Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.47 no.4 Texcoco Jan./Jun. 2013
Biotecnología
Lipophilic constituents and some biological activities of hexanic extracts from Zaluzania montagnifolia, (SCH. BIP.) SCH. BIP. (Asteraceae)
Constituyentes lipofílicos y algunas actividades biológicas de los extractos hexánicos de Zaluzania montagnifolia (SCH. BIP.) SCH. BIP. (Asteraceae)
Nemesio Villa-Ruano1*, Yesenia Pacheco-Hernández2, Edmundo Lozoya-Gloria3, Efraín Rubio-Rosas4, Nancy Ruiz-González4, Yuriana Martínez-Orea5, Ramiro Cruz-Duran5, S. Alberto Ramirez-Garcia1, L. Guadalupe Ramón-Canúl1
1 Universidad de la Sierra Sur. Guillermo Rojas Mijangos s/n. Ciudad Universitaria. 70805, Miahuatlán de Porfirio Díaz, Oaxaca, México * Author for correspondence: (necho82@yahoo.com.mx).
2 Instituto Tecnológico de Sonora. 5 de Febrero 818 Sur. Colonia Centro. 8500, Ciudad Obregón, Sonora, México.
3 Centro de Investigación y de Estudios Avanzados del IPN. Unidad Irapuato. Km 9.6 Libramiento Norte. Carretera Irapuato-León. 36000, Irapuato México.
4 Benemérita Universidad Autónoma de Puebla. Ciudad Universitaria Avenida San Claudio y 22 sur. 72570, Puebla, México.
5 Universidad Nacional Autónoma de México, Ciudad Universitaria. México DF. Avenida Universidad 3000. 04510, Delegación Coyoacán, México, D.F.
Received: October, 2012.
Approved: March, 2013.
Abstract
Zaluzania montagnifolia, an antidiabetic and abortive shrub 1 to 3 m high, grows actively in xeric scrublands in some states of México. Zulazanins and some flavonoids are the foremost constituents of the polar extracts in this species; however, there is no available information about the lipophilic compounds from non-polar extracts. The objective of this study was to describe the gas chromatography-mass spectrometry (GC-MS) profile of hexanic preparations, exclusivelly with dissolve lipophilic molecules, in order to sustain the empirical uses and the new biological activities reported in this study for the plant. These preparations were obtained from flowers, leaves and roots of Z. montagnifolia which were collected in Oaxaca, México, in October 2011. In addition to the chemical study, antibacterial, allelopathic and antioxidant properties of the hexanic extracts were determined. The chemical profile revealed two majoritarian ent-kaurane diterpenes. According to the relative endogenous levels, grandiflorenic acid (35.5 %) and ent-kaurenoic acid (28.3 %) were the most abundant natural molecules in aerial and underground structures of the plant, and more than 50 different volatile organic compounds (VOC's) were identified according to their mass spectra and retention index. Antibacterial tests were carried out by the broth microdilution method in combination with hexanic extracts and conventional antibiotics as reference standards. The analyisi of results reveal potent inhibitory on Escherichia coli TOP10 F' (MIC=49.2 µg mL-1) and DH5α (MIC= 32.4 µg mL-1) strains as well as for Agrobacterium tumefaciens LBA 4404 (MIC=30.4 µg mL-1) strain. Allelopathic properties were determined using the lettuce seed system, showing that the root extracts had a significant effect (p≤0.01). An acceptable antioxidant activity of leaf and flower extracts (IC50=158.3-253.5 µg mL-1) was found by using 2,2-diphenyl-1-picryl hydrazyl radical (DPPH). The latter biological activities are strongly related to VOC's and ent-kaurane derivatives which play an essential role as antibacterial and allelopathic agents.
Keywords: Zaluzania montagnifolia, grandiflorenic acid, ent-kaurenoic acid, VOC's, biological activities.
Resumen
Zaluzania montagnifolia, un arbusto antidiabético y abortivo de 1 a 3 m de altura, crece activamente en matorrales xerófilo en algunos estados de México. Las zulazaninas y algunos flavonoides son los constituyentes principales de los extractos polares en esta especie; sin embargo, no existe información disponible sobre los compuestos lipofílicos en los extractos no polares. El objetivo de este estudio fue describir el perfil lípofílico por medio de cromatografía de gases acoplada a espectrometría de masa (GC-MS) de extractos hexánicos, exclusivamente con moléculas lipofílicas, para sustentar usos empíricos y nuevas actividades biológicas reportadas en este estudio para la planta. Las preparaciones se obtuvieron de flores, hojas y raíces de Z. montagnifolia recolectadas en Oaxaca, México, en octubre del 2011. Además del estudio químico, se determinaron las propiedades antibacteriales, alelopáticas y antioxidantes de los extractos hexánicos. El perfil químico reveló dos diterpenos del ent-kaureno mayoritarios. De acuerdo con los niveles endógenos relativos, el ácido grandiflorénico (35.5 %) y el ent-kaurenoico (28.3 %) fueron las moléculas naturales más abundantes en las estructuras aéreas y subterráneas, y se identificaron más de 50 compuestos orgánicos volátiles (VOCs) con base en su espectro de masas y su índice de retención. Pruebas antibacteriales se realizaron con el método de microdilución en caldo en combinación con extractos hexánicos y antibióticos convencionales como estándares de referencia. El análisis de los resultados revelan potencial inhibitorio para Escherichia coli TOP10 F' (MIC= 49.2 µg mL-1) y cepas de DH5α (MIC= 32.4 µg mL- 1), y para la cepa Agrobacterium tumefaciens LBA 4404 (MIC=30.4 µg mL-1). Las propiedades alelopáticas se determinaron con el sistema de semilla de lechuga, mostrando que los extractos de raíz tuvieron un efecto significativo (p≤0.01). La actividad antioxidante fue aceptable para los extractos de hoja y flor (IC50=158.3-253.5 µg mL-1), usando el radical 2,2-difenil-1-picril hidrazil (DPPH). Las últimas actividades biológicas están muy relacionadas con los VOCs y los derivados de ent-kaureno, los cuales tienen una función esencial como agentes antibacterianos y alelopáticos.
Palabras clave: Zaluzania montagnifolia, ácido grandiflorénico, ácido ent-kaurenoico, VOCs, actividades biológicas.
INTRODUCTION
The Zaluzania genus is one of the most recurrent Asteraceae groups in Mexican traditional medicine due to its anti-tumoral, antidiabetic and abortive properties (Andrade-Cetto and Heinrich, 2005). Zaluzania montagnifolia, Z. augusta (limpia tuna), Z. triloba, Z. grayana, and Z. robinsonii contain guaianolide sesquiterpene lactones called zaluzanins (from A to E and their dehydro forms), which are the main constituents of such species. These compounds show trypanocidal, fungistatic and smooth relaxant properties (Uchiyama et al., 2002; Krishna-Kumari et al., 2003), as well as effective agents in the P-388 lymphocytic leukemia test system (Jolad et al., 1974). The tea of roots from Z. montagnifolia is orally administered to treat diabetes and to induce abortion, but there is not any chemical approach that sustains the effectiveness of their empirical uses. The ecological roles of the main secondary metabolites from the Zaluzania genus are still unknown, but Juárez-Flores et al. (2010) suggest that the aerial tissues contain insecticidal compounds. Some VOC'S or semi-volatile organic compounds could probably be related to these activities because of its tested insecticidal properties (Pavela, 2005). Morphologically, Z. montagnifolia differs from the other members of the genus due to its upper leaves being conspicuously crenate-dentate. Therefore, the aim of this study was to elucidate the content of the main VOC's and lipophilic molecules dissolved in the hexanic extracts from Z. montagnifolia in order to find compounds putatively involved in its empirical uses, as well as its possible antibacterial, alellopathic and antioxidant activities.
MATERIALS AND METHDOS
Plant material and extraction
Zaluzania montagnifolia was collected in Miahuatlán de Porfirio Díaz, Oaxaca, 16° 20.63' N, 0.96° 34.85' O at 1580 masl, in October 2011. The identity of the plant was corroborated by comparing it with voucher 130910 from the FCME-herbarium at UNAM-México. Dried samples (40 g each) of flowers, leaves and axonomorfous roots were extracted with 100 mL of n-hexanes for 10 d at 4 °C. Hexanic extracts were filtered using Ahlstrom grade 642 filter paper, concentrated using a rotary evaporator Buchi R-200 (Germany) to 10 mL and finally reduced to 1 mL under a N2 stream. Heptadecanoic acid was used as the internal standard for extraction.
GC-MS analysis
Four µL of the hexanic extracts were injected into a Varian CP3800 gas chromatograph equipped with a Factor Four Column VF-5ms (30 m x 0.25 mm x 0.25 µm ID, covered with 5:95 phenyl-dimethylpolysiloxane as stationary phase) coupled to a Varian quadrupole mass spectrometer 320MS model. The carrier gas was He at 1 mL min-1 flow rate. Injector temperature was maintained at 200 °C. The oven temperature program was 150 °C for 3 min and finally ramped up to 250 °C for 20 min. The GC-MS run software was programmed to identify a m/z range of 30-600. Obtained peaks were carefully analyzed according to their mass spectra using the MS data Review and AMDIS 2.64, and subsequently compared to the NIST Search 2.0, Wiley Registry 8th edition, Pherobase, Flavornet, mass spectral data bases from FiehnLib as well as to the retention indexes reported in the literature. The retention index was calculated by running a standard mix of n-alkanes of C -C and C -C from Sigma-Aldrich Co., under identical conditions. After determining the presence of ent-kauranes diterpenes in Z. montagnifolia preparations, these molecules were semi-purified by thin layer chromatography (TLC) using a mix of hexane:ethyl acetate (90:10 v/v) according to reference standards isolated from Montanoa tomentosa which were characterized by GC-MS at 70 eV as described by Villa-Ruano et al. (2009). The mass spectra of sylanized ent-kauranes from Z. montagnifolia were obtained with N, O-Bis (trimethylsilyl) triflouroacetamide with 1% trimethylchlororsilane (BSTFA+1%TMCS, Sigma-Aldrich Co.) as a derivatizing agent and compared with those obtained from M. tomentosa under the same run conditions described above. Relative abundance was calculated from total ion chromatogram (TIC) data.
Antibacterial activity
This activity was determined by the broth microdilution method in combination with hexanic extracts and conventional antibiotics as reference standards. Besides, the minimal inhibitory concentration (MIC) for each extract was calculated (Villa-Ruano et al., 2012).
Allelopathic activity
This activity was determined by using the Lactuca sativa (cv. Great Lakes) system according to Villa-Ruano et al. (2012). An analysis of variance (ANOVA) was performed from dose-response data (curves of 0-300 µg mL-1) obtained from the assayed hexanic extracts and semi-purified ent-kauranes. Besides a Tukey test (p≤ 0.01) was performed using Statistica 6.0 (StatSoft, Inc. 2001).
Antioxidant activity
This activity was determined according to Shafaghat et al. (2011), using butylated hydroxytoluene (BHT) instead of vitamin C as a reference standard, and 2,2-diphenyl-1-picryl hydrazyl (DPPH) was obtained from Sigma-Aldrich Co. Dose-response curves (0-500 µg mL-1) were performed in quintuplicated to obtain the respective half maximal inhibitory concentration (IC50) BHT was used because of its hydrophobic properties.
RESULTS AND DISCUSSION
GC-MS profile
Analysis of hexanic extracts revealed 59 compounds (Table 1), including sesquiterpenes, aliphatic hydrocarbons, fatty acids and diterpenes. The most abundant sesquiterpenes in aerial parts were α-humulene (14.5 %), (-)-α-panasinsene (8.4%), β-bisabolene (7.94 %), humulene epoxide II (6.7 %) and germacrene D (5.9 %), while trans-α-bergamotene (6.51 %), α-cadinene (5.57 %), and italicene (5.32 %) were the most plentiful in roots. Sesquiterpene lactone tomentosin (0.87 %) was exclusively detected in leaves but not in the flowers, whereas η-dodecanal (3.9 %), squalene (3.43 %) and nonacosane (2.6 %) were the most abundant aliphatic hydrocarbons. A low percentage of cuticle-associated hydrocarbons such as pentadecene (1.2 %), octadecene (0.4 %), 1,12-tridecadiene (0.60 %), and (Z)-n-heptadec-8-ene (0.09 %) was also observed. Hexadecanoic acid (0.9 %) and the essential linoleic acid (2.7 %) were detected in aerial parts but not in roots. The gibberellin precursor ent-kaurene was exclusively detected in aerial parts of the plant. In all the studied organs, especially in roots, ent-kaurane derivatives such as grandiflorenic acid (GF) [kaura-9 (11), 16-dien-18-oic acid] and ent-kaurenoic acid (KA) [kaur-16-en-18-oic acid] were the most prevalent.
Grandiflorenic acid possesses uterotonic properties in animal species (Villa-Ruano et al., 2009) and KA is a powerful hypoglycemic agent in rat models (Bresciani et al., 2004). According to these studies, GF could be related to abortive properties due its stability and chemical integrity in aqueous infusions studies supported by high performance liquid chromatography (HPLC), GC-MS and nuclear magnetic resonance (NMR) (Enríquez et al., 1996). Besides, these authors point out that KA exhibits the identical stability of GF and its presence and high abundance suggest its involvement in the antidiabetic effects according to in vivo tests performed by Bresciani et al. (2004). Therefore, the root teas from Z. montagnifolia could probably be influenced by both diterpenes to perform their effects. Zaluzanins have an in vitro effect on smooth muscle contraction (Calesteren et al., 2008), but there is no evidence of its detection in aqueous preparations.
In addition to these findings, epimanool (Labda-8(20),14-dien-13-ol, (13S)-; 4.14 %) was detected in aerial parts of Z. montagnifolia. Epimanool and related labdanes are usually present in oleoresins of many plants and associated with insecticidal and larvicidal effects (Chinou, 2005; Demetzos and Dimas 2007). Plant essential oils with high amounts of a-humulene, coapaene and germacrene D as well as other VOC's also found in Z. montagnifolia extracts, show insecticidal properties (Murugesan et al. , 2012). Further experiments are required to show the involvement of hexanic extracts and its molecules in the insecticidal properties reported by Juárez-Flores et al. (2010). The presence of labdane and ent-kaurane diterpenoids was shown in other Asteraceae such as Stevia spp (Hernández et al., 1998). Results of the present study show that Z. montagnifolia roots are the best source to obtain both majoritarian ent-kaurane diterpenes.
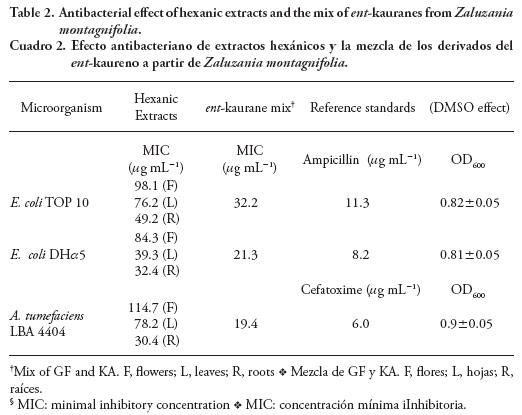
Antibacterial activity
The inhibitory effect of hexanic extracts on the growth of E. scherichia coli and Agrobacterium tumefaciens strains was shown. The low MIC values of leaf and root extracts (Table 2) revealed their antibacterial properties and suggested that those extracts contain natural compounds involved in the observed effect. MIC value refers to the lowest concentration of an antimicrobial agent that will inhibit the visible growth of a microorganism after incubation; this value is inversely proportional to the effectiveness of a particular substance. The detection and abundance of both KA and GF by GC-MS was the first approach that suggested their possible role in antibacterial activity. Antibacterial properties of KA and GF were shown in E. coli and other gram-negative bacteria, especially for the first diterpene (Bremner and Meyer, 2000). The inhibitory effect of the ent-kaurane mix on the assayed bacterial species suggests that the latter compounds are involved in this biological activity. However, there is no discarding the possible synergism with other cytotoxic VOC's such as a-humulene and germacrene D (Vijayakumar et al., 2012) in aerial parts where the endogenous levels of ent-kauranes are relatively lower than in the roots. Agrobacterium tumefaciens LBA 4404 is one of the most virulent strains of the respective genus, the MIC=30.4 mg mL-1 reveals that root extracts are the most effective against this bacteria and could be employed in the biological control of crown gall disease. Interestingly, these data predict that plants with high endogenous levels of both ent-kaurane diterpenes could be hardly transformed genetically by A. tumefaciens.
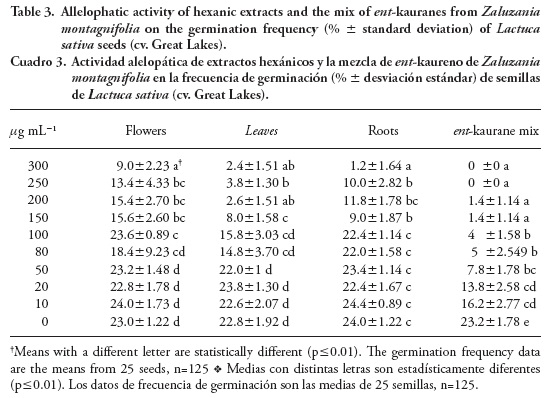
Allelopathic activity
Table 3 shows the data about the allelopathic activity of the hexanic extracts from the three organs studied. The results of an ANOVA coupled to a Tukey test reveal significant differences between the assayed concentrations in the germination frequency of L. sativa seeds (p≤0.01). According to these data, leaf and root extracts were the most effective causing 91-95 % inhibition at 100-150 µg mL-1 whereas the maximum concentration by flower extracts (300 µg mL-1) only resulted in 64 % inhibition. Allelopathic properties of leaf extracts were more significant than those of the roots as shown by the concentration of 200 mg mL-1, observing 89.6 % and 52.8 % inhibition by leaf and root extracts. Considering that ent-kaurane diterpenes were the most abundant compounds in both organs and showed allelopathic properties at the concentrations here reported, the specific effectiveness of leaf extracts suggests that other compounds increase that effect. Some abundant VOC's in this organ such as β-bisabolene and trans-α-bergamotene could probably be involved in the allelopathic properties according to Bradow (1985). Related physiological effects could be expected due the similarities in chemical structure between ent-kauranes and gibberellins. Vieira et al. (2005) report a stimulation of seed germination and shoot elongation of L. sativa (cv. Grand Rapids) by KA and GF as well as by some of its derivatives. According to these studies, concentrations of 0.1 mM, 10 µM and 0.1 µM of KA and GF promoted the shoot growth of L. sativa seeds. In the present study, the highest concentration of mixed ent-kauranes was 300 µg mL-1 (~1 mM) and the lowest 10 µg mL-1 (~30 µM, or ~0.03 mM) and these concentrations inhibited seed germination. Contrary to other studies where micromolar concentrations stimulated the germination of L. sativa (cv. Great Lakes) the lower one in this essay (~30 µM) had no effect on promoting the same process. This effect could be due to the variety of L. sativa seed assayed (cv. Great Lakes) or to the mix itself. Allelopathic properties of crude extracts and semi-purified ent-kaurane diterpenes from Z. montagnifolia have been shown for L. sativa seeds (cv. Great Lakes) but further experiments are required to determine their effects in the growth inhibition of seeds from weeds.
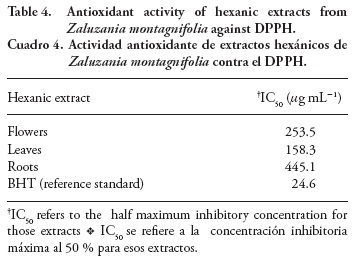
Antioxidant activity
The results of antioxidant activity of hexanic extracts by the DPPH radical scavenging method are shown in Table 4. According to the obtained IC, leaf extracts were the most effective followed by those of flowers and roots. Despite IC50 values being significantly lower than those of BHT, this molecule must be considered as a pure substance, whereas the hexanic extracts consist of diverse molecules that are surely diluted. Due to this fact and considering that other plant preparations with high levels of flavonoids showed similar antioxidant MIC values (García-Mateos et al., 2012) than those of this study, the antioxidant properties of hexanic extracts from Z. montagnifolia are acceptable. According to the chemical profile, the observed effect could be related to some volatiles such as b-bisabolene and germacrene D, since essential oils with high endogenous levels of both sesquiterpenes show the same behavior according to Ricci et al. (2005) and Shafaghat (2011). Flavonoids and related phenolic compounds derived from Shikimate pathway are usually associated with antioxidant activities, despite its quick degradation by moderate temperatures (>40 °C) and its susceptibility to light is known (Deng et al., 2011).
The presence of thermostable lipophilic compounds with antioxidant properties, represent an alternative that plants probably use to avoid oxidative stress and to regulate the physiology under high temperatures. Besides, antioxidant agents from lipophilic preparations could regulate glycemia by affecting the enzymes involved in glucose and lipid metabolism (Chung et al., 2010). The discovery of novel antioxidant extracts and the isolation of their bioactive molecules, open the possibility to perform further experiments in animal models based on its capability to avoid oxidative stress and its relationship with cancer, premature ageing and neurodegenerative diseases (Timsina et al., 2012).
CONCLUSIONS
The first extensive list of the lipophilic compounds from Zaluzania montagnifolia is shown in order to reveal the unknown chemistry of this plant. The accurate identification, high abundance of KA (hypoglycemic diterpene) and GF (uterotonic diterpene) could suggest in a coherent way, that the effects of the root teas as antidiabetic and abortive agents is probably influenced by those diterpenes. There was no chemical evidence that supports these properties in certain natural products from this plant until now. Based on literature, it is suggest that some of the main VOC's of Zaluzania montagnifolia could be involved in the antioxidant properties of the hexanic extracts. Results of this study suggest that ent-kaurane mixes from this plant possesses antibacterial and allelopathic properties. This is the first study showing the presence of ent-kaurane diterpenes in a member of the Zaluzania genus.
ACKNOWLEDGEMENTS
We would like to thank Craig A. Hilts for reviewing the manuscript, as well as Horacio Duque Bautista, Guilibaldo Gabriel Zurita Vásquez and Martha Guadalupe Betancourt Jiménez (from Cinvestav-Irapuato) for their technical support. We specially thank the financial hold from Programa Intergral de Fortalecimiento Institucional (PIFI).
LITERATURE CITED
Andrade-Cetto, A., and M. Heinrich. 2005. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Etnopharmacol. 99: 325-348. [ Links ]
Bradow, J. M. 1985. Germination regulation by Amaranthus palmeri and Ambrosia artemisiifolia. In: Thompson, C. (ed). The Chemistry of Allelopathy. Vol 268. ACS Symposium Series. USA. pp: 285-299. [ Links ]
Bremner, P. D., and J. J. Meyer. 2000. Prenylbutyrylphloroglucinol and kaurenoic acid: two antibacterial compounds from Helichrysum kraussii. South Afr. J. Bot. 66: 115-117. [ Links ]
Bresciani, L. F. V, R. A. Yunes, C. Bürguer, L. E. De Oliveira, K. L. Bof, and V. Cechinel-Filho. 2004. Seasonal variation of kaurenoic acid, a hypoglycemic diterpene present in Wedelia paludosa (Acmela brasiliensis) (Asteraceae). Z. Naturforsch. 59c: 229-232. [ Links ]
Calesteren, M. R. V., C. K. Jankowski, R. Reyes-Chilpa, M. Jiménez-Estrada, M. Campos G., A. Zarazua-Lozada, M. Oropeza, and D. Lesage. 2008. X-ray and high-resolution 1H and 13C NMR of smooth muscle relaxant sesquiterpene lactones. Can. J. Chem. 86: 1077-1084. [ Links ]
Chinou, I. 2005. Lanbdanes of natural origin-biological activities (1981-2004). Curr. Med. Chem. 12: 1295-1317. [ Links ]
Chung, M. J., S. Y. Cho, M. J. Bhuiyan, K. H. Kim, and S. J. Lee. 2010. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 104: 180-188. [ Links ]
Demetzos, C., and K. S. Dimas. 2007. Labdane-type diterpenes: Chemistry and biological activity. In: Atta-ur Rahman (ed). Studies in Natural Products Chemistry, Vol.25. Elsevier. London UK. pp: 235-292. [ Links ]
Deng, S., B. J. West, and C. J. Jensen. 2011. Thermal degradation of flavonol glycosides in noni leaves during roasting. Adv. J. Food Sci. Technol. 3: 155-159. [ Links ]
Enríquez, R. G., E. Miranda, B. Ortiz, I. León, G. Magos, A. Peña, W. F. Reynolds, and D. Gnecco. 1996. The unambiguous detection of kaurenic derivatives in aqueous infusions of Montanoa tomentosa by GC-MS and 2D-NMR spectroscopy: an answer to contradictory reports. Planta Med. 62: 569-571. [ Links ]
García-Mateos, R., L. Aguilar-Santelisis, M. Soto-Hernández, R. Nieto-Angel, and G. Kite. 2012. Total phenolic compounds, flavonoids and antioxidant activity in the flowers of Crataegus spp. from México. Agrociencia 46:651-662. [ Links ]
Hernandez, L. R., C.A. Catalán N., and P. Joseph-Nathan. 1998. The chemistry of the genus Stevia (Asteraceae). Rev. Acad. Colomb. Cien. 22: 229-279. [ Links ]
Jolad, S. D., R. M. Wiedhopf, and J. R. Cole. 1974. Tumor-inhibitory agent from Zaluzania robinsonii (Compositae). J. Pharm. Sci. 63: 1321-1322. [ Links ]
Juárez-Flores, B. I., Y. Jasso-Pineda, J. Aguirre-Rivera, y I. Jasso-Pineda. 2010. Efecto de polvos de asteráceas sobre el gorgojo del maíz (Sitophilus zeamais motsch). Polibotánica 30: 123-135. [ Links ]
Krishna-Kumari, G. N., S. Masilamani, M. Ganesh, S. Aravind, and S. Sridhar. 2003. Zaluzanin D: a fungistatic sesquiterpene from Vernonia arborea. Fitoterapia 74: 479-482. [ Links ]
Murugesan, S., C. Rajeshkannan, D. Suresh Babu, R. Sumathi, and P. Manivachakam. 2012. Identification of insecticidal properties in common weed Lantana camara Linn by Gas Chromatography and Mass Spectrum (GC-MS-MS). Adv. Appl. Sci. Res. 3: 2754-2759. [ Links ]
Pavela, R. 2005. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia 76: 691-696. [ Links ]
Ricci, D., D. Fraternale, L. Giampieri, A. Bucchini, F. Epifano, G. Burini, and M. Curini. 2005. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 98: 195-200. [ Links ]
Shafaghat, A. 2011. Chemical constituents, antimicrobial and antioxidant activity of the hexane extract from root and seed of Levisticum persicum Freyn and Bornm. J. Med. Plant Res. 5: 5127-5131. [ Links ]
Timsina, B., M. Shukla, and V. K. Nadumane. 2012. A review of few essential oils and their anticancer property. J. Nat. Pharm. 3: 1-8. [ Links ]
Uchiyama, N., K. Matsunga, F. Kiuchi, G. Honda, A. Tsubouchi, J. Nakajima-Shimada, and T. Aoki. 2002. Trypanocidal terpenoids from Laurus nobilis L. Chem. Pharm. Bull. 50: 1514-1516. [ Links ]
Vieira, H. S., J. A. Takahashi, L. P. Pimenta S., and M. A. D. Boaventura 2005. Effects of kaurane diterpene derivatives on germination and growth of Lactuca sativa seedlings. Z. Naturforsch. 60c: 72-78. [ Links ]
Vijayakumar, A., V. Duraipandiyan, B. Jeyaraj, P. Agastian, M. K. Raj, and S. Ignacimuthu. 2012. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. F. and Thoms extracts. Asian Pac. J. Trop. Dis 2: 190-199. [ Links ]
Villa-Ruano, N., M. G. Betancourt-Jiménez, and E. Lozoya-Gloria. 2009. Biosynthesis of uterotonic diterpenes from Montanoa tomentosa (zoapatle). J. Plant Physiol. 166: 1961-1967. [ Links ]
Villa-Ruano, N., Y. Pacheco-Hernández, E. Rubio-Rosas, N. Ruiz-González, R. Cruz-Duran, E. Lozoya-Gloria, G. Zurita-Vásquez, and J. Franco-Monsreal. 2012. Alkaloid profile, antibacterial and allelopathic activities of Lupinus jaimehintoniana, BL Turner. (Fabaceae). Arch. Biol. Sci. 64: 1065-1071. [ Links ]














