Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.46 no.5 Texcoco jul./ago. 2012
Fitociencia
Pollen grain viability in accessions of Crotalaria juncea L. (FABACEAE)
Viabilidad del grano de polen en accesiones de Crotalaria juncea L. (FABACEAE)
Ana P. D. Coelho1 , Katiule P. Morais1, Haywood Dail Laughinghouse IV2, Sandro J. Giacomini1 , Solange B. Tedesco1
1 Universidade Federal de Santa Maria. Avenida Roraima, 1000, Cidade universitária, CEP 97105-900, Santa Maria-RS. (apauladurand@yahoo.com.br).
2 MEES Program, College of Computer, Mathematical and Natural Sciencies, University of Maryland, College Park, MD, USA. * Author for correspondence (stedesco@smail.ufsm.br).
Received: June, 2011.
Approbed: July, 2012.
Abstract
Studies on pollen viability are useful for breeding Crotalaria juncea L. since they provide information that will increase the chance of successful intraspecific crossing. The aim of this study was to evaluate pollen viability in C. juncea accessions cultivated in Rio Grande do Sul, Brazil. Inflorescences of 10 accessions of C. juncea were collected, fixed in absolute ethanol: glacial acetic acid (3:1) for 72 h at room temperature and placed in 70 % ethanol. Analysis of pollen viability was performed with slides of anthers using the squashing technique and staining with acetic orcein 2 % and Alexanders stain. Means were compared using the Tukey test (p≤0.05). Pollen viability was high and significantly different among the accessions; the highest value was 99.67 % and the lowest 45.34 %. However, unviable pollen grains were also observed in all accessions.
Key words: Crotalaria juncea Alexander's stain, acetic orcein, pollen viability, plant cytogenetics.
Resumen
Los estudios de la viabilidad del polen son útiles para el mejoramiento genético de Crotalaria juncea L., porque proveen información que aumentará la oportunidad de cruces intraespecíficos exitosos. El objetivo de este estudio fue evaluar la viabilidad del polen de accesiones de C. juncea cultivadas en Rio Grande do Sul, Brasil. Las inflorescencias de 10 accesiones de C. juncea fueron recolectadas, fijadas en etanol absoluto: ácido acético glacial (3:1) por 72 h a temperatura ambiente, y colocadas en etanol al 70 %. El análisis de viabilidad del polen se realizó en rebanadas de anteros mediante la técnica de aplastado y teñidas con orceina acética al 2 % y tinción de Alexander. Las medias se compararon con la prueba de Tukey (p≤ 0.05). La viabilidad del polen fue alta con diferencias significativas entre las accesiones; el valor más alto fue 99.67 % y el menor 45.34 %. Sin embargo, en todas las accesiones se observaron granos de polen no viable.
Palabras clave: Crotalaria juncea, tintura de Alexander, orceina acética, viabilidad de polen, citogenética vegetal.
INTRODUCTION
In an attempt to minimize the accelerated degradation of soil fertility, green manure crops are used to maintain the physical, chemical and biological properties of the soil. Among these crops, Crotalaria juncea L. is frequently used due to its precocity and high green mass production; moreover, it produces highly smooth fiber which is economically important (Mozambani et al., 1993). This species is originally from India and is well adapted to tropical regions. The plant is shrub-like, growing erect; its fiber and high quality pulp are appropriate for the paper industry. It is also recommended as green manure for crops interspersed in cane sugar (Saccharum ojficinarum) or in rotation in grain cultures. It shows fast initial growth reaching 3.0 to 3.5 m in height under normal weather conditions; it is also considered a poor host of galls and cyst-forming nematodes (Braga et al., 2010).
The study of pollen viability is used in plant breeding for various species because of the ease, speed, low cost, and reliability of the technique. An individual's genotype comes from a male and a female gamete; therefore, the higher the pollen viability, the greater the probability of obtaining different combinations of alleles, and genetic variability (Souza et al., 2002). According to Ferreira (2009), pollen grains showed low viability in two out of three species of Crotalaria; this finding could be extended to all the species of the genus such as C. juncea. The determination of pollen viability can be carried out by direct methods, such as in vivo or in vitro induction of pollen germination, and by indirect methods based on cytological variables such as staining (Dafni, 1992; Shivanna and Rangaswamy, 1992; Kearns and Inouye, 1993). According to Techio et al. (2006), there is no description of a universal test of pollen viability using a specific stain.
It is important to verify pollen viability due to the direct link to fertilization efficiency, since they contribute to taxonomic, ecological and palynological studies. These studies also provide information for practical application in genetic conservation and plant breeding (Arroyo, 1981; Guinet, 1989; Auler, 2004). Therefore, the objective of this study was to evaluate pollen grain viability of Crotalaria juncea using two different stains to indicate which one is more efficient.
MATERIALS AND METHODS
The analyses were performed at the Laboratory of Cytogenetics and Plant Genotoxicity, Department of Biology, Center of Natural and Exact Sciences, of the Federal University of Santa Maria (UFSM), Rio Grande do Sul, Brazil.
Flower buds of 10 accessions of C. juncea were sown on 17 January, 2010, in the experimental area of the Department of Lands-UFSM, with UTM coordinates UTM 6709849/238028. This area is characterized by yellow dystrophic arenic Acrisol soil type, gentle undulating topography and Cfa climate according to Köppen climate classification (Köppen, 1936). The flower buds were cultivated in 5 X10 m plots, where they served as a summer cover crop (SCC). The 10 accessions were planted randomly within the plots.
For the pollen viability study, the flower buds were collected in April 2010, fixed in ethanol-acetic acid (3:1 v: v), kept at 23 °C for 72 h, transferred to 70 % ethanol (v/v) and kept under refrigeration. The slides for the analysis were prepared with the technique of squashing the anthers (Guerra and Souza, 2002), and two slides per C. juncea accession. In each type of staining 300 grains of pollen per slide were counted and the variable was the number of viable or nonviable grains. The viability of the pollen was determined comparing the stains: 2 % acetic orcein (w/v) and Alexander's stain: 5 mL ethanol, 0.5 mL green malachite, 25 mL distilled water, 12.5 mL glycerol, 2.5 mL acid fuchsine, 1.5 mL glacial acetic acid and 2.5 g chloral hydrate (adapted from Alexander, 1980). Pollen grains of all accessions of C. juncea were analyzed using light microscopy at 400x and photographed with a digital Cannon camera. A completely randomized design was used and means were analyzed with the Tukey test (p≤ 0.05) using the program Assistat version 7.6.
RESULTS AND DISCUSSION
The grains treated with acetic orcein and showing weak or no staining were considered nonviable (Figure 1A) and those that stained were viable (Figure 1B).

The grains stained with the Alexander's stain were nonviable when the pollen was colored blue-green (Figure 2A) or blue, and partially stained (Figures 2B and 2C); and viable when the pollen stained purple (Figure 2D).

The results of C. juncea pollen grain viability study using acetic orcein and Alexander's stain on the 10 accessions are shown in Table 1. In accessions 1, 7 and 10 there were significant differences between both stains, which could be due to the different mode of action of each stain on the cytological structure of the pollen grains. The values varied between 96 and 100 % viable pollen grains using acetic orcein. Auler (2004) report 97 to 98 % pollen viability with populations of Baccharis trimera Less (DC), which is as high as in our study.
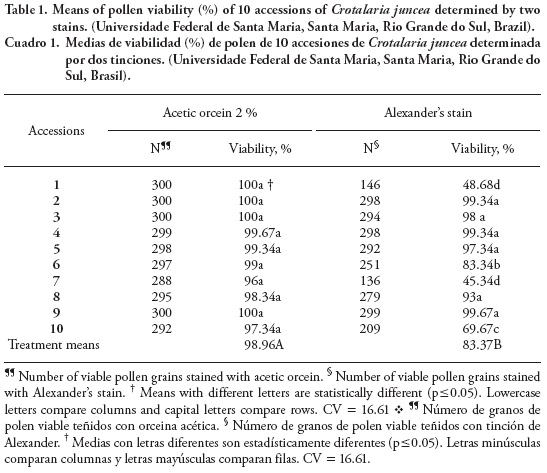
The analysis of pollen viability with Alexander's stain (Table 1; 45.34 to 99.67 %) shows that acetic orcein overestimated pollen viability. Techio et al. (2006) report that there is no description of a universal test for viability using a specific stain and that Alexander's stain (malachite green + acid fuchsin) was more accurate in differential staining where malachite green has an affinity with the cell wall cellulose and stains it green, while the protoplasm is stained pink by the fuchsin acid.
According to Alexander (1980), pollen grains without protoplasm are stained green indicating that some stains have limited application because they stain only functional or viable pollen, while the nonviable pollen grains do not stain. Therefore, such stains are not adequate for species with pollen grains having thick walls which make stain penetration difficult and prevent coloration.
Munhoz et al. (2008) studied the pollen viability of papaya (Carica papaya L.) cv. Sunrise Solo using in vitro germination and colorimetric tests, as well as validation of colorimetric tests compared with that of the germination test. The two culture media differed basically in concentration of essential nutrients and agar. The culture media without essential elements and with a higher concentration of agar had the best pollen germination index (65 %) and the five tested stains were 2, 3, 5-triphenyltetrazolium chloride (TTC), Alexander, acetic carmine, lugol, and Sudan IV. The colorimetric test with TTC supplied a viability estimate (67.5 %) equivalent to the in vitro germination test, and therefore, it is reliable for testing pollen viability. The other stains overestimated pollen viability (>90 %), but they were efficient for determining cellular constituents of the pollen grain.
Pollen viability was high for Crotalaria spectabilis (99.3 %), C. zanzibarica (98.6 %) and C. micans (98 %) according to the different stains, including Alexander's stain, but it was not possible to detect the nonviability with the differential Alexander's stain (Ferreira et al., 2009). According to Twell (1995), pollen can become nonviable during microgametogenesis, where errors in meiotic behavior result in gametes with unbalanced, or anucleate, chromosomes or in pollen grains with a retracted cytoplasm.
Based on the results of our study, acetic orcein showed a higher percentage of viability for all accessions, while Alexander's stain showed lower percentages for 3 accessions but higher for 7 of the 10 accessions. These results illustrate the occurrence of genetic variability in the accessions because most likely in those accessions showing significant difference between the acetic orcein stain and Alexander's stain, the grain wall is probably thicker. Souza et al. (2002), Meletti et al. (2003) and Corrêa et al. (2005) report that pollen viability can vary considerably among individuals of a single species and among samples of an individual.
CONCLUSIONS
Acetic orcein overestimated pollen viability for Crotalaria juncea, whereas Alexander's stain minimized the problems for obtaining data on pollen viability. Due to the gradual decrease of pollen grain viability and the reduction of fertilization efficiency, understanding the specific characteristics of pollen viability will contribute to plant breeding and seed production programs.
LITERATURE CITED
Alexander, M. P. A. 1980. Versatile stain for pollen fungi, yeast and bacterium. Stain Technol. 5(1): 13-18. [ Links ]
Auler N., M. F. 2004. Distribuiçâo da variabilidade genética em populaçôes naturais de Baccharis trimera (Less) DC. (Carqueja) no Sul do Brasil. PhD thesis. Universidade Federal de Santa Maria. Santa Maria. 108 p. [ Links ]
Arroyo, M. T. K. 1981. Breeding systems and pollination biology in Leguminosae. In: Polhill, M., and P. H. Raven (eds). Adv. in Legumes Systematic 2: 723-769. [ Links ]
Braga, N. R., M. A. C. de Miranda, E. B. Wutke, E. J. Ambrosano, and E. A. Bulisani. 2010. http://www.iac.sp.gov.br/Tecnologias/Crotalaria/Crotalaria.htm. (Accessed: April, 2010). [ Links ]
Corrêa, M. G. S., J. Viégas, J. B. Silva, P. F. V. Ávila, G. R. Busato, and J. S. Lemes. 2005. Meiose e viabilidade polínica na família Araceae. Acta Bot. Bras. 19(2): 295-303. [ Links ]
Dafni, A. 1992. Pollination Ecology: A Practical Approach. University Press. New York. 250 p. [ Links ]
Ferreira, K., G. A. Torres, I. V. Carvalho, and L. C. Davide. 2009. Comportamento meiótico anormal em três espécies de Crotalaria. Pesq. Agropec. Bras. 44(12): 1641-1646. [ Links ]
Guerra, M. S., and M. J. Souza. 2002. Como Observar Cromossomos: Um Guia de Técnicas em Citogenética Vegetal, Animal e Humana. FUNPEC. Ribeiráo Preto. 191 p. [ Links ]
Guinet, P. H. 1989. Advances in Legume Biology-Structure Evolution and Biology of Pollen in Leguminosae. Missouri Botanical Garden. 842 p. [ Links ]
Kearns, C. A., and D. Inouye. 1993. Techniques for Pollination Biologists. University Press of Colorado. Niwot. 583 p. [ Links ]
Köppen, W. 1936. Das Geographische Climate das System der Klimate. In: Köppen ,W. and R. Geiger (eds). Handbuch der Klimatologie, Band 5, Teil C. Gebrüder Bornträger. Berlin. 44 p. [ Links ]
Meletti, L. M. M., L. C. Bernacci, and M. D. Soares-Scott. 2003. Variabilidade genética em caracteres morfológicos, agronómicos e citogenéticos de populaçôes de maracujazeiro-doce (Passijlora alata Curtis). ver. Bras. Frut. Jaboticabal 25 (2): 275-278. [ Links ]
Mozambani, A. E., R. Sader, and L. R. Pinto. 1993. A maturaçâo fisiológica e retardamento de colheita de sementes de crotalária (Crotalaria juncea L.). ver. Bras. Sementes 15(1):55-62. [ Links ]
Munhoz, M., C. F. P. da Luz, P. E. Meissner Filho, O. M. Barth, and F. Reinert. 2008. Viabilidade polínica de Carica papaya L.: uma comparaçâo metodológica. ver. Bras. Bot. 31(2): 209-214. [ Links ]
Shivanna, K. R., and N. S. Rangaswamy. 1992. Pollen Biology. A Laboratory Manual. Springer-Verlag. Berlin/New York. 119 p. [ Links ]
Souza, M. M. de, T. N. S. Pereira, and E. R. Martins. 2002. Microsporogênes e microgametogênese associadas ao tamanho do botáo floral e da antera e viabilidade polínica em maracujazeiro-amarelo (Passijlora edulis Sims f. jlavicarpa Degener). Ciênc. Agrotec. 26(6): 1209-1217. [ Links ]
Techio, V. H., L. C. Davide, C. A. Pedrozo, and A. V. Pereira. 2006. Viabilidade do gráo de pólen de acessos de capim-elefante, milheto e híbridos interespecíficos (capim-elefanteX milheto). Acta Scientarium Biol. Sci. 28(1): 7-12. [ Links ]
Twell, D. 1995. Diphtheria toxin-mediated cell ablation indeveloping pollen: vegetative cell ablation blocks generativecell migration. Protoplasma 187: 144-154. [ Links ]














