Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.46 no.1 Texcoco ene./feb. 2012
Recursos naturales renovables
Components of net aerial primary production in a Bambusa oldhamii plantation
Componentes de la producción primaria neta aérea en una plantación de Bambusa oldhamii
Arturo Castañeda-Mendoza, J. Jesús Vargas-Hernández*, Armando Gómez-Guerrero
Forestal. Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México. (arturocm@colpos.mx), (vargashj@colpos.mx) * Author for correspondence: (agomezg@colpos.mx)
Received: july, 2011.
Approved: december, 2011.
Abstract
Biomass production is an important criterion to be considered for the establishment of commercial forestry plantations for bioenergy production. In this study, an estimation of net aerial primary production (NAPP) was made in a young plantation of Bambusa oldhamii Munro from the sum of biomass production from growth of new culms, biomass increment of the pre-existing culms, and production of litter. Biomass production in the incorporated culms was 15.82 Mg ha-1 year-1, of which 13.77 Mg (87 %) corresponded to stems and 2.05 Mg (13 %) to foliage. Biomass increment in the pre-existing culms was 10.88 Mg ha-1. One- and two-year-old cohort production was 7.31 and 3.57 Mg ha-1. Litter production was 5.56 Mg ha-1 year-1. Results show that NAPP was 32.20 Mg ha-1, with 50 % coming from incorporation of new culms, whereas 17 % of the NAPP is recycled in the system through litter. Estimated production of biomass in this plantation is comparable to that registered for high productivity bamboo plantations in other regions of the world.
Key words: bamboo, litter, biomass production, bioenergy, tropical forest species.
Resumen
La producción de biomasa es un factor importante a considerar para el establecimiento de plantaciones forestales comerciales para la producción de bioenergía. En este estudio se realizó una estimación de la producción primaria neta aérea (NAPP, por sus siglas en inglés), en una plantación joven de Bambusa oldhamii Munro a partir de la producción total de biomasa del crecimiento de los culmos nuevos, aumento de la biomasa en los culmos pre-existentes y producción de residuos orgánicos. La producción de biomasa en los culmos incorporados fue 15.82 Mg ha-1 año-1, de las cuales 13.77 Mg (87 %) correspondieron a los tallos y 2.05 Mg (13 %) al follaje. El aumento de la biomasa en los culmos pre-existentes fue 10.88 Mg ha-1. La producción de las cohortes de uno y dos años fue 7.31 y 3.57 Mg ha-1. La producción de residuos orgánicos fue 5.56 Mg ha-1 año-1. Los resultados muestran que la NAPP fue 32.20 Mg ha-1, con 50 % proveniente de los culmos nuevos, mientras que 17 % de NAPP se recicla en el sistema a través de los residuos. La producción estimada de biomasa en esta plantación es comparable a la registrada en las plantaciones de bambú de alta productividad en otras regiones del mundo.
Palabras clave: bambú, residuos orgánicos, producción de biomasa, bioenergía, especies de bosques tropicales.
INTRODUCTION
The environmental deterioration caused by deforestation, the loss of sources of employment in the forestry sector, and the growing importance of fnding renewable energy sources, make the establishment of commercial plantations with fast-growing species an important activity (Christersson and Verma, 2006). The interest in planting fast-growing species is due to their potential for biomass production, providing high-energy outputs to replace conventional energy sources, particularly when they can be established in low fertility sites (Tiarks et al., 1988). In Mexico, approximately 11 million ha have been identifed with high potential for the establishment of forest plantations for wood production, although up to now, less than 450 000 ha had been established (CONAFOR)1.
Productivity is a critical factor in the selection of species for the establishment of commercial plantations; therefore, studies focused on the evaluation of this silvicultural variable are required. Forest productivity refers to the biomass production or carbon balance of a stand that can be realized at a certain site with a given species (genotype) and a specifed management regime (Skovsgaard and Vanclay, 2008). Specifically, net primary productivity (NPP) is defned as the total photosynthetic production that occurs in a given period, minus the losses due to respiration in that time (Clark et al., 2001). However, since it is not easy to directly estimate NPP, measurements of dry mass per area incorporated per time unit is used as an indicator of NPP (Waring et al., 1998).
In recent years, there has been interest in the establishment of fast-growing bamboo plantations in the tropical zones of México. Bamboo plantations can provide building material, replacing traditional building components with high CO2 emission costs (Christersson and Verma, 2006). However, there have not been any studies in this region focused on the biomass productivity that can be achieved so far. Bamboo plantations open the possibility of integrating small property owners of rural communities in the formation of micro-industries with low-cost technologies for bio-energy production in short rotations, helping to mitigate global warming by sequestering atmospheric carbon (Dagilis and Trucke, 1998; Ganapathy et al., 1999; Lobovikov et al., 2009).
The purpose of the present study was to estimate the net aerial primary production (NAPP) in an eight-year-old Bambusa oldhamii Munro plantation, given by the biomass increment of existing culms, the biomass incorporation in new culms and the annual production of litter.
MATERIALS AND METHODS
Site description
The study was carried out in a plantation of B. oldhamii Munro, located in municipality Huatusco, Veracruz, Mexico (10° 09' N and 96° 57' W; 1320 m elevation). The plantation is found on a hillside with a mean slope of 18 % and a north-eastern aspect. According to García (1973), the climate is type (A) C (m)b (i)g, with a mean annual temperature of 19 °C and average annual rainfall of 1746 mm. The soils correspond to ocric and vertic Andosol (FAO-UNESCO, 1988); they have a clay (67 % clay, 23 % sand, and 10 % loam) texture.
Plantation structure
Plantation density is 370 plants ha-1, with a spacing of 6 m between plants and 4.5 m between rows, on an area of 2430 m2. An inventory was made through a random sampling to determine the diametric structure of each cohort of culms (one to four years of age) present at the beginning of the study. Stem DBH was measured in all culms present in 12 randomly selected plants. The dry mass balance of culms was followed by three more years when the plantation was seven-years old. In order to get the initial allometric equations, minimal biomass removal was considered.
Estimation of aerial biomass
The aerial biomass present at the beginning of the study was estimated from the biomass equations developed for the plantation under study by Castañeda-Mendoza et al. (2005), and the data obtained from the diametric structure in the inventory for this work (Table 1). The biomass generated by incorporation of culms emerged during the last growing season was estimated by a biomass prediction equation for the culms belonging to the eighth generation, using a similar equation as for the seventh cohort (Table 1).
Estimation of biomass increment in pre-existing culms
Given that young culms of a bamboo plant increase their biomass in stem, branches, and foliage despite the fact that they do not grow neither in diameter nor in height after their first growing season, it is possible to estimate this increase from the equations generated for each cohort. For one- and two-year-old cohorts, the total biomass equations for cohorts one year older (two- and three-year-old) were applied, assuming that the culms would reach this biomass during the current year of growth. This procedure for biomass increment estimation was proposed by Taylor and Zisheng (1987) in three species of bamboo under natural conditions in a temperate forest in China. Thus, the biomass equation for two-year-old culms was applied to one-year-old culms, and the diference in biomass estimated with both equations was considered to be the increment in biomass during one year of growth. For two-year-old culms, the same procedure was applied using the equation for three-year-old culms; in the case of the three-year-old culms, a null increment was considered, given that the equation obtained for the four-year-old cohort did not allow simulation of the increment in biomass. Finally, four-year-old culms were harvested at the moment of beginning the study. We know that not including the three-year-old culms in the estimation of biomass increment leads to under-estimation of productivity. However, we had to use the data obtained with the allometric equations developed for the study plantation. Previous studies (Lakshamana, 1991; Upadhyaya et al., 2008; Nath and Das, 2011) have shown that biomass increment in bamboo plants is concentrated in the young culms, mainly one- and two-year-old shoots; thus, the efect of the under-estimation is negligible.
Measurement of litter biomass
To estimate the biomass of ground litter three 27 m2-traps (4.5×6 m) were established within the plantation, ensuring that each trap represented the growth space of a plant. At the moment of litter collection, fresh weight was obtained and subsamples were oven dried at 80 °C until constant weight was achieved. Total dry matter was calculated for the litter collected in the trap from the fresh weight and moisture content of subsamples, including branches, foliage and culm sheaths. The litter caught in the traps was collected every 50 d during the period of December to August. Direct field observations in previous years indicated that litter production is concentrated in this dry period; given that in the study site the period of highest water stress is between February and May, it was considered that the sampling period for litter production was appropriate.
Net aerial primary productivity (NAPP)
Net aerial primary production was obtained through the sum of the biomass incorporated by the culms formed in the last growing season (Bnc), the increment in biomass of the two youngest cohorts present at the beginning of the study (BIoc), and litter production (LB) (i.e., NAPP = Bnc + BIoc + LB). The onset of culms sprouting (beginning of July) was used as reference, being the moment at which the youngest cohort reached one year of age.
Given that the study attempts to investigate productivity in the juvenile stage of a plantation that has not yet reached stability in height and diameter structure of different generations of culms, the estimated productivity can be considered conservative. Estimation considers that three-year-old culms do not increase their biomass; however, when biomass equations generated with culms of lower average height are applied to younger (one- and two-year-old) culms (cohorts seven and six, respectively), the biomass increment would be slightly under-estimated. The productivity will be stabilized once the plantation reaches a "normal" production of culms when reaching the maturity stage, and the dimensions of the new culms are more uniform.
RESULTS AND DISCUSSION
Structure of the plantation
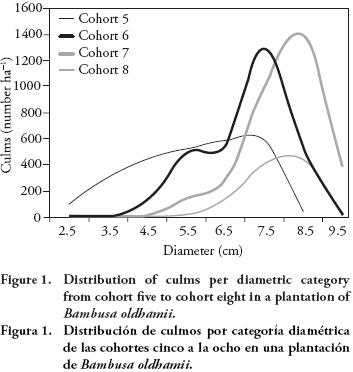
After incorporation of culms from cohort eight and harvest of cohort four, the initial structure of culms in the plantation was modified. The number of culms per ha decreased from 10 101 to 9762, due to a lower production than expected in the youngest cohort (Figure 1). Culms production showed a gradual increase both in average height and diameter, as well as in the number of culms per plant in the first three cohorts (five to seven). However, in cohort eight only the average height increased with respect to the previous generations, with an average of 17.3 m.
Biomass of new culms
The increment in biomass associated with incorporation of new culms in the plantation was 15.82 Mg ha-1, of which 13.77 Mg (87 %) corresponded to stems and 2.05 Mg (13 %) to foliage. Biomass produced by the new cohort of culms was much lower than expected, given that total biomass of previous cohort (generation seven) was 39.4 Mg ha-1 at one year of age, with an average of 8.8 culms per plant and 3256 culms ha-1. In contrast, in the new cohort the average was 2.8 culms per plant and 1028 culms ha-1.
Biomass increment in the pre-existing culms
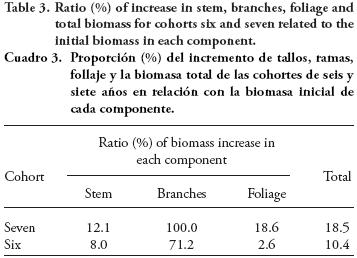
Estimated increase in aerial biomass of pre-existing culms was 10.88 Mg ha-1, of which 3.57 Mg correspond to cohort six (two-year-old at the beginning of the study), and 7.31 Mg to cohort seven, one-year-old at the beginning of the study (Table 2). Including both cohorts, 59.6 % of the increase in biomass was accumulated in the stems, 31.6 % in the branches and less than 10 % in the foliage.
Based on the initial biomass of cohorts six and seven, was drop is observed in the increase of total biomass with age, falling from 18.5% in cohort seven to 10.4 % in cohort six. Stem biomass increases 12.1 % after the frst year of age, and 8.0 % the following year; branches doubled their biomass after the first year and increased an additional 71.2% in the second year, whereas foliage increased 18.6 and 2.6% after one and two years of age (Table 3).
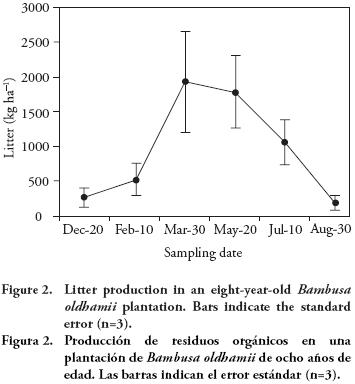
Litter biomass
The period of maximum litter fall in the year occurred from February to July, with a duration of approximately six months (Figure 2). Annual litter production was estimated at 5.56 Mg ha-1 with a minimum amount of 0.19 Mg ha-1 in August and a maximum of 1.93 Mg ha-1 in March. The fallen litter and residues of the annual harvest (branches and foliage) accumulated in the soil, forming a thick layer of organic matter, which conserves soil moisture and minimizes surface run-of, and hence, erosion. Christanty et al. (1996) point out that this litter accumulation is due to the slow decomposition rate of bamboo foliage. In a mature bamboo stand (planted or natural), litter production should be equal to the production of new foliage; however, the total amount depends on culms density and stand management (Hunter and Junqui, 2002).
Net aerial primary productivity
The NAPP of the plantation was estimated in 32.20 Mg ha-1 year-1, of which 15.82 Mg (48.9 %) represent the biomass of new culms, 10.88 Mg (33.8 %) the biomass increase of pre-existing culms, and 5.56 Mg (17.3 %) litter production (Figure 3). The increase in standing aerial biomass was 26.7 Mg ha-1. NAPP is a parameter that depends on diverse ecological factors, among which climatic and soil factors are outstanding, along with the capacity of the species to optimize conversion of available resources into biomass production. When bamboo plantations are established and management is not applied (harvest of culms for production or thinning), the annual rate of biomass production is characterized by a sigmoidal increase up to 3-5 years of age (Kleinhenz and Midmore, 2001). Removal of old culms prevents congestion within the plant, which reduces the production of shoots, afects quality of wood, and makes difficult the management of plantations (Lakshamana, 1991). The annual harvest of culms, both in natural populations and plantations, makes it possible to maintain productivity at an optimum rate during a prolonged period of time, by controlling stand density and competition for growth space. In addition, it reassigns resources to younger culms, which provides an important link between new and productive culms (Hogarth and Franklin, 2009).
In the sympodial species of bamboo, young culms contain relatively younger and more productive foliage, and their transport tissues are more efective than in older culms (Liese, 1998); metabolism residues are accumulated in the stem tissues, progressively reducing water and nutrients transport in the xylem, and photosynthates in the phloem, which fnally causes death of shoots (Liese, 1998). According to Lakshamana (1991), one-year-old culms contribute with 77 % in the production of new shoots, whereas two-year-old and older culms contribute with 20 and 3 %. Other authors (Kleinhenz and Midmore, 2001; Banik and Nurul, 2005; Li et al., 2007) point out the same tendency in other studies, so the contribution of culms over two years of age to total productivity is minimal. Therefore, harvesting the culms over three years of age is recommended; at this age, enough stem maturity is reached for a large variety of products, especially "wood" products.
In this sense, the NAPP assessed in the present study can be considered as a conservative estimate or the lower limit of real productivity in the plantation, considering that the anatomical and phenological characteristics of bamboos make it possible to assume that there is a marginal increase in the cohorts over three years of age. The harvest regime in the plantation under study considers removal of four-year-old culms, an age suggested by diverse authors (Kleinhenz and Midmore, 2001; Wahab et al., 2010; Nath and Das, 2011). However, it is important to investigate the implications of this regime on plantation productivity and quality of products obtained.
The maximum values of NAPP found in bamboo species are between 10 and 30 Mg ha-1 year-1 (Table 4), within the interval registered for plantations with conventional tree species (Hunter and Junqui, 2002). The intensive management of bamboo plantations may result in a greater production of biomass if the high production rates are maintained for a longer period of time. Soil fertility is a factor of great importance for maintaining site productivity, given the continuous nutrient extraction from the system which accumulates in the culms biomass (Krishnankutty and Chundamanil, 2005). Therefore, it is suggested to carry out studies focused on availability of essential nutrients for plant growth and on recycling processes.
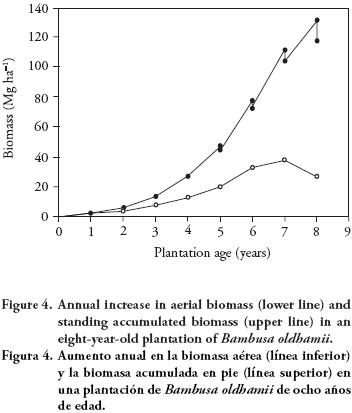
Total aerial biomass in the study plantation after eight years was 144.36 Mg ha-1; however, due to the annual harvest of four-year-old culms, the standing aerial biomass present at that date was 117.28 Mg ha-1. Figure 4 shows the changes in biomass accumulation of the plantation considering the biomass removed at the four harvest cycles done in the last four years. In diverse bamboo species, variation in annual production of culms has been identified, which is explained both by density of plantation and intensity of harvest (Raghubanshi, 1994; Singh and Kochhar, 2005). Harvest intensity has implications both on modifying the age structure of remaining foliage (photosynthetically active tissue), and on distribution and translocation of assimilates among culms of different ages.
CONCLUSIONS
Bamboo species, particularly Bambusa oldhamii, are fast-growing species, with high productivity, and can be used as a species for plantations in some tropical zones of Mexico either in carbon sequestration or biomass and bio-energy projects. Management of bamboo plantations is essential for increasing or maintaining a desired productivity level, given that the highest proportion of aerial biomass is assigned to young culms. Since production of new culms is associated with the amount of pre-existing culms from earlier cohorts, it is important to consider harvest cycles and intensity levels to maintain an adequate stand density. It is necessary to continue productivity studies in these species and evaluate the effects of different management practices in plantations at different development stages.
ACKNOWLEDGEMENTS
The authors would like to express their thanks to Bambuver A.C., for the facilities and logistic support given for this work. The study was financed by the National Forestry Commission (Comisión Nacional Forestal) through project CONAFOR-2002-C01-6541 within the Sectorial Fund for Forest Research (Fondo Sectorial para la Investigación Forestal).
LITERATURE CITED
Banik, R. L., and I. S. Nurul. 2005. Leaf dynamics and above ground biomass growth in Dendrocalamus longispathuz Kurtz. J. Bamboo Rattan 4(2): 143-150. [ Links ]
Castañeda-Mendoza, A., J. J. Vargas-Hernández, A. Gómez-Guerrero, J. I. Valdez-Hernández, and H. Vaquera-Huerta. 2005. Acumulación de carbono en la biomasa aérea de una plantación de Bambusa oldhamii. Agrociencia 39: 107-116. [ Links ]
Christanty, L., D. Maily, and J. P. Kimmins. 1996. 'Without bamboo, the land dies': biomass, litterfall, and soil organic matter dynamics of a Javanese bamboo talun-kebun system. Forest Ecol. Manage. 87: 75-88. [ Links ]
Christersson, L., and K. Verma. 2006. Short-rotation forestry - a complement to "conventional" forestry. Unasylva 223 (57): 34-39. [ Links ]
Clark, D. A., S. Brown, D. W. Kicklighter, J. Q. Chambers, J. R. Tomlinson, and J. Ni. 2001. Measuring net primary production in forests: concepts and feld methods. Ecol. Appl. 11: 356-370. [ Links ]
Dagilis, T. D., and D. J. Trucke. 1998. One bamboo stand does not a planet save: bamboo industrialization in the context of sustainable development. In: VI International Workshop and V International Bamboo Congress. INBAR-FUNBAMBUIBA, Costa Rica. p. 143. [ Links ]
FAO-UNESCO. 1988. Soil map of the world. Revised legend. Report 60. Rome. s/p. [ Links ]
Fujimori, T. , and K. Yamamoto. 1967. Productivity of Acacia dealbata stands. J. Japan. For. Soc. 49: 143-149. [ Links ]
Ganapathy, P. M., Z. Huan-Ming, S. S. Zoolagud, D. Turcke, and Z. B. Espiloy. 1999. Bamboo panel boards: a state-of-the-art review. Technical report No. 12. International Network for Bamboo and Rattan. 115 p. [ Links ]
García, E. 1973. Modifcaciones al sistema de clasificación climática de Köeppen. Instituto de Geografía. Universidad Nacional Autónoma de México. 246 p. [ Links ]
Hogarth, N. J., and D. C. Franklin. 2009. Observations on the clonal parentage of culms in wild stands of a clumping bamboo from northern Australia. J. Trop. For. Sci. 21(2): 139-146. [ Links ]
Hunter, I. R., and W. Junqui. 2002. Bamboo biomass. Working paper No. 36. International Network for Bamboo and Rattan. 11 p. [ Links ]
Isagi, Y., T. Kawahara, K. Kamo, and H. Ito. 1997. Net production and carbon cycling in a bamboo Phylostachys pubescens stand. Plant Ecol. 130 (1): 41-52. [ Links ]
Kleinhenz, V., and D. J. Midmore. 2001. Aspects of bamboo agronomy. Adv. Agron. 74: 99-149. [ Links ]
Krishnankutty, C. N., and M. Chundamanil. 2005. Predicting the weight of a bamboo clump: commercial weight tables for Bambusa bambos. J. Bamboo Rattan 4 (4): 311-316. [ Links ]
Kumar, B. M., G. Rajesh, and K. G. Sudheesh. 2005. Aboveground biomass production and nutrient uptake of thorny bamboo (Bambusa bambos (L.) Voss) in the homegardens of Trissur, Kerela. J. Trop. Agric. 43: 51-56. [ Links ]
Lakshamana, A. C. 1991. Culm production of Bambusa arundinacea in natural forest of Karnataka, India. In: Proceedings of the 4th International Bamboo Workshop on Bamboo in Asia and the Pacific. Chiangmai, Tailand. Technical Document. Forspa Publication. No. 6; Tailand. pp: 100-103. [ Links ]
Li, X. B., T. F. Shupe, G. F. Peter, C. Y. Hse, and T. L. Eberhardt. 2007. Chemical changes with maturation of the bamboo species Phyllostachys pubescens stand. J. Trop. For. Sci. 19 (1): 6-12. [ Links ]
Liese, W. 1998. The anatomy of bamboo culms. International Network for Bamboo and Rattan. INBAR Technical Report No. 18. Beijing, China. 205 p. [ Links ]
Lobovikov, M., Y. Lou, D. Schoene, and R. Widenoja. 2009. The poor man's carbon sink. Bamboo in climate change and poverty alleviation. FAO-INBAR. Non-wood Forest Products working document no. 8, 52 p. [ Links ]
Nath, A. J., and A. K. Das. 2011. Carbon storage and sequestration in bamboo-based smallholder homegardens of Barak Valley, Assam. Curr. Sci. 100(2): 229-233. [ Links ]
Nath, A. J., G. Das, and A. K. Das. 2009. Above ground standing biomass and carbon storage in village bamboos in North East India. Biomass Bioenerg. 33: 1188-1196. [ Links ]
Parrotta, J. A. 1992. Leucaena leucocephala (Lam.) de Wit. Leucaena, tantan. USDA, Forest Service, Southern Forest Experiment Station. 8 p. [ Links ]
Raghubanshi, A. S. 1994. Effect of bamboo harvest on dynamics of nutrient pools, N mineralization, and microbial biomass in soil. Biol. Fert. Soils 18: 137-142. [ Links ]
Shanmughavel, P. , R. S. Peddappaiah, and T. Muthukumar. 2001. Biomass production in an age series of Bambusa bambos plantations. Biomass Bioenerg. 20: 113-117. [ Links ]
Singh, A. N., and J. S. Singh. 1999. Biomass, net primary production and impact of a bamboo plantation on soil redevelopment in a dry tropical region. For. Ecol. Manage. 119: 195-207. [ Links ]
Singh, K. A., and S. K. Kochhar. 2005. Effect of clump density/ spacing on the productivity and nutrient uptake in Bambusa pallida and the changes in soil properties. J. Bamboo Rattan 4 (4): 323-334. [ Links ]
Skovsgaard, J. P. , and J. K. Vanclay. 2008. Forest site productivity: a review of the evolution of dendrometric concepts for even-aged stands. Forestry 81: 12-31. [ Links ]
Stape, J. L., D. Binkley, and M. G. Ryan. 2004. Eucalyptus production and the supply, use and efficiency of use of water, light and nitrogen across a geographic gradient in Brazil. Forest Ecol. Manag. 193: 17-31. [ Links ]
Taylor, A. H., and Q. Zisheng. 1987. Culm dynamics and dry matter production of bamboos in the Wolong and Tangjiahe giant panda reserves, Sichuan, China. J. Appl. Ecol. 24: 419-433. [ Links ]
Tiarks, A., E. K. S. Nambiar, and C. Cossalter. 1988. Site management and productivity in tropical forest plantations. Center for International Forestry Research. Occasional paper No. 16. Jakarta. 11 p. [ Links ]
Tripathi, S. K., and K. P. Singh. 1994. Productivity and nutrient cycling in recently harvested and mature bamboo savannas in the dry tropics. J. Appl. Ecol. 31: 109-124. [ Links ]
Upadhyaya, K., A. Arunachalam, K. Arunachalam, and A. K. Das. 2008. Aboveground biomass and productivity appraisal of four important bamboo species growing along different altitudinal regimes in Arunachal Pradesh. J. Bamboo Rattan 7: 219-234. [ Links ]
Virtucio, F. D., B. M. Manipula, and F. M. Schlegel. 1991. Culm yield and biomass productivity of laak (Sphaerobambos philipinensis). In: Proceedings of the 4th International Bamboo Workshop on Bamboo in Asia and the Pacific. Chiangmai, Tailand. Technical Document. Forspa Publication. No. 6; November 27-30. pp: 95-99. [ Links ]
Wahab , R., M. Mustapa, O. Sulaiman, A. Mohamed, A. Hassan, and I. Khalid. 2010. Anatomical and physical properties of cultivated two- and four-year-old Bambusa vulgaris. Sains Malaysiana 39(4): 571-579. [ Links ]
Waring, R. H., J. J. Landsberg, and M. Williams. 1998. Net primary production of forests: a constant fraction of gross primary production? Tree Physiol. 18: 129-134. [ Links ]
1 CONAFOR. 2008. Programa Institucional 2007-2012. Comisión Nacional Forestal- SEMARNAT. Zapopan, Jalisco.59 p.