Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.46 no.2 Texcoco Fev./Mar. 2012
Protección vegetal
Identification, pathogenicity, and histopathology of Lasiodiplodia theobromae on mamey sapote grafts in Guerrero, México
Identificación, patogenicidad e histopatología de Lasiodiplodia theobromae en injertos de zapote mamey en Guerrero, México
Juan M. Tovar-Pedraza1*, José A. Mora-Aguilera1, Cristian Nava-Díaz1, Daniel Téliz-Ortiz1, Guadalupe Valdovinos-Ponce1, Ángel Villegas-Monter2, Javier Hernández-Morales1
1 Fitopatología, * Author for correspondence. (jmtovar@colpos.mx)
2 Fruticultura, Campus Montecillo, Colegio de Postgraduados. Carretera México-Texcoco. Km. 36.5, Montecillo, Estado de México.
Received: February, 2011.
Approved: January, 2012.
Abstract
Dieback of mamey sapote (Pouteria sapota) grafts is the most important disease during vegetative propagation in commercial nurseries. In 2008, Lasiodiplodia theobromae identified by cultural, morphological and molecular characterization was associated with this disease in 97 % of necrotic rootstocks and scions samples from a nursery in Guerrero, México. The fungus was inoculated onto the binding site of El Mexicano grafted plants. Dieback symptoms and gradual drying with cracking of the bark from the apex to the base of the scion were observed 30 d after inoculation. Abundant mycelia and pycnidia were found on inoculated scions and graft union areas, in the periderm, and on leaf scars. Transverse sections of inoculated scions tissues showed pycnidia. Parenchyma cells of cortex and phloem collapsed and died. L. theobromae mycelia and red inclusions, probably of phenolic nature, were observed in both xylem vessels and pith parenchyma cells. To our knowledge, this is the first study that provides pathogenicity and histopathological data about a disease caused by L. theobromae during the grafting process.
Key words: dieback, nursery, vegetative propagation, Pouteria sapota, Lasiodiplodia theobromae.
Resumen
La muerte descendente de injertos de zapote mamey (Pouteria sapota) es la enfermedad más importante durante la propagación vegetativa en viveros comerciales. En 2008, Lasiodiplodia theobromae identificado por cultivo, morfológica y molecularmente, se asoció con esta enfermedad en 97 % de las muestras de portainjertos y varetas necrosadas en un vivero de Guerrero, México. El hongo se inoculó en el sitio de unión de plantas injertadas de la selección El Mexicano. Síntomas de muerte descendente con deshidratación gradual y agrietamiento de la corteza que inició en el ápice y desplazó hacia la base de la vareta, se observaron 30 d después de la inoculación. Abundante micelio y picnidios se encontraron en varetas, zonas de unión del injerto, peridermis, y en cicatrices foliares. Secciones transversales de tejidos de varetas inoculadas mostraron abundantes picnidos. Las células parenquimatosas de la corteza y floema colapsaron y necrosaron. Micelio de L. theobromae e inclusiones rojas, de probable naturaleza fenólica, se observaron en vasos del xilema y en células parenquimatosas de la médula. Para nuestro conocimiento, este es el primer estudio que provee datos de patogenicidad e histopatología acerca de una enfermedad causada por L. theobromae durante el proceso de injerto.
Palabras clave: muerte descendente, vivero, propagación vegetativa, Pouteria sapota, Lasiodiplodia theobromae.
INTRODUCTION
Mamey sapote [Pouteria sapota (Jacq.) H. E. Moore and Stearn] is a tropical fruit tree native to México and Central America (Popenoe, 1974). In 2009, the cultivated area with this plant in México was 1524 ha, distributed mainly in the states of Guerrero, Yucatán, Chiapas, Michoacán, Puebla, Oaxaca, Morelos and Veracruz (SIAP, 2010). Mamey sapote trees can be propagated either by sexual or asexual means (Villegas and Mora, 2008). However, members of Sapotaceae are considered difficult to graft, and mamey sapote is particularly complicated (Ogden et al., 1986). Besides the difficulty in propagating this fruit, the success of grafting is limited by the presence of diseases.
Information about mamey sapote diseases is rather scarce: 1) leaf spot by Phyllosticta sp. and Phyllachora sp., anthracnose by Colletotrichum gloeosporioides, and root rot caused by Pythium sp., in Florida, USA (Farr et al., 1989); 2) Phyllosticta sapotae induces leaf spot in Cuba and the Bahamas; 3) Uredo sapotae causes curly leaves in El Salvador (Azurdía, 2006); 4) Botryosphaeria sp. and Hypoxylon sp. were associated to dieback syndrome, bark splitting and stem canker, in Guatemala (Álvarez, 1997); 5) in México, there is vegetative and floral proliferation induced by Uredo baruensis (Pereyda et al., 2008), dieback by Lasiodiplodia theobromae (Vázquez et al., 2009), floral necrosis by Alternaria alternata, Pestalotiopsis paeoniicola and Penicillium olsonii (Mora et al., 2008) and fruit rot by L. theobromae and P. paeoniicola (Bautista et al., 2002; Gómez et al., 2009).
Death of scions is a severe disease during propagation by grafting of mamey sapote in Alpoyeca, state of Guerrero, México. Lasiodiplodia theobromae (Pat.) Griff. & Maubl. has been associated with symptoms of dieback and necrosis at the binding site of grafting on cashew (Anacardium occidentale L.) propagative material (Freire et al., 2002), as well as in guava (Psidium guajava L.) (Cardoso et al., 2002), citrus (Davis et al., 1987), and grapevine (Aroca et al., 2008). However, there are not experimental bases to determine its role as the causal agent of death of scions of mamey sapote. Based on this information, the aims of this study were to determine the etiology and anatomic abnormalities associated with dieback of grafted scions of mamey sapote.
MATERIALS AND METHODS
Study site and sampling
Alpoyeca, Guerrero, México, is located at 17° 40' N and 98° 31' W, at an altitude of 960 m, with an average temperature of 25.5 °C and an annual rainfall of 780 mm. In March 2008, a commercial mamey sapote nursery was sampled and 10 asymptomatic grafted plants and 20 grafted plants with scion dieback symptoms and dead tissue in the graft union were collected for isolation purposes. In order to know the fungal diversity in asymptomatic scions, field sampling was carried out in three commercial mamey sapote orchards, from which 30 scions with latent apical buds were collected.
Isolation
Tissue samples of 5 mm3 were cut from the graft union area including necrotic tissues. Samples were disinfested by immersion in a 3 % sodium hypochlorite solution for 4 min, washed in sterile distilled water for 3 min and placed in petri dishes with potato-dextrose-agar culture medium (PDA). The dishes were incubated 48 h under continuous black light at 30 ±2 °C. Most frequent fungal colonies were purified by monosporic cultures and transferred to new petri dishes with PDA.
Pathogenicity test
In October 2008, pathogenicity of L. theobromae was verified in grafted plants of El Mexicano selection in a nursery in Alpoyeca. The inoculum concentration used was 190 mycelial colony-forming units mL-1. In the inoculation test 15 scions were used per treatment: T1, scions were washed by hand using a natural fiber and water, immersed 15 min in mancozeb (1 mL L-1), and 100 µL of the inoculum were placed on the upper part of the grafting site; T2, scions were washed by hand using a natural fiber and water, immersed 15 min in mancozeb and 100 µL of sterile distilled water were placed on the upper part of the grafting site; T3, scions did not have any prophylactic measures and the grafted plants were not disinfested or inoculated. The 45 scions were grafted by using the side veneer technique described by Villegas and Mora (2008). All grafts were covered with clear plastic bags to avoid dehydration. Grafted plants were maintained under 75-80 % shade and continuous irrigation. Fungal infection signs and symptoms were registered 30 d after inoculation (dai). The fungus was re-isolated from the infected tissues; the colony and its reproductive structures were compared with the colony originally inoculated.
Incidence of diseased grafts with natural infection was calculated with the following equation: Ii = ∑ni/Ni; where Ii = incidence of diseased scions at the moment i; ni = number of diseased scions at the moment i; Ni = total population of grafted scions.
Morphological characterization
In vitro
Characteristics of fungal colonies grown on PDA under continuous black light at 30 ±2 °C were recorded. Type of mycelial growth, pigmentation and formation of reproductive structures were registered every 24 h.
In vivo
Signs and symptoms observed on natural and experimentally infected scions were evaluated using a stereoscopic microscope (Nikon Eclipse E400®, USA). Longitudinal sections of pycnidia were made by hand and morphological characteristics of 10 pycnidia, 10 germinative tubes, 10 conidiophores, and 100 conidia (50 immature and 50 mature) were observed with a compound microscope (Nikon SMZ800®, USA). Identification of the genus was made according to taxonomic keys by Sutton (1980) and Barnett and Hunter (2006), and of the species, those by Burgess et al. (2006).
Scanning electron microscopy (SEM)
Longitudinal and transversal pycnidia sections were fixed in 3 % glutaraldehyde and washed in 0.1M, Sorensen's phosphate buffer. The specimens were dehydrated through a graded ethyl alcohol series, dried in a critical-point dryer (Sandri-780A®, USA) with CO2, coated with gold (Ion Sputter JFC-1100, JEOL®, Japan) and examined by a scanning electron microscope (JEOL JSM- 6390®, Japan) at the Electron Microscope Unit of Colegio de Postgraduados.
Molecular characterization
DNA of the re-isolated fungus was extracted from monosporic cultures according to the protocol described by Ahrens and Seemüller (1992). Isolated DNA quality was evaluated by electrophoresis on a 1 % agarose gel (Agarose Ultra Pure, Invitrogen®) and quantified in a Perkin Elmer® spectrophotometer (Lambda BIO 10®, USA). Amplification of the ITS1 and ITS2 regions of the ribosomal genes (rRNA) was done by polymerase chain reaction (PCR) using the ITS4 and ITS5 primers (White et al, 1990). The amplification and visualization of final products were done according to the protocol by Ahrens and Seemüller (1992), with the modifications in the PCR reactions suggested by Vásquez et al. (2009). The amplified product was purified by using the Wizard kit (Promega®) protocol and sequenced with the Genetic Analyzer model 3100®, Applied Biosystem®. The sequence generate from this study was deposited in GenBank (NCBI, 2011) under accession number JQ245975.
Histopathology
Sample preparation for light microscopy
Transverse sections 10 mm long and 10-15 mm thick were cut off from mamey sapote grafts from T1, T2 and T3. The sections were fixed in FAA (absolute ethanol, glacial acetic acid, formaldehyde and distilled water), incubated 24 h at room temperature (20±2 °C), washed with tap water for 20 min and infiltrated in an automatic tissue processor (Tissue-Tek® II, Model 4640-B®, Japan) as described below. Dehydration was carried out gradually in ethyl alcohol solutions. The samples passed through a mixture of absolute ethanol-xylene (1:1) and three changes in pure xylene and after the last change in xylene, samples were placed in Paraplast (SIGMA®) for 48 h. Tissue sections were floated 1 min in a water bath at 65 °C with 3.0 g of grenetin, and mounted on glass slides. The technique of differential staining safranin-fast green was performed as described by Johansen (1940) and Curtis (1986). The sections were dewaxed in three changes of xylene (3 min each) and hydrated in a graded series of ethyl alcohol. Samples were stained with 1 % Safranin (Technical Chemistry®) in 50 % ethyl alcohol for 4 h. Then, sections were dehydrated in a graded series of ethyl alcohol at 50, 70 and 96 % (3 min each) and 3-4 drops of 1 % fast green (Technical Chemistry®) were added in 96 % ethyl alcohol for 30 s. Excess dye was decanted, the sections were washed in 96 % ethyl alcohol, dehydrated 3 min in absolute alcohol, and passed through three changes of xylene for 3 min each. Finally, sections were mounted in resin and examined by a compound microscope (Nikon SMZ800®, USA).
RESULTS AND DISCUSSION
Symptoms on plants in nursery
Necrosis appeared in recently grafted plants. Wilting and death of apical bud followed by dieback, gradual drying and cracking of the bark from the apex and moving down toward the base of the scion (Figure 1A), developed 22 d after grafting. The union of the graft presented longitudinal extended necrosis (Figure 1B). The vascular tissue of diseased scions showed hard texture (mummification of xylem and cortex) with a dark gray to black necrosis (Figure 1C). Thirty days after grafting, abundant light gray mycelial growth was observed covering most of the scion and the graft union (Figure 1E).
Isolates
Lasiodiplodia sp. (97 %) and Pestalotiopsis sp. (3 %) were found in 200 isolated colonies from 20 plants that showed typical necrosis symptoms at the graft union. Pestalotipsis sp. (82 %) and Lasiodiplodia sp. (18 %) were isolated from the 200 colonies, obtained from 10 asymtomatic scions collected in nursery. Also, Pestalotiopsis sp. (94 %) and Lasiodiplodia sp. (6 %) were isolated from 300 isolated colonies obtained from asymptomatic scions collected from the field. Morphological characteristics of Pestalotiopsis sp. coincided with those reported by Gómez et al. (2009) who state that P. paeoniicola is an endophytic organism in mamey sapote in Guerrero.
Pathogenicity test
Wilting symptoms in apical buds, dieback of scions, and abundant grayish mycelial growth at the graft union were observed at 30 d in 60 % of the inoculated plants (T1). Rapid dieback after inoculation of grafted plants was probably due to the fungus infection during the formation of new vascular tissue in the rootstock and the scion, and prior to lignification, which facilitated the development of this pathogen. The deep and extensive cuts made in the scions for the purpose of propagation probably facilitated pathogen penetration to vascular tissue (Ploetz et al., 1996; Pavlic et al., 2004). Besides, the union of the inoculated graft was covered with a plastic bag during 30 d, which created a microenvironment (>30 °C and 100 % relative humidity) that favored L. theobromae infection. Sub-immersed and erumpent pycnidia were observed on the graft union area, on foliar scars (Figure 1D), and on the periderm (Figures 1F-G) with necrosis extending along the vascular tissue of the scions and rootstock of T1 plants. These findings correlate with the symptoms reported in grafted plants infected with L. theobromae (Cardoso et al., 2002; Freire et al., 2002).
Grafted scions treated with fungicide and inoculated with distilled water (T2) did not develop symptoms, whereas 67 % of T3 scions became symptomatic. These data suggest that L. theobromae is a natural pathogen of this fruit species, which penetrates the vascular tissue through wounds or cuts made by the propagator or grafter. This coincides with Freire et al. (2002), Fourie and Halleen (2004) and Retief et al. (2006), who affirm that the infected scions and rootstocks are the source of inoculum of pathogens that cause diseases during the vegetative grapevine propagation.
In mamey sapote, the most likely source of primary inoculum are the scions collected from orchards with deficient agronomic management and high incidence of dieback of branches caused by L. theobromae. The pressure of inoculum by this pathogen was lower in the spring grafting season (February-March) with an incidence of 69 %, whereas in the autumn (September-November) the incidence was 87 % in naturally infected scions, coinciding with the period of June to November when the highest number of L. theobromae conidia were found in volumetric spore traps in mamey orchards (Vásquez et al., 2009). The present study did not verify the pathogenicity of P. paeoniicola because of its endophytic nature in mamey trees in the zone (Gómez et al., 2009).
Morphological characterization
Cultural characteristics
Fungal culture showed a rapid grown and abundant aerial mycelium (1-3 d). The aerial mycelium was initially gray, then becoming olive gray and denser in the center of the dish. Pycnidial conidiomata were observed after 14 d (Figure 1H). Pycnidia were produced in stroma, simple or compound, scattered, and often aggregated.
In vitro
Pycnidia were black, obpyriform and ostiolate. Immature conidia (18 d) were hyaline, ellipsoidal to subovoid, amerospore, 22.73-27.04 ×11.88-15.84 µm, thick-walled with a granular content. Mature conidia (23 d) were dark brown, ellipsoid to ovoid, didymospore, 19.66-26.35×11.3-14.17 µm, with longitudinal and irregular striations.
In vivo
Natural and experimentally infected scions showed globose pycnidia, immersed in the host tissue (Figure 1I); they were errumpent, simple and clustered, and dark brown to black, 235-380×190 to 335 µm, with a long neck ostiole. Conidiophores were hyaline, cylindrical, simple, sometimes septate, arising from the inner layer of cells lining the pycnidial cavity. Paraphyses were hyaline, cylindrical and aseptate. Immature conidia were hyaline, subovoid to ellipsoid, aseptate, granular (Figure 1J), and measured 23.4-27.32×12.26-15.61 µm. Mature conidia were dark brown, ovoid to ellipsoid, uniseptate, 21.19-26.76×11.15-13.38 µm, and showing longitudinal and irregular striations (Figure 1K).
Observations with SEM showed erumpent and globose pycnidia, unilocular (Figure 2A) and multilocular with elongated ostiolar neck (Figure 2B), with conidia extruding in a mass (Figure 2C). Mature conidia had irregular and longitudinal striations from apex to base (Figure 2D), and an internal septum (Figure 2E). Conidia germinate through simple germ tubes (Figure 2F).
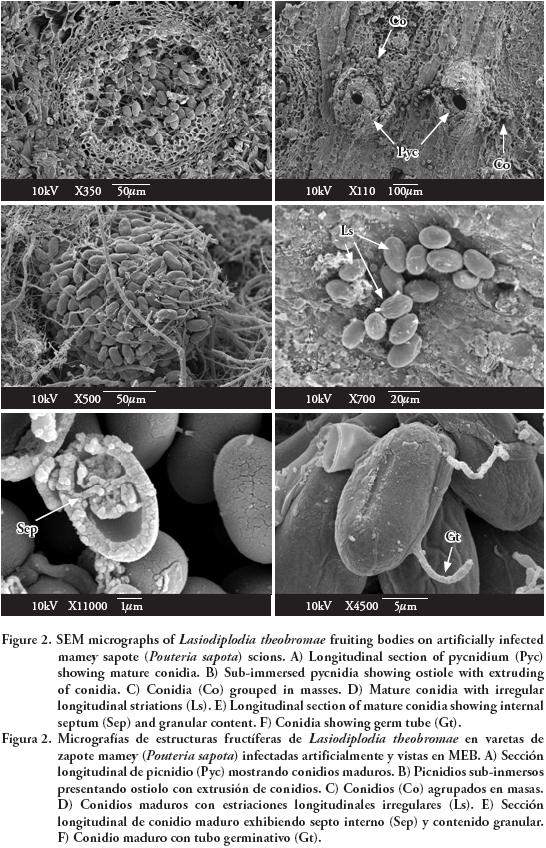
Cultural characteristics and structures of asexual reproduction coincided with those reported by Burgess et al. (2006) for L. theobromae. The isolate used in this study is maintained in the culture collection (Accession No. CB007) of the herbarium (CMPH) at Colegio de Postgraduados, Campus Montecillo, Fitopatología, Texcoco, Estado de México.
Molecular characterization
The molecular analysis confirmed that the fungus which causes dieback of mamey sapote scions in Guerrero was L. theobromae. The sequence (GenBank Accession No. JQ245975) obtained from this study showed 99 % of similarity to the sequence of L. theobromae with accession number GQ469934.
Histopathology
Structural abnormalities were not observed in asymptomatic tissues (Figures 3A-C).
Anatomical description of artificially infected scions and rootstocks
Pycnidia of L. theobromae at various stages of development (Figures 3D-E) were found 30 dai, which correlates with Biggs and Britton (1988), Michailides (1991) and Rayachhetry et al. (1996), who report the presence of reproductive structures of various Botryosphaeria spp. anamorphs between 12-56 dai. Our findings contrast with those observed by Vásquez et al. (2009) who found no pycnidia in mamey sapote samples with dieback symptoms 24 months after inoculation. In our study, the deep cuts made during the grafting process could facilitate an extensive route of penetration. It is possible that the host susceptibility has been greater during the formation of new vascular tissues of rootstock and scion, prior to lignification, which could help the development of L. theobromae.
Histological damages observed on the cortex of artificially infected scions (Figure 3D) could be attributed to the presence of pycnidia as well as mechanical injuries caused during the grafting process. Parenchyma cells of the cortex and phloem collapsed and became necrotic. Red inclusions, probably of phenolic nature, were observed in 10 % of the xylem vessels (Figure 3G) and in 13 % [percentage with respect to asymptomatic tissues (Figure 3H)] of pith parenchyma cells (Figure 3I). The presence of these compounds has been explained as a plant response to colonization of fungal, bacterial and oomycete pathogens.
Lasiodiplodia theobromae quickly penetrated and colonized all tissues of the inoculated scion. The mycelium, characterized by developing long and branched hyphae, grew intracellularly in xylem vessels and medulla parenchyma cells (Figure 3I). The presence of inclusions and hyphae of the pathogen in the xylem vessels may explain the restriction of the flow of water, mineral salts and other solutes to the rest of the plant, resulting in wilt and dieback of branches (Rayachhetry et al., 1996; Pandit and Samajpati, 2005). Hyphae of Botryosphaeria spp. has been observed in the cortex, xylem vessels, pith, phloem and xylem parenchyma of apple, peach and Melaleuca quinquenervia (Brown and Hendrix, 1981; Biggs and Briton, 1988; Rayachhetry et al., 1996), confirming that these species are specialized pathogens that colonize all the tissues in branches and stems of woody plants. Vásquez et al. (2009) did not observe hyphae in mamey sapote branch tissues infected with L. theobromae, possibly because their observation was made 24 months after inoculation on collapsed necrotic tissues.
Tyloses were not observed in the xylem vessels, as reported in other interactions: Botryosphaeria spp.-blueberry and apple (Milholland, 1972; Brown and Hendrix, 1981) and L. theobromae-grapevine (Atia et al., 2003), as a major cause of obstruction of water flow and dieback.
Pith parenchyma of asymptomatic rootstocks contained starch granules (Figure 3J), which were not observed in artificially infected rootstocks. The fungus probably uses starch as a carbon source for energy and growth, since some fungi can use the starch by amylase production (Deacon, 2006). Red inclusions of probably phenolic nature were also observed in 7 % of pith parenchyma cells in rootstocks (Figure 3K).
Anatomical description of naturally infected scions and rootstocks
Lasiodiplodia theobromae hyphae showed a similar growth pattern to that observed in artificially infected tissue. However, pycnidia were not present in the periderm. The absence of these structures could be explained by the process of penetration and infection, which occurred slowly as the pressure of inoculum was lower compared with the scions inoculated with the suspension of hyphae in the wounds.
Accumulation of red inclusions 5 % (percentage with respect to asymptomatic) was lower in pith parenchyma compared with artificially infected grafts, which could be due to a lower extent of colonization by fungus in the tissues. The subsequent formation of pycnidia on twigs that survived natural infection in the field is perhaps the main source of inoculum for the spread of the disease in mamey sapote orchards in Guerrero.
CONCLUSIONS
Pathogenicity and histopathological data showed that Lasiodiplodia theobromae is the causal agent of dieback of mamey sapote grafts. This is the first study that provides a better understanding of a disease caused by L. theobromae during the grafting process.
ACKNOWLEDGEMENTS
This research was financially supported by Fundación Produce de Guerrero, México (Project PM 1731). The authors are grateful to Greta Hanako Rosas Saito (Unidad de Microscopía Electrónica, Colegio de Postgraduados) for her technical assistance with the SEM.
LITERATURE CITED
Ahrens, U., and E. Seemüller. 1992. Detection of DNA of plant pathogenic mycoplasmalike organisms by polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82: 828-832. [ Links ]
Álvarez, V. G. 1997. La muerte descendente y el cáncer del tallo en el zapote. Tikalia 15: 37-46. [ Links ]
Aroca, A., R. Raposo, D. Gramaje, J. Armengol, S. Martos, and J. Luque. 2008. First report of Lasiodiplodia theobromae on rootstocks mother grapevines in Spain. Plant Dis. 92: 832. [ Links ]
Atia, M. M. M., A. Z. Aly, M. R. A. Tohamy, H. El-Shimy, and M. A. Kamhawy. 2003. Histopathological studies on grapevine die-back. J. Plant Dis. Protect. 110: 131-142. [ Links ]
Azurdía, C. 2006. Tres Especies de Zapote en América Tropical. Southampon Centre for Underutilised Crops, Southampon University, Southampon, UK. 216 p. [ Links ]
Barnett, L. H., and B. B. Hunter. 2006. Illustrated Genera of Imperfect Fungi. Fourth Edition. The American Phytopathological Society. St. Paul, Minnesota, USA. 218 p. [ Links ]
Bautista-Baños, S., J. C. Díaz-Perez, and L. L. Barrera-Nencha. 2002. Postharvest fungal rots of sapote mamey Pouteria sapota H. E. Moore & Stearn. Postharvest Biol. Thechnol. 24: 197-200. [ Links ]
Biggs, A. R., and K. O. Britton. 1988. Presymptom histopathology of peach trees inoculated with Botrysphaeria obtusa and B. dothidea. Phytopathology 78: 1109-1118. [ Links ]
Brown, E. A. II., and F. F. Hendrix. 1981. Pathogenicity and histopathology of Botryosphaeria dothidea on apple stems. Phytopathology 71: 375-379. [ Links ]
Burgess, T. I., P. A. Barber, S. Mohali, G. Pegg, W. de Beer, and M. J. Wingfield. 2006. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98: 423-435. [ Links ]
Cardoso, J. E., C. M. Maia, and M. N. G. Pessoa. 2002. Occurrence of Pestalotiopsis psidii and Lasiodiplodia theobromae causing stem rot of guava plants in the State of Ceará, Brazil. Fitopatol. Bras. 27: 320. [ Links ]
Curtis, P. J. 1986. Microtécnica Vegetal. Ed. Trillas, México. 106 p. [ Links ]
Davis, R. M., C. J. Farrald, and D. Davila. 1987. Botryodiplodia trunk lesions in Texas citrus. Plant Dis. 71: 848-849. [ Links ]
Deacon, J. 2006. Fungal Biology. Fourth Edition. Blackwell Publishing. Cornwall, England. 372 p. [ Links ]
Farr, D. F. , G. F. Bill, G. P. Chamuris, and A.Y. Rossman. 1989. Fungi on Plants and Plant Products in the United States. APS Press. St. Paul, Minnesota, USA. 1252 p. [ Links ]
Fourie, P. H., and F. Halleen. 2004. Occurrence of grapevine trunk disease pathogens in rootstock mother plants in South Africa. Austral. Plant Pathol. 33: 313-315. [ Links ]
Freire, F. C. O., J. E. Cardoso, A. A. dos Santos, and F. M. P. Viana. 2002. Diseases of cashew nut plants (Anacardium occidentale L.) in Brazil. Crop Protection 21: 489-494. [ Links ]
Gómez, J. R., D. Nieto A., D. Téliz O., A. Mora A., M. T. Martínez D., y M. Vargas H. 2009. Evaluación de la calidad e incidencia de hongos en frutos refrigerados de zapote mamey (Pouteria sapota (Jacq.) H. E. Moore and Stearn. Agrociencia 43: 37-48. [ Links ]
Johansen, D. A. 1940. Plant Microtechnique. McGraw Hill, New York, USA. 503 p. [ Links ]
Michailides, T. J. 1991. Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology 81: 566-573. [ Links ]
Milholland, R. D. 1972. Histopathology and pathogenicity of Botryosphaeria dothidea on blueberry stems. Phytopathology 62: 654-660. [ Links ]
Mora, A. J. A., A. Vásquez L., J. Pereyda H., y D. Téliz O. 2008. Algunas enfermedades del zapote mamey (Pouteria sapota (Jacq.) en México. El Zapote Mamey en México: Avances de Investigación. Morelos, México. pp: 103-114. [ Links ]
NCBI (National Center for Biotechnology Information). 2011. GenBank. http//:www.ncbi.nlm.nih.gov/. (accessed: December 2011). [ Links ]
Ogden, M. A. H., C. W. Campbell, and S. Lara P. 1986. Grafting techniques for mamey sapote (Calocarpum sapota [Jacq.] Merr.) under Florida conditions. Proc. Interamerican Soc. Trop. Hort. 30: 215-221. [ Links ]
Pandit, P. K., and N. Samajpati. 2005. Wilt disease of guava caused by Botryodiplodia theobromae Pat. J. Mycopathol. Res. 43: 41-43. [ Links ]
Pavlic, D., B. Slippers, T. A. Coutinho, M. Gryenhout, and M. J. Wingfield. 2004. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycol. 50: 313-322. [ Links ]
Pereyda, H. J., J. A. Mora A., J. G. Florencio A., C. Nava D., D. Téliz O., S. Sandoval I., y A. Villegas M. 2008. Proliferación vegetativa y floral del zapote mamey (Pouteria sapota (Jacq.)) en México. El Zapote Mamey en México: Avances de Investigación. Morelos, México. pp: 115-130. [ Links ]
Ploetz, R. C., D. Benscher, A. Vazquez, A. Colls, J. Nagel, and B. Schaffer. 1996. A reexamination of mango decline in Florida. Plant Dis. 80: 664-668. [ Links ]
Popenoe, W. 1974. Manual of Tropical and Sub-tropical Fruits, Excluding the Banana, Coconut, Pineapple, Citrus Fruit, Olive and Fig. Hafner Press, New York, USA. 542 p. [ Links ]
Rayachhetry, M. B., G. M. Blankslee, and T. Miller. 1996. Histopathology of Botryosphaeria ribis in Melaleuca quinquenervia: Pathogen invasion and host response. International J. Plant Sci. 157: 219-227. [ Links ]
Retief, E., A. McLeod, and P. H. Fourie. 2006. Potential inoculum sources of Phaeomoniella chlamydospora in South African grapevine nurseries. Eur. J. Plant Pathol. 115: 331-339. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2010. www.siap.sagarpa.gob.mx. (Accessed: July 2011). [ Links ]
Sutton, B. C. 1980. The Coleomycetes: Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute, Kew, Surrey, England. 696 p. [ Links ]
Vásquez, L. A., J. A. Mora A., E. Cárdenas S., y D. Téliz O. 2009. Etiología e histopatología de la muerte descendente de árboles de mamey (Pouteria sapota (Jacq.) H.E. Moore and Stearn) en Guerrero, México. Agrociencia 43: 717-728. [ Links ]
Villegas, M. A., y J. A. Mora A. 2008. Propagación vegetativa del zapote mamey. El Zapote Mamey en México: Avances de Investigación. Morelos, México. pp: 1-16. [ Links ]
White, T. J., B. S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., D. A. Gelfand, J. J. Sninsky, and T. J. White (eds). PCR Protocols: A Guide to Methods and Applications, Academic Press, CA, USA. pp: 315-322. [ Links ]














