Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.48 n.2 Texcoco Feb./Mar. 2014
Fitociencia
Embryogenic induction of torch ginger (Etlingera elatior) callus
Inducción embriogénica en callo de jengibre antorcha (Etlingera elatior)
Gabrielen de M. Gomes-Días1*, Filipe Almendagna-Rodrigues1, J. Dória Rodrigues-Soares1, Moacir Pasqual1, A. Cristina Portugal- Pinto de Carvalho2
1 Federal University of Lavras (UFLA), Departamento of Agriculture, Postal Office 3037, 37200-000, Lavras-MG, Brazil. * Author for correspondence (gabriellen@gmail.com).
2 Embrapa Tropical Agroindustry (CNPAT), Dra. Sara Mesquita Street, 2270, 60511-110, Fortaleza-CE, Brazil.
Received: February, 2013.
Approved: February, 2014.
Abstract
Torch ginger Etlingera elatior (Jack) R.M. Smith is a tropical herb-like, rhizome-like and perennial plant that possesses inflorescences that are both pretty and showy at different colour shades, and have a great visual appeal. Producing seedlings at a high enough level when done conventionally by the division of tussocks, has high costs and phytosanitary problems. The objective of this study was to evaluate the induction of somatic embryogenesis and its potential for subsequent plant regeneration in torch ginger. Rhizomes, leaves and roots from the seedlings grown in vitro were used as explant segments. The different explants were cultured on MS medium supplemented with 30 g L-1 sucrose, and it was solidified with 5.5 g L-1 of agar. The effect of 2.4-D and picloram concentrations of 0.0, 1.0, 2.0 and 4.0 mg L-1 was studied. Astructural analysis was performed by scanning electron microscopy (SEM). Cytochemical analysis were performed to detect nucleeous with carmine acetic, starch with lugol and lipids with Sudan III, and a flow cytometry analysis to verify the stability of ploidy in the callus. Results indicate that the culture medium with 2.4-D yielded the induction of embryogenic callus with the characteristics of the rhizome segments of the explant. Regarding the ultrastructural analysis of embryogenic callus characteristics, the cells showed a similar isodiametric form as somatic embryos in the globular stage and cytochemical analysis. The presence of callus cells in pro-embryogenic explants containing each of the regulators was confirmed. In the cytometry analysis, the average DNA content of callus ranged from 5.43 to 10.15 pg, and the coefficients of variation varied between 0.3 to 2.26. Callus induced with 2.4-D showed 86 % stability, and with picloram was 64 %. The callus formation was more evident after application of 2.4 D. This is the first study of embryogenic potential in cells of the torch ginger, and showed that 2,4-D induced callus with higher starch content and genetically stable.
Keywords: Zingiberaceae, Etlingera elatior, viability.
Resumen
El jengibre antorcha Etlingera elatior (Jack) R. M. Smith es una planta tropical perenne, similar al rizoma, que tiene inflorescencias que son bellas y llamativas, de diferentes colores y matices y de un gran atractivo visual. La producción de plántulas en un nivel lo suficientemente alto cuando se hace convencionalmente utilizando la división de matas tiene altos costos y problemas fitosanitarios. El objetivo de este estudio fue evaluar la inducción de la embriogénesis somática y su uso potencial para la reproducción posterior de plantas de jengibre antorcha. Los rizomas, hojas y raíces de las plántulas cultivadas in vitro se utilizaron como segmentos de explantes. Los diferentes explantes se cultivaron en un medio MS al que se le agregaron 30 g L-1 de sacarosa y fue solidificado con 5.5 g de agar L-1. Se estudió el efecto del 2.4-D y picloram en concentraciones de 0.0, 1.0, 2.0 y 4.0 mg L-1. El análisis estructural se llevó a cabo por microscopía electrónica de barrido (SEM). Se realizaron análisis citoquímicos para detectar núcleos con carmín acético, almidón con lugol y lípidos con Sudán III; y un análisis de citometría de flujo para verificar la estabilidad de la ploidía en el callo. Los resultados indicaron que el medio de cultivo con 2.4-D produjo la inducción de callo embriogénico con las características de los segmentos de rizomas del explante. En el análisis ultraestructural de las características del callo embriogénico, las células mostraron una forma isodiamétrica similar a embriones somáticos en etapa globular y análisis citoquímico. Se confirmó la presencia de células de callo en explantes pro- embriogénicos conteniendo cada uno de los reguladores. En el análisis de citometría, el contenido medio de ADN del callo varió de 5.43 a 10.15 pg, y los coeficientes de variación oscilaron entre 0.3 y 2.26. El 2.4-D indujo callos con estabilidad de 86 %, y con picloram fue 64 %. La formación de callo fue más evidente después de aplicar 2.4-D. Éste es el primer estudio del potencial embriogénico de células del jengibre antorcha, y mostró que el 2.4-D indujo la formación de callo con mayor contenido de almidón y genéticamente estable.
Palabras clave: Zingiberaceae, Etlingera elatior, viabilidad.
INTRODUCTION
The main species of tropical flowers in Brazil belong to the families Araceae, Heliconiaceae, Musaceae and Zingiberaceae; they are found either as natural vegetation or in conventional plantations in tropical America, Asia and the western Pacific (Assis et al., 2002). Among these plants, there is propagation and cultivation of the Etlingera genus that belongs to the Zingiberaceae family; in particular, the torch ginger (Etlingera elatior (Jack) R. M. Smith) and is propagated by rhizomatous clumps division or seeds. This vegetative propagation spread pests and diseases causing production problems (Lins and Coelho, 2004).
Somatic embryogenesis is an in vitro propagation technology with promising results for improving plant cloning. It has elicited interest, especially from research institutions and Brazilian companies with advanced breeding and cloning programs (Titon et al., 2007). Somatic embryogenesis is an important system for vegetative propagation in which somatic cells can generate embryos due to totipotency of plant cells. This process provides a useful experimental model to investigate the events of plant embryogenesis; besides, it is one of the most important techniques for mass propagation of elite or genetically modified plants (Santos et al., 2002). In vitro regeneration is achieved via organogenesis (Yunus et al., 2012); however, in the literature reviewed no references were found about somatic embryogenesis in torch ginger.
The objective of this study was to evaluate different types of explants and growth regulators concentrations in relation to the potential for callus induction in torch ginger (Etlingera elatior (Jack) RM Smith) var. Porcelain.
MATERIALS AND METHODS
The study was carried out in the Laboratory of Plant Tissue Culture of the Department of Agriculture (DAG) at the Federal University of Lavras (UFLA), between January to July 2010.
The plants established in vitro from shoot meristems of torch ginger var. Porcelain. These were provided by the Brazilian Unit of Agricultural Research (EMBRAPA), located in Fortaleza, Ceará. These plants were subcultured monthly for 60 d under aseptic conditions on MS medium (Murashige and Skoog, 1962) containing 2.5 mg L-1 of 6-benzilamonopurine (BAP) (SIGMA®) solidified with agar (5.5 g L-1). The cultures were maintained in a growth chamber at 25 ±2 °C, irradiance of 36 mmol m-2 s-1 and for a 16-h photoperiod.
Leaf segments (0.7 cm2), root segments (1.0 cm) and rhizome segments (0.5 cm) were removed from the micropropagated plants, and the rhizome was cut longitudinally into three segments corresponding to positions R1 (apical), R2 (middle) and R3 (basal). The segments were transferred to test tubes containing MS medium supplemented with four concentrations of 2.4-D (SIGMA®) and and four of picloram (SIGMA®) (0.0, 1.0, 2.0 and 4.0 mg L-1). The cultures were incubated in a growth chamber at 25±2 °C in complee darkness. At 120 d after inoculation, the percentage of callus formation was measured in all treatments.
The experimental design was completely randomized with 40 treatments (eight auxin concentrations and five types of explants) and five replicates per treatment (two test tubes each). The data were statistically analyzed for variance and regression using the program SISVAR (Ferreira, 2011).
Aditionally a scanning electron microscopy (SEM) study, a cytochemical test and a ploidy study level by flow cytometry were performed on each treatment at 120 d. For the SEM study, the callus tissue was fixed in Karnovsky (Karnovisky, 1965), fixative solution (pH 7.2) for 24 h inder refrigeration (4 °C). Then, samples were washed in 0.056 M cacodylate buffer (three times) for 10 min each and postfixed in 1 % osmium tetroxide in 0.05 M cacodylate (SIGMA®) buffer for 4 h at room temperature (20 °C). Afterwards, the samples were washed three times in distilled water and dehydrated in a serial of acetone solutions (25, 50, 75 and 90 %) for 10 min each and three times in 100 % acetone for 1 min. The samples were dried in a critical point drier using an Instrument Bal-Tec (model CPD 030 Critical Point Dryer, Germany) and gold coated. The specimens were observed with SEM (LEO Evo 040, Germany), operating at 10 and 20 kV, at the Laboratory of Electron Microscopy and Ultrastructural Analysis (LME) of the Plant Pathology Department (UFLA).
For the cytochemical analysis callus fragments of 100 mg were double stained with acetic carmine blue Evans (positive reaction indicates embryogenic potential of cells), lugol for starch and Sudan III for lipids, according to Steiner et al. (2005). The photomicrographs were made with a digital camera EIREKAM 3.0 (effective pixels 2048 x 1536, USA) coupled to a light microscope (Olympus BX 60).
The determination of DNA amount and ploidy level was performed in three replicates of 300 mg from callus of each treatment. The samples were crushed in a petri dish containing 1 mL of extraction buffer LB01 (Dolezel et al., 1989). A leaf weight of pea (Pisum sativum) was used as reference standard quantity of DNA=9.9 pg. Histograms were obtained on a FACSCalibur cytometer (Becton Dickinson, USA) with the Cell Quest software (Dickinson, 1998). The amount of DNA (pg) was calculated with the equation: Amount of DNA (pg) = (G1 peak position of sample / G1 peak position pea (Pisum sativum)) x9.09. The statistical analysis of the data was performed using WinMDI 2.8 software (Trotter, 2000).
RESULTS AND DISUSSION
In the absence of growth regulators, there was no callus in any of the five tested explants butthe rhizomes formed whole plants and lateral shoots (Figure 1 D). Most species require growth regulators to initiate the process of callus formation, since the concentrations of endogenous auxin and cytokinins are not sufficient to perform this process (George, 2008). The use of 2.4-D and picloram was effective to induce friable embryogenic callus in the different tested explants, after 120 d of in vitro inoculation (Figure 1 B). In contrast, Miachir et al. (2004) found no induction of callus explant in any of Curcuma zedoaria when using only 2.4-D in the culture medium.
There was an increase in the percentage of callus (100 %) on the surface of the explant R1 rhizome as the concentrations of picloram or 2.4-D were raised to 2.5 mg (Figure 2 A). These results are different to those obtained by Lincy et al. (2009), who found that the use of 2.4-D at 1.0 and 2.0 mg L-1 in rhizomes of Zingiber officinale Rosc. favored callus induction by 70 and 100 %.
A similar tendency was observed for R2 rhizomes, with an increase in callus formation (100 %) for 1.0 and 2.0 mg L-1 2.4-D and 4.0 mg L-1 picloram, and there were significant differences between the treatments (Figure 2 B). This result is analogous to that obtained by Roopadarshini (2010) who, using in vitro vegetative buds of Curcuma longa L., reported 86 % callus with 3.0 mg L-1 2.4-D and 38 % with 0.5 mg L-1 2.4-D.
There were significant differences for callus formation in explants of rhizome R3. Thus, the best response (100 %) was for 4.0 mg L-1 picloram (Figure 2 C), whereas with 2,4-D callus formation was similar for 1.0 and 2.0 mg L-1 (90 %) but with 4.0 mg L-1 it decreased to 60 %. Different results were obtained by Rostiana and Syahid (2008) who found a significant difference for meristems of Zingiber officinale Rosc. with 1.0 (87 %), 2.0 (47 %) and 3.0 mg L-1 (28 %) of 2.4-D.
For root segments, there were also significant differences between treatments at the concentrations of 1.0 mg L-1 2.4-D and 4.0 mg L-1 picloram (100 %) (Figure 2 D). This result is different to that reported by Miachir et al. (2004) who found no callus formation in root segments in Curcuma zedoaria when using 2.4-D.
The high percentages of embryogenic callus arising from root segments and sections of rhizome increase the availability of sources of torch ginger explants, since it was obtained in 60 to 100 % of embryogenic callus with the regulators utilized in the present study.
The increase in the concentrations of 2.4-D and picloram caused only a small increase in callus formation in leaf explants, but there was no significant difference between treatments and the maximum was 20 % with 4.0 mg L-1 of both growth regulators. Different results were reported by Sultana et al. (2009) who found that leaf explants of Zingiber officinale Rosc. induced 67 % of embryogenic callus.
The effects 2.4-D and picloram were studied in somatic embryogenesis of Cleome rosea Vahl by Simões et al. (2010), who found that both regulators were effective in callus formation but only 2.4-D caused somatic embryogenesis. According to Jimenez (2005), 2.4-D is the most used auxin in the induction of callus and somatic embryos, combined with picloram, which may be more effective than 2.4-D (George, 2008). However, in the present study, picloram was more efficient for all segments used (except for leaves). The callus formed in the presence of both 2.4-D and picloram had a white-yellowish coloration without oxidation, a friable appearance, and formation of a structure similar to globular embryos (Figure 1G, H and I). These structures are characteristic of the first stage of tissue differentiation and are the first indication of success in a protocol that aims for somatic embryogenesis.
A more friable texture was fund in callus grown at 1.0 and 2.0 mg L-1 2.4-D. Root-like structures were observed in callus masses that formed in roots segments on medium with 4.0 mg L-1 2.4-D, which were probably originated via somatic embryogenesis. This would agree with Reis et al. (2007), who showed roots originated from callus on Schizolobium parahyba grown at 6.0 mg L-1 2.4-D. These roots had no vascular communication with the explant.
In the analysis by SEM, it was observed that the cell masses developed under the influence of 2.4-D had a rounded shape in all explants tested (Figure 1 N, O, P, Q, R and S). It was possible to distinguish regions with a spherical organization presenting isodiametric cells with a similar shape to the globular stage of somatic embryos, which, according to Evert (2006), is characteristic of meristematic cells.
Similar results were also found by Ulisses et al. (2010), who used 2.4-D to induce callus in Heliconia chartaceae and obtained round cells and in cell division. According to Zuo et al. (2002), 2.4-D has the function of inducing somatic embryogenesis. Nogueira et al. (2007) also found that it was possible, with SEM, to observe clear cellular changes in shape over time of exposure in leaf segments of Byrsonima intermediate with the use of 2.4-D. This indicates that the presence of auxin is a crucial factor in signaling cell dedifferentiation and subsequent resumption of meristematic characteristics and it also favors somatic embryogenesis.
The cell masses under the influence of picloram showed cells in clusters with rounded shape, demonstrating the morphogenic ability of both auxins and, according to Steiner et al. (2005), isodiametric cells with such a shape are characteristic of embryogenic formations. Besides, it was observed that callus formation in the treatments containing picloram presented cells surrounded by a thin layer, typical of cells at the beginning of callus formation (Figure 1 R and S). The analysis with SEM confirmed the presence of proembryos in the callus and morphogenetic capacity of rhizome segments of torch ginger var. Porcelain. All callus originated with the addition of 2.4-D (Figure 1 J) and picloram showed a positive reaction to acetocarmine stain, which confirmed the presence of pro-embryogenic cells. According to Besson et al. (2010), friable callus with granular texture exhibit small isodiametric cells, which react with acetocarmine and are indicative of the presence of glycoproteins associated with the embryogenesis route. Steiner et al. (2005) pointed out that the positive reaction to acetocarmine staining is associated with cell development competence. Besides, it was observed that there were two cell types in embryogenic cultures. For 2.4-D, the cells were less clustered (friable), for picloram, the cells had a certain clustering (compact).
The presence of starch was detected in embryogenic cells from both growth regulators. Starch grains were mainly found in the inner part of the proembiogenic tissue (Figure 1 L). Pescador et al. (2008) showed remarkable differences in nonstructural carbohydrates, with lower values of soluble sugars and a higher starch content in somatic embryos of Acca sellowiana when compared to zygotes. The possible role of starch in the somatic embryogenesis process is still unclear, but it is known that the reserve components found here are necessary for reorganization and cell differentiation. A positive reaction with Sudan III detected the presence of lipids, shown in a dark orange color (Figure 1 M), what highlights its importance as a reservesubstance. The starch synthesis can be directly associated with the mobilization of the lipid content via the glyoxylate cycle, since starch grains were detected in larger amounts than the lipid grains at the same development stage (Taiz, 2010).
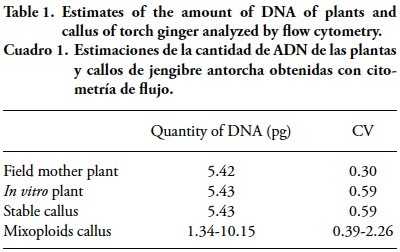
The ploidy analysis by flow cytometry indicated that the amount of DNA of the individual plant and the in vitro plant showed no change, remaining at 5.42 ± 0.01 pg. The amount of DNA of callus, estimated with three samples, ranged from 5.43 to 10.15 pg (Table 1).
These results show evidence of the genetic stability of the callus in relation to the Mother plant in the field, which is important for plants requiring DNA and no genetic variability. Quantification of the amount onf nuclear DNA may replace chromosome counting, which facilitates the work with a very large number of individuas (Schifino-Wittmann, 2001). The coefficient of variation (CV) from 0.3 to 2.26 (Table 1) indicated the uniformity of the population and reliability of the data. Marie and Brown (1993) and Ulrich and Ulrich (1991) showed that a CV between 1 and 2 % indicates high quality results, and approximately 3 % is routine, while Galbraith et al. (1983) define a CV below 5 % as an acceptance criterion for international publications.
The peaks of the mother plant and in vitro plant indicated that they were diploid plants (Figure 3) with scarce mitotic divisions in their tissue. Callus induced by 2.4-D showed 86 % stable and 14 % mixoploids (Table 2), while those induced by picloram were 64 % stable and 36 % mixoploids. These results show greater variability in ploidy in callus induced by picloram. Lo Schiavo et al. (1989) indicated that the methylation degree increased as concentration of 2.4-D raised in the culture medium, whereas Muller et al. (1990) found that 2.4-D tends to accelerate cell division, which may indirectly affect genetic alteration and the methylation degree, which may interferes with DNA quantification.
The variations found in callus induced by 2.4-D and picloram may be related to the exposure time in the culture medium or to the high cell division or the explant. The mixoploidy found in the callus appeared from explants of the rhizome segments, which is consistent with the quote from Gutierrez and Ferrero (2005) that explants, as leaf tissue, roots and rhizomes, can accumulate a large number of somatic mutations.
The best results were obtained with the use of 2.4-D with rhizome explant of in vitro seedlings, where more evidence of somatic embryos was exhibited. With the flow cytometric technique, it was possible to obtain the ploidy level of the embryogenic cells of torch ginger, and the regulator 2.4-D reached 84 % of stable callus. The callus induced by picloram showed greater mitotic activity (mixoploids) in the genetic material, compared to callus induced by 2.4-D.
CONCLUSIONS
The growth regulator 2,4-D induced callus geneticaly stable with high starch content and great potential for embryogenic callus induction, which inables the development of future methodologies.
ACKNOWLEDGMENTS
We thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the granting of a scholarship and Embrapa Tropical Agroindustry Corporation for providing the seedlings for this work.
LITERATURE CITED
Assis, S. M. P., R. R. L. Marinho, M. G. C. Goim Júnior, M. Menezes, e R. C. T. Rosa. 2002. Doenças e Pragas de Helicônias. Diseases and Pests of Helicônias. Recife UFRPE. 102 p. [ Links ]
Besson, J. C. F., L. K. Oliveira, F. Cavalini, W. Neiverth, A. F. Neves, e S. Stefanello. 2010. Influência de reguladores de crescimento e extratos vegetais na resposta morfogenética de calos de Solanum sessiliflorum DUNAL. Rev. Agr. Meio Amb. 3(3): 311-322. [ Links ]
Dickinson, B. 1998. CellQuest Software Reference Manual, rev. B. San Jose [s.n.] 227 p. [ Links ]
Dolezel, J., P. Binarova and S. Lucretti. 1989. Analysis of nuclear DNA content in plant cells by flow cytometry. Biol. Plantarum 31(2): 113-120. [ Links ]
Evert, R. F. 2006. Esau's Plant Anatomy, 3rd edn. Meristems, Cells and Tissues of the Plant Body — their Structure Function and Development. Wiley-Interscience, New Jersey.
Ferreira, D. F. 2011. SISVAR: A Computer Statistical Analysis System. Ciência Agrotecnologia 35(6): 1039-1042. [ Links ]
Galbraith, D. W., K. R. Harkins, J. M. Maddox, N. M. Ayres, D. P. Sharma, and E. Firoozabady. 1983. Rapid flow cytometric analysis of the cell-cycle in intact plant-tissues. Science 220(4601): 1049-1051. [ Links ]
Gutierrez, L. G. L., and V. M. Ferrero. 2005. Análise de ploidia por citometria de flujo de callos embriogénicos de Aliso andino (Alnus acuminata H. B. K.). Sci. Tech. 11(28):157- 161. [ Links ]
George, E. F. 2008. Plant Propagation by Tissue Culture. 3. ed. Hants Exegetics. 1, 501 p. [ Links ]
Jiménez, V. M. 2005. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul. 47(1/2): 91-110. [ Links ]
Karnovsky, M. J. 1965. A formaldehyde-glutaraldehyde fixative in high osmolality for use in electron microscopy. J. Cell Biol. 27: 137-138. [ Links ]
Lincy, A. K., A. B. Remashree, and B. Sasikumar. 2009. Indirect and direct somatic embryogenesis from aerial stem explants of ginger (Zingiber officinale Rosc.). Acta Bot. Croatica 68(1): 93-103. [ Links ]
Lins, S. R. O., e R. S. B. Coelho. 2004. Ocorrência de doenças em plantas ornamentais tropicais no Estado de Pernambuco. Fitopatol. Bras. 29: 332-335. [ Links ]
Lo Schiavo, F., L. Pitto, G. Giuliano, G. Torti, V. Nuti-Ronchi,, D. Marazziti, R. Vergara, S. Orselli, and M. Terzi. 1989. DNA methylation of embryogenic carrot cell cultures and its varation as caused by mutation, differetation, hormones and hypomethylating drugs. Theor. Appl. Genet. 77: 325-331. [ Links ]
Marie, D., and S. A. Brown. 1993. A cytometric exercice in plant DNA histograms, with 2C values for 70 species. Biol. Cell 78: 41-51. [ Links ]
Miachir, J. I., V. L. M. Romani, A. F. de C. Amaral, M. O. Mello, O. J. Crocomo, and M. Melo. 2004. Micropropagation and callogenesis of Curcuma zedoria Roscoe. Scientia Agricola 61: 427-432. [ Links ]
Muller, E., P. T. H. Brown, S. Hartke, and H. Lorz. 1990. DNA variation in tissue culture-derived rice plants. Theor. Appl. Genet. 80: 673-679. [ Links ]
Murashige, T., and F. A. Skoog. 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiologia Plantarum 25: 473-497. [ Links ]
Nogueira, R. C., R. P. Paiva, J. M. P. Porto, P. M. Nicioli, V. C. Stein, S. Deuner, and E. Alves. 2007. Análise ultraestrutural de calos embriogênicos de murici-pequeno (Bysonima intermédia a. Juuss.). Rev. Bras. Biociên. 5: 48-50. [ Links ]
Pescador, R., G. B. Kerbauy, J. E. Kraus, W de W. M. Ferreira, M. P. Guerra, and R. de C. L. F. Ribeiro. 2008. Changes in soluble carbohydrates and starch amounts during somatic and zygotic embryogenesis of Acca sellowiana (Myrtaceae). Vitro Cellular Develop. Biology-Plant 44: 289-299. [ Links ]
Reis, I. N. R. de S., O. A. Lameira, e I. M. C. C. Cordeiro. 2007. Efeito do 2,4-D na indução de calos in vitro de paricá (Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby). Rev. Bras. Biociên. 5: 498-500. [ Links ]
Roopadarshini, V. 2010. High frequency shoot multiplication and callus regeneration of turmeric. Int. J. Biotechnol. Biochem. 6: 723-733. [ Links ]
Rostiana, O., and S. F. Syahid. 2008. Somatic embryogenesis from meristem explants of ginger. Biotropia 15: 12-24. [ Links ]
Santos, A. L. W, V. Silveira, N. Steiner, M. Vidor, and M. P. Guerra. 2002. Embriogênese somática em araucária (Araucaria angustifolia (Bert.) O. Kuntze). Braz. Arch. Biol. Technol. 45: 97-106. [ Links ]
Simões, C., N. Albarello, C. H. Callado, T. C. Castro, and E. Mansur, E. 2010. Somatic embryogenesis and pant regeneration from callus cultures of cleome rosea vahl. Brazilian Arch. Biol. Technol. 53: 679-686. [ Links ]
Steiner, N., F. N. Vieira, S. Maldonado, and M. P. Guerra. 2005. Effect of carbon source onmorphology and histodifferenciation of Araucariaangustifolia embriogenic cultures. Braz. Arch. Biol. Technol. 48: 895-903. [ Links ]
Sultana, A., L. Hassan, S. D. Ahmad, A. H. Shah, F. Batool, M. A. Islam, R. Rahman, and S. Moonmoon. 2009. In vitro regeneration of ginger using leaf, shoot tip and root explants. Pak. J. Bot. 41: 1667-1675. [ Links ]
Titon, M., A. Xavier, W. C. Otoni, e S. Y. Motoikes. 2007. Efeito dos reguladores de crescimento dicamba e picloram na indução de embriogênese somática em Eucalyptus grandis. Revista Árvore 31: 417-426. [ Links ]
Trotter, J. 2000. WinMDI© Version 2.8. La Jolla, CA: The Scripps Research Institute. http://facs.scripps.edu/software.html. [ Links ]
Ulrich, I., and W. Ulrich. 1991. High-resolution flow cytometry DNA in higher plants. Protoplasma 165: 212-215. [ Links ]
Ulisses, C., T. R. Camara, C. C. de A. Wiladino, and J. Z. Brito. 2010. Early somatic embryogenesis in Heliconia chartacea Lane ex Barreiros cv. Sexy Pink ovary section explants. Braz. Arch. Biol. Technol. 53: 11-18. [ Links ]
Yunus, M. F., M. A. Aziz, M. A. Kadir, and A. A. Rashid. 2012. In vitro propagation of Etlingera elatior (Jack) (torch ginger). Scientia Horticulturae 135: 145-150. [ Links ]
Zuo, J., Q. W. G. Niu, and N. H. C. Frugis. 2002. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. The Plant J. 30: 349-359. [ Links ]