (Boletim de Pesquisa e Desenvolvimento, 55). [ Links ]
Silva, S. D. A., G. Casagrande Jr. J., B. Scivittaro W. 2007. A cultura da mamona no Rio Grande do Sul. Embrapa Clima Temperado. Pelotas. 115 p. (Embrapa Clima Temperado. Sistemas de Produção, 11). [ Links ]
Silva, S. M. S., R. Gheyi H., E. M. Beltrão N., W. Santos J., A. L. Soares F. 2009. Dotações hídricas em densidades de plantas na cultura da mamoneira cv. BRS Energia. Rev. Bras. Cienc. Agrar. 4: 338-348. [ Links ]
Xavier, J. F., A. V. Azevedo C., E. M. Beltrão N., R. S. Andrade A., L. A. Lima V. 2009. Crescimento da mamoneira sob diferentes tipos de águas residuárias e níveis de água no solo. Rev. Amb. Água 4: 196-210. [ Links ]
Zhu, J. K. 2002. Salt and drought stress signal transduction in plants. Ann. Rev. Plant Bio. 53: 247-273. [ Links ]
Zuchi, J., A. P. Bevilaqua G., C. Zanuncio J., T. Peske S., D. A. Silva S., S. Sediyama C. 2010. Características agronômicas de cultivares de mamona em função do local de cultivo e da época de semeadura no Rio Grande do Sul. Ciência Rural 40: 501-506. [ Links ]














