Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.48 n.4 Texcoco Jun. 2014
Ciencia de los alimentos
Physicochemical evaluation of cooking and dessert bananas (Musa sp.) varieties
Evaluación fisicoquímica de variedades de plátanos (Musa sp.) de cocción y postre
O. Lidia Rosales-Reynoso1, Edith Agama-Acevedo1*, Andres Aguirre-Cruz2, Luis A. Bello-Perez1, Dominique Dufour3,4, Olivier Gibert3
1 Instituto Politécnico Nacional, CEPROBI, Km. 6.5 Carretera Yautepec-Jojtla, Colonia San Isidro, Calle Ceprobi Núm. 8, Yautepec, Morelos, México. * Author for correspondence (agama@ipn.mx).
2 Instituto de Química Aplicada, Universidad del Papaloapan (UNPA), Circuito Central 200, Colonia Parque Industrial, Tuxtepec, Oaxaca, 68301, México.
3 Centre de Cooperation Internationale en Recherche Agronomique pour le Developpement (CIRAD), UMR QUALISUD, 73 Rue Jean-Francois Breton, TA B-95/15 F-34398 Montpellier, France.
4 International Center for Tropical Agriculture (CIAT), Km17 Recta Cali-Palmira, A. A. 6713, Cali, Colombia.
Received: August, 2013.
Approved: March, 2014.
Abstract
In México, banana (Musa sp.) varieties ate used for human consumption as well as for traditional medicine, but the literature lacks information on local diversity and functional justification for their use. Three varieties of dessert bananas (Valery, Morado, and Enano) and one cooking banana (Macho) were collected in a commercial farm in Tuxtepec, Oaxaca, México, at the agronomic maturity stage, and they were physically and chemically evaluated. A random sampling, ANOVA, and Tukey tests were used. As compared to the dessert bananas, the cooking banana (Macho) showed a lower number of hands per banana bunch (6) and of fingers per hands (6), one of the smallest bunch yields (about 12.4 kg), a higher average finger weight (349 g), length (31.7 cm), girth (17 cm), starch amount (75.7 %, dry basis), resistant starch (59.2 % db), and greater firmness (10.2 N). Values of extractable polyphenols (EP), condensed tannins (CT), and hydrolysable tannins (HT) were higher for Morado variety, followed by Macho. The antioxidant capacity of EP, CT, and HT fluctuated among varieties. The Morado variety exhibited the lowest pasting temperature, lowest onset temperature, highest peak viscosity, and highest breakdown than those of the other varieties. The cooking variety exhibited the highest pasting and onset temperature (86.2 and 74.8 °C), and cooking ability (88.6 s) (p≤0.05). The results revealed the differentiation of edible Mexican banana varieties and for their potential acceptability.
Key words: Musa sp., starch properties, polyphenol, antioxidant capacity.
Resumen
En México, las variedades de plátano (Musa sp.) se usan para el consumo humano y para la medicina tradicional, pero la literatura carece de información sobre la diversidad local y la justificación funcional para su uso. Tres variedades de plátanos de postre (Valery, Morado y Enano) y una de cocción (Macho) se recolectaron en una granja comercial en Tuxtepec, Oaxaca, México, a la edad de madurez agronómica, y se realizó una evaluación fisicoquímica. La muestra fue aleatoria y se realizaron pruebas ANDEVA y de Tukey. En comparación con los plátanos de postre, el plátano de cocción (Macho) mostró un menor número de manos por racimo (6) y de dedos por mano (6), uno de los rendimientos más bajos de racimo (alrededor de 12.4 kg), un mayor peso promedio de dedos (349 g), longitud (31.7 cm), circunferencia (17 cm), cantidad de almidón (75.7 % base seca), almidón resistente (59.2 % db), y mayor firmeza (10.2 N). Los valores de poli-fenoles extraíbles (PE), taninos condensados (TC) y taninos hidrolizables (TH) fueron mayores para la variedad Morado, seguida de la Macho. La capacidad antioxidante de PE, TC y TH fluctuó entre las variedades. La variedad Morado exhibió la temperatura de formación de pasta más baja, la menor temperatura de inicio, la mayor viscocidad pico y la mayor de rompimiento, en comparación con las otras variedades. La variedad de cocción presentó la mayor temperatura de formación de pasta y de inicio (86.2 y 74.8 °C), y habilidad de cocción (88.6 s) (p≤0.05). Los resultados revelaron la diferenciación de las variedades comestibles de plátano mexicanas, y para su potencial aceptabilidad.
Palabras clave: Musa sp., propiedades del almidón, polifenol, capacidad antioxidante.
INTRODUCTION
Banana (Musa sp.) is a tropical fruit widely consumed in México (García-Mata et al., 2013) when ripe, a characteristic obtained after storage and depends on temperature and relative humidity. Dessert bananas are usually consumed raw at a full stage of maturity but other varieties, called cooking bananas, are cooked for consumption at various stages of maturity (Gibert et al., 2009). Exporting bananas from México has decreased because of major volume lost due to high banana ripening rate, thus decreasing income for farmers. There are different banana varieties used in Mexico (Vázquez-Castrejon et al., 2005), but little is known about their composition, functionality and acceptability. So far, starch is the main unripe banana compound known to contribute to functional properties of processed banana products, such as textural, thermal and rheological (Dufour et al., 2009; Gibert et al., 2009; Gibert et al., 2010). However, banana fruit has polyphenols compounds with antioxidant capacity (Rodriguez-Ambriz et al., 2008) that can increase its nutraceutical benefits, information which is almost unknown for Mexican varieties.
It is worthwhile to study unripe banana flour and starch isolated from unknown Mexican Musa sp. varieties due to their high starch content (around 74 %, dry basis), and resistance of starch to hydrolysis by digestive enzymes (Aparicio-Saguilan et al., 2005; Ovando-Martinez et al., 2009), as well as antioxidant capacity of the unripe banana flour. Moreover, the banana fruit is available year round at low cost, and the production of raw flour and starch from unripe banana fruit can be a technological alternative for nutraceutical food products (Bello-Pérez and Paredes-López, 2009). Banana varieties were evaluated for resistance to plagues, biochemical changes during ripening, flour physicochemical specificities for a given genotype, and physicochemical and functional characteristics of starch (Ayo-Omogie et al., 2010; Zhang and Hamaker, 2012; Gibert et al., 2013). Thus, the aim of this study was to evaluate the physical and chemical characteristics of the banana varieties (cooking and dessert) growing in México.
MATERIALS AND METHODS
Plant materials
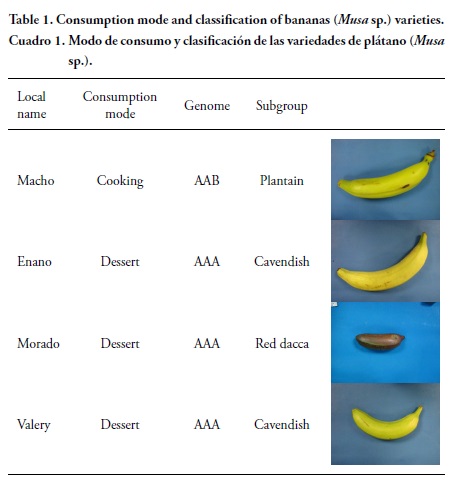
Four banana varieties (three bunches), at agronomic maturity stage, were collected from a commercial farm in Tuxtepec (Oaxaca, México) using random sampling (Table 1). Bananas samples were analyzed 24 h after harvest and the pulp (10 kg) was dehydrated to perform chemical analysis.
Physical characterization
Bunch weight was determined by weighing individual clusters. The number of hands (pads) and fingers (fruits) were obtained by counting number of hands on each bunch and fingers of each hand. Individual fingers were weighed, the length and the circumference of the fruits was determined according to Dadzie and Orchard (1977). The fruit (in the middle part) was cut in slices of 1 cm thickness (five slices), to evaluate the firmness of the pulp using a texture analyzer (model TA-XT2i) and measuring the force required to penetrate the tissue with a 2 mm diameter cylindrical probe. On average six fruits were evaluated.
Chemical composition
Banana starches and flours were prepared according to Aparicio-Saguilan et al. (2005) and Ovando-Martinez et al. (2009). Ash content (08-01), fat (30-25), and protein (Nx6.25) (46-143) were determined by official methods of the AACC (2000). Total starch was measured using K-TSTA 04/2009 kit Megazyme, according to "C determination" for samples containing resistant starch, but not D-glucose or maltodextrins or both. Total, soluble and insoluble fiber (32-05) were determined according to AOAC (2000).
Determination of polyphenols content
Samples were extracted with methanol-water acidified with HCl (50:50 v/v, pH 2, 50 mL g-1 sample, 60 min) and acetone-water (70:30 v/v, 50 mL g-1 sample, 60 min) at room temperature (25 °C) under constant stirring. After centrifugation (15 min, 25 °C, 3000 x g) supernatants were pooled and used to determine extractable polyphenols content and antioxidant capacity. Extractable polyphenols were determined by the Folin-Ciocalteau procedure (Singleton et al., 1999).
Condensed tannins
To obtain condensed tannins, residues from the methanol/ acetone/water extraction were treated with 5 mL L-1 HCl-butanol for 3 h at 100 °C (Reed et al., 1982) and absorbance was measured at 550 nm. Condensed tannins from Mediterranean carob pod (Ceratonia siliqua L.) were supplied by Nestlé S.A. (Switzerland) and analyzed at the Department of Nutrition, Faculty of Pharmacy, Universidad Complutense de Madrid, Spain.
Hydrolysable polyphenols were released from the food matrix by strong acidic hydrolysis (methanol/H2SO4 90:10 (v/v) at 85 °C for 20 h) from the residues of methanol/acetone/water extraction (Hartzfeld et al., 2002). The sample was centrifuged 15 min at 25 °C and 3000 x g. The supernatant was used for determination of hydrolysable polyphenols by Folin Ciocalteu method (Montreau, 1972). The result was expressed as gallic acid equivalents.
Antioxidant capacity assay (ABTS
The antioxidant capacity was evaluated in terms of free radical scavenging activity following the procedure described by Re et al. (1999) with some modifications (Pulido et al., 2003).
Thermal analysis and granule size distribution of starch
Gelatinization properties were assessed by a differential scanning calorimetry (DSC 8500 Pyris, Perkin-Elmer Corp., Norwalk, CT). Starch (≈8.0 mg db) was weighed accurately into an aluminum DSC pan and moistened with 40 μL of de-ionized water. The pan was hermetically sealed and allowed to stand for 30 min prior to thermal analysis. Samples were heated from 25 to 140 °C at a rate of 10 °C min-1. The characteristics for starch crystallinity loss during gelatinization transition, including onset (To), peak (Tp), conclusion (Tc) temperatures, and enthalpy of gelatinization (ΔH in J g-1 db starch) were computed using the Pyris software v. 9.1.
Starch granule size distribution was performed using a Malvern Mastersizer 3000 at room temperature (25 °C). A small amount of native starch was suspended in water, and an aliquot of this suspension was fed into the mixing cell to reach a 2 % about obscuration level. Volume distribution was determined using the Fraunhofer scattering theory, while considering opaque starch granules. The granule size corresponded to the average granule diameter.
Pasting profile
The pasting characteristics of 7 % db starch suspension (1.125 g db of pure starch adjusted with sample moisture content and purity, 15 mL distilled water) were determined with a MCR301 rheometer (Physica, Anton Paar GmbH, Austria) using an Rapid Visco Analyser (RVA) profile with a starch cell (C-ETD160/ST) and a specific paddle (ST24-2D/2V/2V-30) at a 3 mm gap. The slurry was maintained at 50 °C for 1 min, heated from 50 to 90 °C in 3 min, maintained 5 min at 90 °C and cooled to 50 °C in 3 min, prior to a holding stage at 50 °C for 3 min. Variables measured on the viscoamylogram (Dufour et al., 2009) were:pasting temperature (PT) and pasting time (Pt), peak viscosity (PV) and peak viscosity time PVt), cooking ability CA=PVt-Pt), hot paste viscosity (HPV), viscosity at the end of the plateau (VEP), breakdown (BD=PV-HPV), CPV the cold paste viscosity at final 50 °C 5CB, setback (SB=CPV-PV), and consistency (CS=CPV-HPV).
Statistical analysis
Data was analyzed using one way ANOVA and means were compared with HSD Tukey test (p≤0.05).
RESULTS AND DISCUSSION
Physical characterization
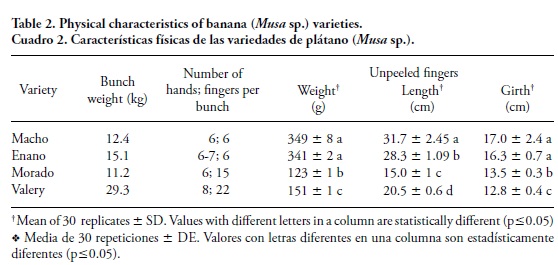
Shape of the banana varieties was different (Table 1) and Morado banana was purple. Valery banana showed a higher bunch weight yield (29 kg; Table 2) due to a higher number of hands and fingers per bunch. This variety is economically important in Mexico because of its high yield per hectare. Macho banana showed fewer fingers per bunch (6), greater length (28-31 cm) and girth (16-17 cm), and were heavier (340 g) than the dessert types (15-22 fingers, 14-20 cm lenght, and 12-13 cm circumference, and 123-151 g). The peel content was similar among varieties, which is about 34 % of a finger weight (data not shown). Gibert et al. (2009) and Dufour et al. (2009) reported higher peel content (3845 % range) for cooking and dessert banana varieties grown at higher altitude in Colombia. Peel values above 40 % produce average to low yields and a greater amount of waste, which is a disadvantage for industrial purposes.
Chemical composition and texture of banana pulp
The Macho banana showed lower ash content (2.6-2.7 g 100 g-1, db) and protein (3.05-3.3 g 100 g-1, db), but higher starch content (72-75 g 100 g-1, db) than the dessert varieties (Table 3). Gibert et al. (2009) reported slightly higher ash content for six cultivated dessert bananas (3.2 g 100 g-1, db) and equivalent ash content for the Macho variety, which might be related to the higher mineral content reported for dessert varieties. A similar pattern was observed for the protein content, with a higher amount in Valery (4.13 g 100 g-1) and Morado (4.75 g 100 g-1) than in Macho (3.30 g 100 g-1) and Enano (3.05 g 100 g-1). According to Yomeni et al. (2004), protein content in cooking bananas is 1-3 g 100 g-1 at the green stage of maturity, and increased to 4 g 100 g-1 at the mature stage. Those proteins are usually associated with the amount of enzymes involved in catabolism and anabolism of polysaccharides in the fruit. The fat content in the pulp was low (0.17-0.46 g 100 g-1), without significant differences (p>0.05) between Enano and Valery.
Total starch content (Table 3) was higher in Macho banana (75.68 g 100g-1) than in Enano (72.21 g 100 g-1) and Morado (66.27 g 100 g-1) varieties. Dufour et al. (2009) report a similar pattern, with higher starch amounts in Macho (86.5 g 100 g-1) than in dessert bananas (81.9 g 100 g-1), using an alternative analytical approach. In the first hours after harvest starch is hydrolyzed in dessert bananas, which could be related to a moisture content increase in the pulp after being harvested (Aparecida et al., 2011), whereas higher protein content (including amylolitic enzymes) could be related to a lower starch content in these varieties. In cooking bananas, hydrolysis of starch and sugar synthesis continues at the ripe stage of maturity, including senescence; thus, it is inferred that enzymatic degradation system for starch is more efficient in dessert bananas. However, the structure of the starch itself can influence its hydrolysis rate in the fruit pulp.
The Morado variety showed the lowest resistant starch (RS) content (14 g 100 g-1) and the Macho variety the highest one (59 g 100 g-1) (Table 4). In banana pulp, there is a wide range of RS content (17- 50 g 100 g-1) (Juarez-Garcia et al., 2006: Pelissari et al., 2012). The RS content in diverse banana varieties can be related to the shape and size of the starch granules, amylose/amylopectin ratio and starch structure (Zhang and Hamaker, 2012), as will be discussed later. Additionally, some extrinsic factors such as the presence of non-starch polysaccharides can act as a physical barrier which could contribute to an increase in the viscosity of the medium or induce the formation of a network, thus inhibiting the action of the enzymes in starch substrate hydrolysis. The high RS content in the banana varieties can be an important issue since this would contribute to reduce human obesity (Hendrich et al., 2010).
Total, insoluble and soluble dietary fiber contents (Table 3) were higher in the Macho (9.35, 5.09 and 4.26 g 100 g-1) and Morado varieties (10.24, 5.20 and 5.04 g 100 g-1). Dietary fiber content in the bananas ranged between 1.8 and 17 g 100 g-1 (Aguirre-Cruz et al., 2008; Dufour et al., 2009). Pectin and gums are part of the soluble dietary fiber, and RS is included in this fraction, while cellulose, lignin and hemicellulose are included in the insoluble dietary fiber. The insoluble-soluble dietary fiber ratios were different, 50.8:49.2 g 100 g-1 for Morado and 65.6:34.4 g 100 g-1 for Enano. The amount and structure of these components in the soluble and insoluble dietary fiber fractions, as well as the ratio of both fractions, determine its functionality (expansion of baked products, amount of water retained, and texture of the final product) and physiological properties (fermentation, absorption of minerals, intestinal transit) (Champ et al., 2003).
Greater firmness (Table 3) was found for Macho (10.15 N) and Enano (8.94 N) varieties than for Morado (4.95 N) and Valery (4.46 N). The difference in firmness among these varieties could be due to a combination of factors, including turgor pressure of the tissues, structural components and their interactions in the cell wall (Dadzie and Orchard, 1977). Moreover, a correlation was established between cultivated banana firmness and dry matter content by Gibert et al. (2010), who also indicated that starch is the main component of green bananas, thus contributing to the firmness of the varieties. Among the structural components, pectin is responsible for 95-97 % of the firmness of the fruits (Huang and Bourne, 1983). The high value of firmness in cooking bananas makes them less susceptible to mechanical damage and confers greater thermal resistance during postharvest handling and processing. The low strength of dessert bananas could be attributed to their high moisture content, which may increase enzymatic reactions rate, such as hydrolysis of polysaccharides, which confer rigidity to the pulp (Ayo-Omogie et al., 2010; Gibert et al., 2010).
Polyphenols and tannins content and their antioxidant capacity
Morado and Macho bananas showed higher extractable polyphenols content (1.59 and 3.46 mg gallic acid equivalents g-1) than those of Valery and Enano (0.97 and 0.70 mg gallic acid equivalents g-1) (Table 4). Polyphenols content in bananas range from 7.47 to 14 mg catechin g-1 (Balasundrum et al., 2006), and 0.11 to 0.9 mg gallic acid equivalents g-1 (Haslinda et al., 2009). Banana fruit is an important source of polyphenols; however, there are polyphenolic compounds of high molecular weight with low solubility (tannins) that are not taken into account in most chemical and biological studies of extractable polyphenols. Tannins are associated with dietary fiber and other indigestible compounds such as proteins (Haslinda et al., 2009). In this study condensed tannins (proanthocyanidins) of high molecular weight, whose basic structure is represented by flavan-3-ol and flavan-3-4diol, presented higher content than hydrolysable tannins (HT) (gallic and ellagic acids). The Morado variety showed higher values of EP, CT and HT, followed by Macho, whereas Valery showed the lowest EP, CT, and HT, but HT was highest for Enano.
The antioxidant capacities of EP, CT, and HT were different. Thus, EP of Morado variety (3.46 mg GAE g-1 of sample, db) produced an antioxidant capacity of 44.75 (Table 4), and the high CT amount for the same variety produced 57.87, suggesting that a low value of EP produced a high antioxidant capacity. For tannins, HT presented higher antioxidant capacity than CT. The different antioxidant capacity of the phenolics compounds depends on the numbers and positions of the OH- groups present in their structure. However, for some flavonoids determining the structure-activity relationship is more complicated (Saura-Calixto et al., 2007).
The antioxidant capacity of each variety could be influenced by the type of phenolic compound present than the amount ofphenolic compounds evaluated. In banana, gallocatechin, catechin and epicatechin were present in the highest level, and they contributed to the antioxidant activity (Someya et al., 2002). Fiber binds to such compounds while providing protection during the digestive process, and when reaching the colon, fiber is fermented and tannins are released to perform their antioxidant function (Arranz et al., 2010). Thus, green banana could significantly contribute to prevention of gastrointestinal diseases, due to presence of dietary fiber, resistant starch and compounds with antioxidant activity.
Thermal analysis
As described by Dufour et al. (2009), the onset temperature (Table 5) of cooking banana (Macho, 74.8 °C ) was higher than that of Enano (70.7 °C), Morado (59.4 °C) and Valery (71.5 °C) whereas there was no significant differences in enthalpy (adjusted with the starch purity) in J g-1 db of starch. With the highest onset, the Macho variety exhibited the smallest gelatinization range (9.5 °C), which fluctuated from 10.7 to 14.7 °C for dessert bananas. The gelatinization variables (linked to the loss of crystallinity by DSC) are highly dependent on total starch content, higher in cooking banana than in dessert bananas (Table 3). However, dessert banana starch presented a slightly higher onset temperature than cooking banana starch (72.1 versus 69.6 °C), but no difference was found in peak and conclusion temperatures or in enthalpy value. However, the four banana starches had different gelatinization range (Tc-To), indicating that Morado variety has starch granules more heterogenous than Macho variety (Espinoza-Solis et al., 2011).
This difference could be related to the variety used for starch isolation. Thermal variables are also important for the development of new varieties for food processing (according to the thermal resistance and energy needed for cooking). Thermal variables would help for selection of banana varieties with desired properties (i.e., food processing for baby foods or jams).
Pasting profile
The Morado variety presented the lowest pasting temperature (PT) followed by Enano= Valery, and Macho varieties (Table 6). A similar trend was observed when comparing the PT of starches, with starch onset temperature evaluated by differential scanning calorimetry (Table 5). Although both variables (PT and onset temperature) test the starch disorganization due to the heating, they present different values. Macho variety exhibited the highest pasting temperature, the lowest peak and final viscosity, and a low cooking ability. Morado and Enano exhibited the highest peak viscosity, reflecting high swelling of starch granules that could be due to the high amylose content, especially in the cooking type (Dufour et al., 2009). High peak viscosity is often related to the granule size, which was here confirmed for the dessert banana variety, exhibiting the highest distribution of large granule sizes (>40 /μm) among banana varieties, whereas the smallest granule (<7 /μm) and small (7-20 /μm) to intermediate (20-40 /μm) granule size distribution were equivalent (Figure 1). Morado variety presented the highest breakdown (BD) followed by the Enano, whereas Macho and Valery showed the lowest BD, reflecting the fragility of the swollen granules under continuous shear at hot temperatures. The greatest setback was found for Valery and the lowest for Macho and Enano, since setback is an indicator of reorganization of lineal chains, mainly amylose.
Final viscosity and setback pattern was similar between banana varieties. Usually, final viscosity reflects the ability of starch to produce a gel after being cooked and cooled. Final viscosity indicated that Valery produces a stronger gel, which was confirmed by it highest consistency value.
CONCLUSIONS
Macho cooking banana could be highly appreciated for processing and industrial uses according to its high yield (weight, length and circumference, starch, protein, and dry matter content). The beneficial health presence of resistant starch depends on the variety more than the consumption uses and preferences. A similar pattern was found for total dietary fiber, extractable polyphenols and pasting profile. The cooking banana was confirmed, presenting a greater firmness and higher gelatinization temperature than those of the dessert banana. Such specific traits assessed were relevant to the differentiation of edible Mexican banana varieties and for their potential acceptability.
ACKNOWLEDGEMENTS
The authors thank the support from CONACYT (grant 131762), SIP-IPN, COFAA-IPN and EDI-IPN. OLRR also acknowledges the scholarship from CONACYT-México.
LITERATURE CITED
Aguirre-Cruz, A., A. Álvarez-Castillo, H. Yee-Madeira, and L.A. Bello-Pérez. 2008. Production of fiber-rich powder by the acid treatment of unripe banana flour. J. Appl. Polym. Sci. 109: 382-387. [ Links ]
AACC American Association of Cereal Chemists. 2000. Approved Methods of the AACC. 10th ed. The Association. St. Paul, MN. [ Links ]
Aparecida, C., F. Peroni-Okita, R. Barba, M. Shitakubo, F., Lajolo, and B. Cordenunsi. 2011. Plantain and banana starches granule structural characteristics explain the differences in their starch degradation patterns. J. Agric. Food Chem. 59: 6672-6681. [ Links ]
Aparicio-Saguilan, A., E. Flores-Huicochea, J. Tovar, F. García-Suárez, F., Gutiérrez-Meraz, and L.A. Bello-Pérez. 2005. Resistant starch-rich powders prepared by autoclaving of native and linterized banana starch: partial characterization. Starch/Stärke. 57: 405-412. [ Links ]
Arranz, S., J. M. Silván, and F. Saura-Calixto. 2010. Non extractable polyphenols, usually ignored, are the major part of dietary polyphenols a study on the Spanish diet. Mol. Nutr. Food Res. 54: 1-13. [ Links ]
AOAC, Association of Official Analytical Chemists. 2000. Official Methods of Analysis, seventeenth ed. Gaithersburg MD, USA. [ Links ]
Ayo-Omogie, H. N., L. A. Adeyemi, and E. T. Otunola. 2010. Effect of ripening on some physicochemical properties of cooking banana (Musa ABB Cardaba) pulp and flour. Int. J. Food. Sci. Tech. 45: 2605-2611. [ Links ]
Balasundrum, N., K. Sundrum, and S. Samman. 2006. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence and potential uses. Food Chem. 99: 191-203. [ Links ]
Bello-Pérez, L. A., and O. Paredes-López. 2009. Starches of some food crops, changes during processing and their nutraceutical potential. Food Eng. 1: 50-65. [ Links ]
Champ, M., A. M. Langkilde, F. Brouns, B. Kettlitz, and Y. Be Bail Collet. 2003. Advances in dietary fibre characterization. 1. Definition of dietary fibre, physiological relevance, health benefits and analytical aspects. Nutr. Res. Rev. 16: 71-82. [ Links ]
Dadzie, B. K., and J. E. Orchard. 1977. Routine post-harvest screening of banana/plantain hybrids: criteria and methods In: INIBAP Technical Guidelines. International Plant Genetic Resources Institute (ed.). Rome, Italy. pp: 1-77. [ Links ]
Dufour, D., O. Gibert, A. Giraldo, T. Sanchez, M. Reynes, J. P. Pain, A. Gonzalez, A. Fernadez, and A. Diaz. 2009. Differentiation between cooking bananas and dessert bananas. 2. Thermal and functional characterization of cultivated Colombian Musaceae (Musa sp.). J. Agric. Food Chem. 57: 7870-7876. [ Links ]
Espinoza-Solis, V., S.L. Sanchez-Ambriz, B. Hamaker, and L.A. Bello-Pérez. 2011. Fine structural characteristic related to digestion properties of acid-treated fruit starches. Starch/ Stärke. 63: 717-727. [ Links ]
García-Mata, R., M. F. González-Machorro, R. C. García-Sánchez, J. S. Mora-Flores, A. González-Estrada, and M. A. Martinez-Damian. 2013. El mercado del plátano (Musa paradisiaca) en México, 1971-2017. Agrociencia 47: 399410. [ Links ]
Gibert, O., D. Dufour, A, Giraldo, T. Sanchez, M. Reynes, J. P. Pain, A. Gonzalez, A. Fernandez, and A. Díaz. 2009. Differentiation between cooking bananas and dessert bananas. 1. Morphological and compositional characterization of cultivated Colombian musaceae (Musa sp.) in relation to consumer preferences. J. Agric. Food Chem. 57: 7857-7869. [ Links ]
Gibert, O., D. Dufour, M. Reynes, A. Prades, L. Moreno-Alzate, A, Giraldo, A. Escobar, and A. González. 2013. Physicochemical and functional differentiation of dessert and cooking banana during ripening — A key for understanding consumer preferences. Acta Hortic. 986: 269-286. [ Links ]
Gibert, O., A. Giraldo, J. R. Uclès-Santos, T. Sanchez, A. Fernandez, P. Bohuon, M. Reynes, A. Gonzalez, J. P. Pain, and D. Dufour. 2010. A kinetic approach to textural changes of different banana genotypes (Musa sp.) cooked in boiling water in relation to starch gelatinization. J. Food Eng. 98: 471-479. [ Links ]
Hartzfeld, P. W., R. Forkner, D. M. Hunter, and A. E. Hagerman. 2002. Determination of hydrolizable tannins (gallotannins and ellagitanins) after reaction with potassium iodate. J. Agric. Food. Chem. 50: 1785-1790. [ Links ]
Haslinda, W. H., L. H. Cheng, L. C. Chong, and A. A. Noor-Aziah. 2010. Chemical composicion and physicochemical properties of green banana (Musa acuminata x balbisiana Colla cv. Awak) flour. Int. J. Food Sci. Nutr. 60: 232-239. [ Links ]
Hendrich, S. 2010. Battling obesity with resistant starch. Food Tech. 64: 22-30. [ Links ]
Huang, Y. T., and M. C. Bourne. 1983. Kinetics of thermal softening of vegetables. J. Texture Stud. 14: 1-9. [ Links ]
Juarez-Garcia, E., E. Agama-Acevedo, S. G. Sayago-Ayerdi, S. L. Rodriguez-Ambriz, and L. A. Bello-Perez. 2006. Composition, digestibility and application in bread making of banana flour. Plant Food Hum. Nutr. 61. 131-137. [ Links ]
Montreau, F.R., 1972. Sur le dosage des composés phénoliques totaux dans les vins par la méthode Folin-Ciocalteau. Connaiss Vigne. Vin. 24: 397-404. [ Links ]
Ovando-Martinez, M., S. Sayago-Ayerdi, E. Agama-Acevedo, I. Goñi, and L. A. Bello-Perez. 2009. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 113: 121-126. [ Links ]
Pelissari, F. M., M. M. Andrade-Mahecha, P. J. do Amaral-Sobral, and F. C. Manegalli. 2012. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch/Stärke 64: 382-392. [ Links ]
Pulido, R., M. Hernández-García, and F. Saura-Calixto. 2003. Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur. J. Clin. Nut. 57: 1275-1282. [ Links ]
Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 26: 1231-1237. [ Links ]
Reed, J., R.E.McDowell, P. J. Van Soest, and P.J. Horvarth. 1982. Condensed tannins: A factor limiting the use of cassava forage. J. Sci. Food Agric. 33: 213—220. [ Links ]
Rodríguez-Ambriz, S.L., J.J. Islas-Hernández, E. Agama-Acevedo, J. Tovar, and L.A. Bello-Pérez. 2008. Characterization of a fibrerich powder prepared by liquefaction of unripe banana flour. Food Chem. 107: 1515-1521. [ Links ]
Saura-Calixto, F., J. Serrano, and I. Goñ. 2007. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem.101: 492-501. [ Links ]
Singleton, V. L., R. Orthofer, and R. M. Lamuela-Ravento. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 299: 152-178. [ Links ]
Someya, S., Y. Yoshiki, and K. Okubo. 2002. Antioxidant compounds from banana (Musa cavendish). Food Chem. 79: 351-354. [ Links ]
Vázquez-Castrejón, R., A. Romero-Cadena, y J. Figueroa-Viera. 2005. Paquete tecnológico para el cultivo de Plátano. Gobierno del Estado de Colima. http://eiag.edu.ni/Pwebs/Carreras/FRUTYWEB/CONFERENCIAS%202011/UNIDAD%20II.%20Musaceas/Materiales%20Musaceas/Paquete%20tecnologico%20del%20cultivo%20del%20P%C3%A1tano.pdf. (Consulta: noviembre 2012). [ Links ]
Yomeni, M. O., J. Njoukam, and J. Tchango-Tchango. 2004. Influence of stage of ripeness of plantains and some cooking bananas of sensory and physicochemical characteristics of processed products. J. Sci. Food Agric. 84: 1069-1775. [ Links ]
Zhang, P., and B. Hamaker. 2012. Banana starch structure and digestibility. Carbohyd. Polym. 87: 1552-1558. [ Links ]














