Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.46 no.2 Texcoco feb./mar. 2012
Ciencia animal
Physiological and genotoxic effects of molybdenum-induced copper deficiency in cattle
Efecto fisiológico y genotóxico de la deficiencia de cobre inducida por molibdeno en bovinos
Sebastián Picco1,2,3, M. Virginia Ponzzinibio1,2, Guillermo Mattioli3, Diana Rosa3, Leonardo Minatel4, Luis Fazzio3, Analía Seoane1,2*
1 IGEVET (Instituto de Genética Veterinaria), UNLP-CONICET. Calle 60 y 118, B-1900-AVW, La Plata, Argentina.
2 CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Avenida Rivadavia 1917, C-1033-AAJ, Buenos Aires, Argentina. * Author for correspondence: (aseoane@fcv.unlp.edu.ar).
3 Cátedra de Fisiología, Facultad Cs. Veterinarias, UNLP, Calle 60 y 118, B-1900-AVW, La Plata, Argentina.
4 Cátedra de Patología, Facultad Cs. Veterinarias, Universidad de Buenos Aires, Avenida Chorroarín 280, C-1427-CWO, Buenos Aires, Argentina.
Received: april, 2011.
Approved: january, 2012.
Abstract
Molybdenosis is a disease caused by the depressing effect of molybdenumn (Mo) on the physiological availability of Copper (Cu). The present study was carried out in order to analyze the ability of Mo to cause damage on the DNA integrity and changes in membrane fatty acids by oxidative damage. Holstein male calves were fed a Mo-supplemented diet for 9 months. Variables evaluated were plasma Cu concentration, erythrocyte Cu content and SOD activity, comet assay and analysis of the fatty acid composition of erythrocyte membranes. The statistical design was a completely randomized with one single factor and two replications. Copper plasma concentration, erythrocyte copper concentration and Cu/Zn SOD activity were analyzed using the t test. Chi-square test was used to compare the number of cells with DNA damage, and one-way analysis of variance and Tukey test (p≤0.05) for fatty acid composition and lipid peroxidation. Results showed that Mo in the diet induced a depletion of hepatic Cu storage, a decrease of Cu plasma and erythrocyte levels, a fall in Cu/Zn-SOD activity, changes in membrane fatty acids composition and DNA damage. These results are in agreement with the three phases model of Cu deficiency and validate the occurrence of molybdenosis or secondary hypocuprosis. Further studies will be necessary to explore the mechanisms involved in the DNA damage and to distinguish primary molybdenum toxicosis from the molybdenum-induced copper deficiency.
Key words: molybdenum, copper deficiency, oxidative damage, DNA damage, Bos taurus, SOD activity.
Resumen
La molibdenosis es una enfermedad causada por el efecto depresivo del molibdeno (Mo) en la disponibilidad fisiológica del cobre (Cu). El presente estudio se realizó para analizar la capacidad del Mo para causar daño en la integridad del ADN y cambios en los ácidos grasos de la membrana por el daño oxidativo. Becerros machos Holstein recibieron una dieta con un suplemento de Mo durante 9 meses. Las variables evaluadas fueron la concentración de Cu en el plasma, el contenido de Cu en eritrocitos y la actividad SOD, un ensayo cometa y el análisis de la composición de ácidos grasos de las membranas de eritrocitos. El diseño estadístico fue completamente al azar con un solo factor y dos réplicas. La concentración de cobre en el plasma, la concentración de cobre en eritrocitos y la actividad Cu/Zn-SOD se analizaron con la prueba de t. La prueba de Chi cuadrado se usó para comparar el número de células con daño del ADN, y el análisis de varianza en un sentido y la prueba de Tukey (p≤0.05) para la composición de ácidos grasos y la peroxidación de lípidos. Los resultados mostraron que el Mo en la dieta indujo una reducción en el almacenaje hepático de Cu, una disminución de los niveles de Cu en el plasma y eritrocitos, una caída en la actividad Cu/Zn-SOD, cambios en la composición de ácidos grasos de la membrana y daño del ADN. Estos resultados coinciden con el modelo de tres fases de deficiencia del Cu y validan la ocurrencia de molibdenosis o hipocuprosis secundaria. Se requieren más estudios para explorar los mecanismos involucrados en el daño del ADN y distinguir la toxicosis primaria por molibdeno de la deficiencia de cobre inducida por Mo.
Palabras clave: molibdeno, deficiencia de cobre, daño oxidativo, daño del ADN, Bos taurus, actividad SOD.
INTRODUCTION
Molybdenum (Mo), an essential trace mineral in mammals (Turnlud et al., 1995; NRC, 2005), is an integral component of several oxidase enzymes (e.g. xanthine oxidase, sulfte oxidase and aldehyde oxidase) which catalyze basic metabolic reactions in the carbon, sulfur and nitrogen cycles (Turnlund et al., 1995; Tompson and Turnlund 1996; Barceloux, 1999). Molybdenum is slightly toxic to humans, whereas ruminant and non-ruminants animals are quite sensitive to ingesting of excessive amounts (Underwood and Suttle, 1999).
Molybdenum poisoning can be either natural, due to its accumulation in plants growing in areas of molybdeniferous rocks, or anthropogenic, as a result of industrial pollution when an excess of Mo containing fertilizer or lime or both are applied to the soil and appear to increase Mo uptake by plants (NRC, 2005; Raisbeck et al., 2006; Kirchmann et al., 2009). Herbage values of Mo can be greater than 230 mg kg-1 of dry matter (DM) in areas with industrial contamination, while the normal values are less than 1 mg kg-1 DM (NRC, 2005).
Molybdenosis (also called conditioned copper deficiency or secondary hypocuprosis) is a disease mostly characterized by the depressing effect of Mo on the physiological availability of copper (Cu), and it is similar to Cu deficiency in several respects (Erdman et al., 1978). Most of the clinical signs are similar to those of hypocuprosis and include pale coat, anemia, spontaneous fractures, poor capillary integrity, myocardial degeneration, and spinal cord hypomyelinization, impaired reproductive performance, lessening resistance to infectious disease, diarrhea and generalized ill health (Tessman et al., 2001; Underwood and Suttle, 1999).
According to NRC (2001), the antagonistic action of Mo on Cu metabolism increases when sulfur levels are high; besides, in the rumen molybdate and sulfde interact to form thiomolybdates, whereas Cu would react with these compounds to produce insoluble complexes. Furthermore, the absorbed thiomolybdates affect the systemic metabolism of Cu resulting in Cu being tightly bound to plasma albumin rendering it unavailable for biochemical functions (Gooneratne et al., 1989). Copper availability is required for structural and catalytic properties of many enzymes (e.g. Cu/Zn-SOD, ceruloplasmin) linked to the antioxidant defense system (Uauy et al., 1998; Gaetke and Chow, 2003; Lopez-Alonso et al., 2005). Consequently, the association between copper deficiency and oxidative damage has been proposed (Webster et al., 1996; Pan and Loo, 2000; Picco et al., 2004). Therefore, the objective of this study was to analyze the ability of Mo to cause damage on the DNA integrity and changes in membrane fatty acids by way of oxidative damage.
MATERIALS AND METHODS
Experimental design
The experimental design was completely randomized and 20 Holstein male calves (n=20; 70-day-old; 68 kg mean body weight), placed in partially roofed 6 m2 boxes (two calves per box) with a concrete floor, received during 9 months a diet with 60 % corn grain, 15 % commercial feed, 22 % wheat straw and 3 % mineral-vitamin mixture. Concentrations of Cu, Mo and S in the diet, verified by fame atomic absorption spectrophotometer, were as follows: Cu 3.8, Mo 0.4, S 1.8 g kg-1 DM. After a three-week adaptation period, treatments were randomly assigned to calves as follows: control group (A; n=10) and Mo-supplemented group (B; n=10). The mineral-vitamin mixture (3 % of the total diet) increased Cu concentration to 10 mg kg-1 DM in the 20 calves, whereas calves in group B also received 11 mg Mo kg-1 DM and 3 g S kg-1 DM.
Blood samples and liver biopsies
Samples taken from the jugular vein were handled as follows: 1) 10 mL samples from each calf in a test tube containing EDTA (Gibco BRL, NY) were placed in an icebox, brought to the laboratory, centrifuged 10 min at 3000 rpm for separating the plasma and stored at 4 °C for further analysis. 2) Samples were placed in test tubes (Eurotubo®, Barcelona, Spain) containing lithium heparin as an anticoagulant, for determining fatty acid composition of erythrocytes. 3) Samples were collected in heparinized Vacutainer® tubes (Franklin Lakes, NJ, USA) for comet assay. Liver biopsies were obtained in four calves from each group following the protocol described by Swanson and coworkers (2000).
Analysis of plasma copper concentration
Blood samples were centrifuged, the plasma fraction treated with 10 % trichloroacetic acid (w/v) to separate the supernatant and then plasma Cu concentration was determined by a fame atomic absorption spectrophotometer (double beam atomic absorption spectrophotometer; GBC 902) using internal quality controls (Piper and Higgins, 1967).
Erythrocyte copper content and superoxide dismutase activity
The Cu content was determined in erythrocyte samples diluted in 1 mL of a solution of 4 mM manganese sulfate (Sigma-Aldrich®, Saint-Louis, USA) prepared in acetic acid (1 mM) and 1 mL of trichloroacetic acid 10 %. The samples were centrifuged 30 min at 3600 rpm and the supernatant was used for Cu determinations by atomic absorption spectrophotometry. In order to determine the erythrocyte Cu/Zn superoxide dismutase (SOD) activity, the method described by Jones and Suttle (1981) was employed. This method evaluates the competitive inhibition of SOD in the reaction of the superoxide anion produced by the aerobic oxidation of xantine. The violet iodonitrotetrazolium changes its color when it is reduced by the superoxide anion (Beauchamp and Fridovich, 1971). The samples were analyzed with a spectrophotometer (Metrolab UV240) at 500 nm during 5 min (Xin et al., 1991). The standard used for the assay was obtained from bovine red cells (Sigma, Saint-Louis, USA) and the results were expressed as IU mg-1 hemoglobin. Hemoglobin in red cells and in whole blood samples was determined by the cianohemoglobin method using a commercial kit (Wiener Lab., Argentina) in a spectrophotometer (Metrolab UV240).
Comet assay
The method used for this assay was that described by Singh et al. (1988), with minor modifications. Samples were stored in the dark at 4 °C for up to 30 min and 15 mL aliquots of whole blood were mixed with 75 µL of 0.5 % low melting point agarose (Gibco BRL, NY), seeded on a slide coated with 0.5 % normal melting point agarose (Promega) and cooled until solidification. Two slides per calf were prepared and the cells lysed by incubation in a detergent solution [100 mM EDTA (Gibco BRL, NY), 2.5 M NaCl (Gibco BRL, NY), 10 mM Tris (USBiological, MA), 1 % Triton X-100 (Sigma, St Louis, MO) and 10 % dimethyl sulfoxide] for at least 1 h and stored until electrophoresis. Before electrophoresis, the slides were equilibrated in alkaline electrophoresis solution [1 mM EDTA (Gibco BRL, NY), 300 mM NaOH (Carlo Erba, Milano, Italy), pH > 13] for 20 min. The electrophoresis was carried out for 30 min at 25 V and 300 mA (1.25 V/cm). Then, the slides were neutralized by washing them three times with Tris buffer (pH 7.5) for 5 min each time and once with distilled water. Slides were stained with 1/1000 SYBR Green I (Molecular Probes, Eugene, OR) solution (Ward and Marples 2000) and the analysis was performed with an Olympus BX 40 microscope equipped with a 100 W high pressure mercury lamp (USHIO USH 102 D). Images were captured with a Sony CCD camera and saved using Image Pro Plus® software. The data for comet assay was recorded blind on coded slides. In total, 100 cells per calf were scored. DNA damage values were established according to Collins (2004).
Analysis of the fatty acid composition of erythrocyte membranes
The erythrocytes were isolated from the whole blood by centrifugation at 1000 g for 10 min at 4 °C. The buffy-coat and plasma were discarded, and the red cells washed three times in isotonic phosphate buffer (PBS 5 mM pH 7.4, 150 mM NaCl). The pellet was resuspended in isotonic phosphate buffer and the preparation of the erythrocyte membranes (ghosts) was carried out according to the method of Dodge et al. (1963) with minor modifications. Briefly, packed washed erythrocytes were lysed by mixing 10 vol of 5 mM phosphate buffer pH 7.4 at 4 °C. After incubation on ice for 30 min, the erythrocyte membranes were centrifuged and the supernatant containing hemoglobin was removed. The membranes were washed three times in fresh buffer (10 vol) followed by centrifugation at 20 000 g and 4 °C for 10 min. Finally, the membranes were resuspended in isotonic 5 mM buffer (same volume used for lyses) after which they were centrifuged under the same conditions and resuspended in isotonic 5 mM phosphate buffer pH 7.4. Erythrocyte membranes were quantifed taken into account the protein concentration determined by Lowry's method (Lowry et al., 1951). Lipids from erythrocyte membrane samples were extracted with chloroform/methanol (2:1, v/v) (Folch et al., 1957). Fatty acids were transmethylated with 20 % F3B in methanol at 65 °C for 3 h. Fatty acid methyl esters were analyzed with GC-14A gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a packed column (1.8m × 4mm i.d.; J and V Scientific, Folsom, CA, USA) GP 10% DEGSPS on 80/100 Supelcoport, using nitrogen as carrier gas. The injector and detector temperatures were kept at 250 °C, and the column temperature was held 60 min at 200 °C. Fatty acid methyl esters peaks were identifed by comparison of their retention times with the ones of standard compounds and the composition expressed as percentage by area of total fatty acid.
Statistical analysis
The experimental design was completely randomized with one single factor and two replications. Means of copper plasma and erythrocyte copper concentration, fatty acid composition, lipid peroxidation and Cu/Zn SOD activity were analyzed using the t test (SSPS®). For this analysis, treatment was considered as a fixed variable and each calf as the random variable. Chi-square test was used to compare the number of cells with DNA damage from groups.
RESULTS AND DISCUSSION
Analysis of plasma copper concentration
Calves were classified as normocupremic: 60-120 mg dL-1; moderate hypocupremic: 30-59 mg dL-1; severe hypocupremic: <30 mg dL-1, following the Suttle's classification (Suttle, 1983). Data are shown in Table 1.
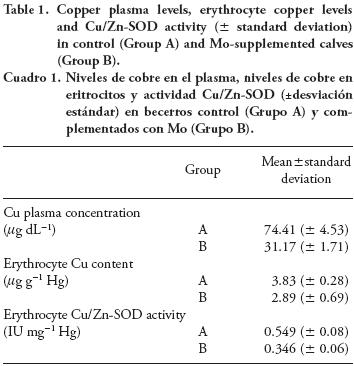
After three months, control calves (Group A) presented a Cu plasma level ≥60 µg dL-1 (normocupremic) while those fed Mo supplement (Group B) were hypocupremic (p≤0.01). In addition, results from four calves per group showed a marked decrease in hepatic Cu concentrations in Group B (10.91 ±2.43 ppm) compared to Group A (153.5±61.42 ppm).
Erythrocyte Cu content and Cu/Zn-SOD activity
Copper content and Cu/Zn-SOD activity were higher (p=0.047 and p≤0.01) in erythrocytes obtained from control calves (Group A) than in erythrocytes from those fed Mo supplement (Group B).
Fatty acid composition of erythrocyte membranes
The concentration of monounsaturated C18 fatty acids decreased while that of C18 saturated fatty acids was increased. Besides, the rate of saturated fatty acids was higher in Group B calves than in those from Group A which showed a lower total unsaturated fatty acid incidence (Table 2).
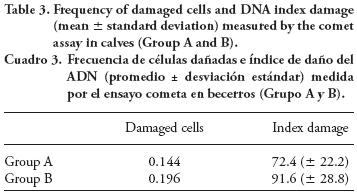
DNA damage
Results from the comet assay (Table 3) show that the number of cells with DNA damage was higher in Group B calves than in Group A (196 vs. 144; X2= 5.13; p=0.023), whereas the index damage was 91.6 in Group B and 72.4 in Group A (p≤0.05).
Results presented here are in agreement with the three phases model of copper deficiency (depletion, deficiency and dysfunction) described by Underwood and Suttle (1999); and confrm the occurrence of molybdenosis or secondary hypocuprosis in the calves. Hepatic copper storage was fourteen fold lower in Mo-supplemented than in the control calves. Hepatic copper is used in the synthesis of ceruloplasmin (Cp), which is the main form of Cu storage in plasma (it contains about 95 % of plasmatic Cu). Thus, during extended periods of copper depletion, low Cu plasma levels generate a fall in tissue Cu content and erythrocyte Cu concentration.
Along with low erythrocyte Cu content, there was a decrease in the Cu/Zn-SOD activity which catalyzes the destruction of the O2- free radical and protects cells against the harmful effects of superoxide free radicals (Fridovich, 1972; Paschen and Weser, 1973; Petkau et al., 1975). This finding, coupled with the increase of C18:0 and total saturated fatty acids concentration, could indicate an oxidative effect of Mo-induced Cu deficiency. There is no evidence that molybdenosis per se nor Cu deficiency are capable of inducing changes in membrane fatty acids composition in cattle. However, there is a similar pattern of C18:0 and C18:1 in thymic lymphocyte membranes of Cu-deficient mice (Korte and Prohaska, 1987) and adipose tissue of pigs (Ovecka et al., 1988). Some findings in rats allowed to associate changes in membrane fatty acids composition with altered D9-desaturase activities (Whale and Davies, 1975); however, further studies did not confirm this result (Ovecka et al., 1988; Korte and Prohaska, 1987). Besides, hypocuprosis is associated with an increase in lipid peroxidation and oxidative damage in cattle and rats (Jain and Williams, 1988; Picco et al., 2004).
There is no evidence about Mo genotoxicity in mammalian cells. Only some molybdenum salts would induce genotoxicity at relatively high doses both in vitro in human cells and in vivo in mice (Titenko-Holland et al., 1998). It is therefore reasonable to assume that Mo-induced copper deficiency would explain the increase of DNA damage found in Mo-supplemented calves.
Oxidative stress might explain the increase in DNA damage considering two lines of evidence that suggest extracellular and intracellular pathways leading to genotoxic effect. First, Cp is the main cupremic determinant and appears to be one of the most sensitive enzymes to copper deficiency (Bingley and Anderson, 1972; Blakley and Hamilton, 1985). According to Blakley and Hamilton (1985), Weiss (1989) and Saenko et al. (1994), Cp acts as an extracellular scavenger of free radicals in plasma, thus protecting the cells against reactive oxygen species released from neutrophils and macrophages. In addition, the ferroxidase activity of Cp mediates oxidation of ferrous ions to the ferric state, preventing ferrous ion-dependent formation of hydroxyl radicals via the Fenton reaction. And a reduction of up to third in Cp activity can be expected during molybdenosis (NRC, 2005).
In addition, we found a significant decrease in one of the main antioxidant enzymes, Cu/Zn-SOD. These findings agree with the increase in the activity of the xantine oxidase (XO) enzyme and the production of H2O2 during molybdenosis (NRC, 2005); and with the fact that enzymes with antioxidant activity not requiring Cu as a cofactor (such as catalase and glutathione peroxidase) are negatively infuenced by Cu unavailability, increasing free radicals in the cells (Strain, 1994).
Changes of membrane fatty acid composition, low Cu/Zn-SOD activity and DNA damage were due to the presence of Mo in the diet that may cause a reduction of the Cu availability at the cellular level in the calves. Therefore, an increase in the Mo-induced oxidative stress might explain genotoxic effect and fatty acid modifications by taking into account the relation between Mo-induced Cu deficiency and impairment of the antioxidant system. Models similar to that reported here, successfully explained the progress of copper deficiency and its consequences although they did not consider molybdenosis per se as an important factor (Arthington et al., 1996; Gengelbach and Spears, 1998).
CONCLUSIONS
Molybdenum could induce DNA damage through copper deficiency, being this condition caused by oxidative mechanisms. Changes of membrane fatty acid composition and decrease in Cu/Zn-SOD activity support this point. Further studies will be necessary to explore the mechanisms involved in the oxidative damage generation when the amount of dietary Mo is higher than the normal expected value; and to distinguish primary molybdenum toxicosis from the molybdenum-induced copper deficiency.
ACKNOWLEDGMENTS
This study received support through grants from Agencia Nacional de Promoción Científica y Tecnológica de la República Argentina (PICT 1841-2006), Ministerio de Ciencia, Tecnología e Innovación Productiva de la Nación Argentina. Authors are grateful to Prof. Ana Insausti for the correction of the manuscript.
LITERATURE CITED
Arthington, J. D., L. Corachet, and F. Blecha. 1996. The effect of molybdenum-induced copper deficiency on acute-phase protein concentrations, superoxide dismutase activity, leukocyte numbers and lymphocyte proliferation in beef heifers inoculated with bovine herpesvirus-1. J. Anim. Sci. 74: 211-217. [ Links ]
Barceloux, D. G. 1999. Molybdenum. J. Toxicol. Clinical Toxicol. 37: 231-237. [ Links ]
Beauchamp, C., and E. Fridovich. 1971. Superoxide dismutase: improved assays an assay applicable to acrylamide gels. Ann. Biochem. 44: 276-287. [ Links ]
Bingley, L., and M. Anderson. 1972. Clinically silent hipocuprosis and the effect of molybdenum loading on beef calves in Gippsland, Victoria. Austr. J. Agric. Res. 23: 885-904. [ Links ]
Blakley, B., and D. Hamilton. 1985. Ceruloplasmin as an indicator of copper status in cattle and sheep. Can. J. Comparative Medicine 49: 405-408. [ Links ]
Collins, A. 2004. The comet assay for DNA damage and repair. Principles, applications, and limitations. Molecular Biotechnol. 26: 249-261. [ Links ]
Dodge, T., D. Mitchell, and D. Hanahan. 1963. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophysics 100: 119-30. [ Links ]
Erdman, J., R. Ebens, A. Arthur and A. Case. 1978. Molybdenosis: A potential problem in ruminants grazing on coal mine spoils. J. Range Manage. 31: 34-36. [ Links ]
Folch, J, N. Lees, and G. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497-509. [ Links ]
Fridovich, I. 1972. Superoxide radical and superoxide dismutase. Accounts Chem. Res. 5: 321. [ Links ]
Gaetke, L., and C. Chow. 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189: 147-163. [ Links ]
Gengelbach, G., and J. Spears. 1998. Effects of dietary copper and molybdenum on copper status, cytokine production and humoral immune response of calves. J. Dairy Sci. 81: 3286-3292. [ Links ]
Gooneratne, R, W. Buckley, and D. Christensen. 1989. Review of copper deficiency and metabolism in ruminants. Can. J. Anim. Sci. 69: 819-845. [ Links ]
Jain, S. K., and D. M. Williams. 1988. Copper deficiency anemia: altered red blood cell lipid and viscosity in rats. Am. J. Clinical Nutr. 48: 637-640. [ Links ]
Jones, D., and N. Suttle. 1981. Some effects of copper deficiency on leucocyte function in sheep and cattle. Res. Vet. Sci. 31: 151-156. [ Links ]
Korte, J., and J. Prohaska. 1987. Dietary copper deficiency alters protein and lipid composition of murine lymphocyte plasma membranes. J. Nutr. 117: 1076-1084. [ Links ]
Lopez Alonso, M., F. Prieto, M. Miranda, C. Castillo, J. Hernandez, and J. Benedito. 2005. The role of metallothionein and zinc in hepatic copper accumulation in catlle. Vet. J. 169: 262-269. [ Links ]
Lowry, O., N. Rosebrough, A. Farr, and R. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265-275. [ Links ]
NRC. 2001. Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition, National Research Council: Washington DC. National Academic Press (eds). Chapter 6, Nutrient Requirements of Dairy Cattle: Seventh Revised Edition. pp: 140-141. [ Links ]
NRC. 2005. Committee on Minerals and Toxic Substances in Diets and Water for Animals, National Research Council: Washington DC. National Academic Press (eds). Chapter 21, Mineral Tolerance of Animals: Second Revised Edition. pp: 262-275. [ Links ]
Ovecka, G., G. Miller, and D. Medeiros. 1988. Fatty acids of liver, cardiac and sdipose tissues from copper-deficient rats. J. Nutr. 118: 480-486. [ Links ]
Pan, Y., and G. Loo. 2000. Effect of copper deficiency on oxidative DNA damage in Jurkat T-lymphocytes. Free Radical Biol. Med. 28: 824-830. [ Links ]
Paschen, W., and U. Weser. 1973. Singlet oxygen decontaminating activity of erythrocuprein (superoxide dismutase). Biochem. et Bioph. Acta 327: 217. [ Links ]
Petkau, A., W. Chelack, S. Pleskach, B. Meeker, and C. Brady. 1975. Radioprotection of mice by superoxide dismutase. Biochem. Biophys. Res. Commum. 65: 886. [ Links ]
Picco, S. J., G. Mattioli, L. Fazzio, D. Rosa, J. De Luca, and F. Dulout. 2004. Association between copper plasma level and DNA damage in cattle. Mutagenesis 19: 453-456. [ Links ]
Piper, H., and G. Higgins. 1967. Estimation of trace metals in biological material by atomic absorption spectrophotometry. Proc. Assoc. Clin. Biochem. 7: 190-195. [ Links ]
Saenko, E., A. Yaropolov, and E. Harris. 1994. Biological functions of caeruloplasmin expressed through copper-binding site and cellular receptor. J. Trace Elem. Exp. Med. 7: 69-88. [ Links ]
Singh, N. P. , M. McCoy, R. Tice, and E. Schneider. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175: 184-191. [ Links ]
Strain, J. 1994. Newer aspects of micronutrients in chronic disease: copper. Proc. Nutr. Soc. 53: 583-598. [ Links ]
Swanson, K. S., N. Merchen, J. Erdman, J. Drackley, F. Orias, G Douglas, and J. Huhn. 2000. Thechnical note: a technique for multiple liver biopsies in neonatal calves. J. Anim. Sci. 78: 2459-2463. [ Links ]
Thessman, R. K., J. Lakritz, J. Tyler, S. Casteel, J. Williams, and R. Dew. 2001. Sensitivity and specifcity of serum copper determination for detection of copper deficiency in feeder calves. J. Am. Vet. Med. Assoc. 218: 756-760. [ Links ]
Thompson, K., and J. Turnlund. 1996. Kinetic model of molybdenum metabolism developed from dual stable isotope excretion in men consuming a low molybdenum diet. J. Nutr. 126: 963-972. [ Links ]
Titenko-Holland, N., J. Shao, L. Zhang, L. Xi, H. Ngo, N. Shang, and M. T. Smith, 1998. Studies on the genotoxicity of molybdenum salts in human cells in vitro and in mice in vivo. Environ. Mol. Mutagen. 32: 251-259. [ Links ]
Turnlund, J., W. Keyes, G. Peifer, and G. Chiang. 1995. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men during depletion and repletion. Am, J. Clin. Nutr. 61: 1102-1109. [ Links ]
Uauy, R., M. Olivares, and M. Gonzalez. 1998. Essentiality of copper in humans. Am. J. Clinical Nutr. 67: 952S-959S. [ Links ]
Underwood, E., and N. Suttle. 1999. The Mineral Nutrition of Livestock. Tird ed. CABI Publishing. Wallingford, UK. pp: 214-235. [ Links ]
Webster, R., M. Gawde, and R. Bhayfacharya. 1996. Modulation by dietary copper of aflatoxin B1 activity of DNA repair enzymes poli(ADPribose) polymerase, DNA polymerase B and DNA ligase. In Vivo 10: 533-536. [ Links ]
Weiss, S. L. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320: 365-376. [ Links ]
Whale, K., and N. Davies. 1975. Effect of dietary copper deficiency in the rat on fatty acid composition of adipose tissue and desaturase activity of liver microsomes. Br. J. Nutr. 34: 105-112. [ Links ]
Xin, Z., D. Waterman, R. Hemken, and R. Harmon. 1991. Effects of copper status on neutrophils function, superoxide dismutase and copper distribution in steers. J. Dairy Sci. 74: 3078-3085. [ Links ]














