Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.45 no.6 Texcoco ago./sep. 2011
Biotecnología
Addition of benzyladenine to coconut explant cultured In vitro improves the formation of somatic embryos and their germination
La adición de benciladenina a explantes de cocotero cultivados In vitro mejora la formación de embriones somáticos y su germinación
Mayra I. Montero–Cortés, José L. Chan–Rodríguez, Ivan Cordova–Lara, Carlos Oropeza–Salin, Luis Sáenz–Carbonell*
Unidad de Biotecnología, Centro de Investigación Científica de Yucatán. 97200. Calle 43 No. 130. Colonia Chuburna de Hidalgo, Mérida, Yucatán, México. *Author for correspondence: vyca@cicy.mx.
Received: January, 2011.
Approved: July, 2011.
Abstract
Coconut (Cocos nucífera L.) production has been declining in México and other countries due to deadly diseases; therefore, massive production of palms resistant to diseases is needed for replanting. Production of somatic embryos and micropropagation could be a very useful technique to produce free disease coconut population. Explants (embryogenic–structures) of Pacific Tall 2 ecotype were cultured in an induction medium defined as I (semisolid Y3 medium containing 0.65 mM 2,4–dichlorophenoxyacetic acid) and treated with the cytokinin benzyladenine (BA) at 0, 25, 100 or 200 ,µM to evaluate its effect on embryogenic callus formation. The BA treatments did not affect the percentage of embryogenic callus formation. In order to evaluate the effect of the BA treatments on the late formation and germination of somatic embryos, the embryogenic calluses obtained in medium I were transferred to medium II (semisolid Y3 medium, containing 6 µM 2,4–dichlorophenoxyacetic acid and 300 ,µM BA). The best treatment was obtained from callus treated with 100 ,µM BA; 72 % of them produced 27 embryos per responding callus at 150 d while with 25 /µM BA and the control (0 µM BA) only 4 embryos were formed. The highest percentage (50 %) of calluses with germinating embryos occurred with 100 µM BA at 150 d; other treatments produced only 20 %. In this BA concentration, the number of germinating embryos per callus was 10, while with the other treatments only 2–3 embryos were formed at 150 d. The total yield of embryos and germinating embryos from 100 explants were 73.6 and 19.0, with 0 µM BA, and 753.2 and 249.0 with 100 µM BA. Pre treatment of coconut explants with BA (100 µM) and further treatment with 6 µM 2,4–D and 300 µM BA, improves the efficiency of coconut somatic embryo formation and germination.
Key words: Cocos nucífera, clonal propagation, somatic embryogenesis, cytokinin.
Resumen
La producción de palmas de cocotero (Cocos nucífera L.) ha disminuido en México y otros países debido a enfermedades letales; por tanto, la producción masiva de palmas resistentes a enfermedades es necesaria para replantar. La producción de embriones somáticos y la micropropagación puede ser una técnica muy útil para producir una población de cocoteros libre de enfermedades. Explantes (estructuras–embriogénicas) del ecotipo Alto Pacífico 2 se cultivaron en un medio de inducción definido como I(medio Y3 semisólido que contiene 0.65 µM 2,4–diclorofenoxiacético) y tratados con la citocinina benciladenina (BA) a 0, 25, 100 o 200 µM para evaluar su efecto sobre la formación de callos embriogénicos. Los tratamientos BA no afectaron el porcentaje de la formación de callos embriogénicos. Para evaluar el efecto de los tratamientos BA en la formación tardía y germinación de embriones somáticos, los callos embriogénicos obtenidos en el medio de cultivo I se transfirieron al medio II (medio Y3 semisólido que contiene 0.65 µM 2,4–de ácido diclorofenoxiacético y 300 µM BA). El mejor tratamiento se obtuvo de callos tratados con 100 ,µM BA; 72 % de ellos produjeron 27 embriones por respuesta del callo a 150 d, mientras que con 25 µM BA y con el testigo (0 µM BA) se formaron sólo 4 embriones. El mayor porcentaje (50 %) de callos con embriones en germinación se produjeron con 100 µM BA en 150 d; los otros tratamientos produjeron sólo 20 %. En esta concentración de BA, el número de embriones en germinación por callo fue 10, mientras que con los otros tratamientos sólo 2–3 embriones se formaron en 150 d. El rendimiento total de los embriones y embriones en germinación de 100 explantes fueron 73.6 y 19.0, con 0 µM BA, y 753.2 y 249.0 con 100 µM BA. El pre–tratamiento de explantes de coco con BA (100 µM) y el tratamiento posterior con 6 ,µM 2,4–de ácido diclorofenoxiacético y 300 M BA, mejoró la eficiencia de la formación y germinación de embrión somático del coco.
Palabras clave: Cocos nucífera, propagación clonal, embriogénesis somática, citocinina.
INTRODUCTION
The coconut palm (Cocos nucífera L.) is a very important crop in tropical areas, besides its potential use as biodiesel obtained from coconut oil in the Philippines (Tan et al., 2004). Millions of palms are disappearing mainly due to deadly diseases; the main cause in México is the lethal yellowing disease (LY) (Harrison and Oropeza, 2008). Therefore, there is a growing need to produce resistant palms to these diseases for replanting.
In México, a nearly 10–year study screening LY resistant coconut germplasm allowed the identification of highly resistant ecotypes, in particular the Mexican Pacific Tall 2 (MPT2) (Zizumbo et al., 1999). In order to obtain enough selected palms for replanting, more efficient propagation methods are needed since only a small number of seeds per palm are produced annually. Micropropagation and protocols via somatic embryogenesis have been reported using different explants: immature leaf (Raju et al., 1984), immature inflorescence (Verdeil et al., 1994), plumule (Chan et al., 1998) and unfertilized ovary (Perera et al., 2006). The plumule explant when cultured in medium containing the synthetic auxin 2,4–dichlorophenoxyacetic acid (2,4–D) without any other growth regulator, show a better response for the production of embryogenic calluses and somatic embryos, although embryos yield is very low (Chan et al., 1998). In order to overcome this limitation there is a protocol with a cumulative effect on the number of somatic embryos produced, mainly by subsequent steps of embryogenic callus multiplication, for which embryogenic–structures were used as explants (Perez–Nuñez et al., 2006). However, each individual step still shows a low efficiency: only 40–60 % explants form embryogenic callus (EC), 1–7 somatic embryos are formed per EC and embryo germination is 1228 % (Perez–Nuñez et al., 2006). Therefore, it is important to improve the protocol to produce more coconut plantlets in order to support the coconut replanting programs in México.
Cytokinins such as benzyladenine (BA) are included in media formulations in combination with auxins to induce embryogenic cultures (Gaj, 2004). BA and other cytokinins added to a medium formulation that includes 2,4–D inhibite the formation of EC on plumule explants (Azpeitia, 2003; Chan et al., 1998). However, BA has not been tested on embryogenic–structure explants derived from multiplying calluses. Therefore the objective of the present study was to evaluate the effect of BA on the formation of embryogenic callus, somatic embryos and their germination rate using embryogenic–structures as explants.
MATERIALS AND METHODS
Initial plant material
Coconut fruit were harvested 12–14 months after controlled pollination of Mexican Pacific Tall palms at least 15 year old. Cylinders (1.6 cm diameter) of endosperm containing the embryo were obtained in the field, placed in a 0.6 % NaClO (w/v) solution and rinsed with sterile water. Under aseptic condition, the cylinders were washed 3 min in 70 % ethanol, 20 min in a 6 % NaClO solution and rinsed with sterile water. The embryos were excised from the endosperm, washed 10 min in a 0.6 % NaClO solution and rinsed with sterile water. The plumules were excised from these embryos under a stereoscopic microscope and placed directly on culture medium I.
Media preparation and culture conditions
Media preparation and culture conditions were set up according to Pérez–Nuñez et al. (2006). Media I and II were each prepared using Y3 medium (Eeuwens, 1976) supplemented with 3 g L –1 gelrite and 2.5 g L –1 charcoal (acid–washed, plant cell culture tested); medium I contained 600 µM 2,4–D, while medium II contained 6 ,µM 2,4–D and 300 µM BA. All chemicals were supplied by Sigma (USA). The medium pH was adjusted to 5.75 with KOH before autoclaving for 20 min at 120 °C. For callus induction, each explant was cultured in 35 mL glass vessel containing 10 mL of medium I for 90 d. Cultures were kept under complete darkness at 27 ±2 °C without subculturing. For induction of somatic embryos, calluses were subcultured in a 100 mL glass vessel containing 25 mL of medium II. Cultures were kept in a 16 h photoperiod (45–60 µMol m–2 s–1 PPFD) at 27±2 °C, and explants were subcultured once every 2 months.
Callus multiplication
Embryogenic–structures, excised from embryogenic callus formed in medium I, were subcultured in medium I. This procedure was repeated twice and the resulting embryogenic calluses were subcultured in medium II for somatic embryo formation and germination. Media and conditions were as described above.
Experiment with BA
The cytokinin BA, benzylaminopurine (0, 25, 50, 100 and 200 µM) was added to medium I (protocol callus multiplication) and the effect of BA treatments was evaluated in the formation of EC. The embryogenic calluses formed under the BA treatments were transferred to medium II (same formulation as above for all treatments) to allow the formation of somatic embryos and their germination. Three repetitions with 20 explants each (60 explants total) were used per treatment in medium I. Then, from each treatment 30 embryogenic calluses were subcultured into medium II (three repetitions with 10 explants).
Quantitative evaluation
Every 30 d, under sterile conditions and using a stereoscopic microscope, the following variables were determined: percentage of explants forming EC, percentage of embryogenic calluses with somatic embryos, number of somatic embryos per EC, percentage of embryogenic calluses with germinating embryos, number of germinating embryos per EC responding, total yield of somatic embryos, and total yield of germinating somatic embryos.
Statistical analysis
The experimental design was completely randomized, data were subjected to analysis of variance and means were compared using LSD test (p≤0.05.) using SPSS version 11.5 software for all statistical analysis.
RESULTS AND DISCUSSION
Morphological development of coconut cultures
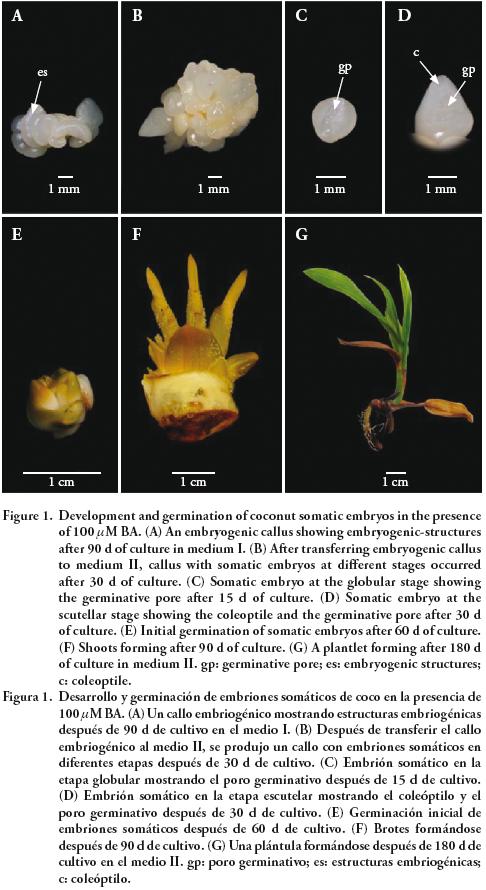
Embryogenic–structures derived from embryogenic calluses obtained from plumule explants were used as explants. After 90 d of culture in medium I, embryogenic callus were produced. The embryogenic–structures are protruding characteristic features of embryogenic coconut calluses and were described by Sáenz et al., (2006) (Figure 1A). This occurred with media supplemented with the BA treatments. After transfer to medium II, these calluses formed several somatic embryos at 30 d of culture (Figure 1B), which developed through a globular stage (Figure 1C) followed by a scutellar stage showing the coleoptiles and the germinative pore (Figure 1D). Embryos on the calluses were already germinating at 60 d (Figure 1E) and later developed into shoots (Figure 1F) and young plantlets (Figure 1G).
The effect of BA (0, 25, 100 or 200/µM in medium I) on the formation of EC was evaluated after 90 d and no significant differences were observed. The control had 50 % of explants forming EC, whereas with 25, 100 or 200 µM BA the responses were 50, 46 and 53 %. From the developmental and morphological point of view, there were no significant differences between BA treatments and the control. Within 90 d of culture in medium I, 50 % of explants formed EC that first developed translucent structures with ear–like shape. Then, pearly globular structures appeared on the surface of translucent structures, and later they became elongated. This developmental pattern was similar to what was reported by Sáenz et al. (2006), who define these elongated structures as embryogenic–structures, since somatic embryos eventually are formed from them. Occurrence of these structures is also found in Elaeís guínenesís (Schwendiman et al., 1990) and Hevea brasílíensís (Michaux–Ferriere and Carron, 1989).
Effect of BA on the formation of somatic embryos
The embryogenic calluses obtained in medium I in presence of BA, were transferred to medium II in order to evaluate the effect of initial BA treatments on the formation of SE over a 150 d of culture. Somatic embryos on embryogenic calluses were observed after 30 d (Figure 2A). The percentages of EC forming embryos ranged from 24 to 36 % for 25 µM and 200 µM BA and control (Figure 2A); afterwards, there were not significant changes. With 100 µM BA the percentage of calluses with embryos was above 50 % and increased up to 72 % at 90 d.
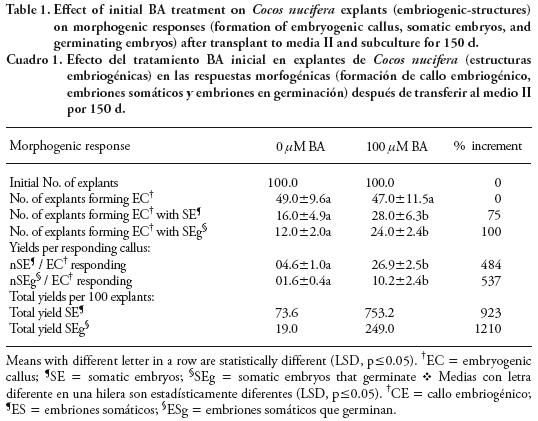
With 100 µM BA, responding calluses had 20 embryos at 30 d (Figure 2B) and it increased to 27 at 150 d. A similar pattern was observed with the other treatments but with lower amounts of embryos per callus: 16 embryos at 150 d with 200 µM BA; 4 embryos at 30 d with 25 /µM BA and the control (Figure 2B). In control the numbers of somatic embryos formed from 100 explants at 150 d were 73.6, but the presence of 100 /µM BA increased the number increased to 753.2 embryos, improving the efficiency in embryo formation by 923 % (Table 1).
Effect of BA on the germination of somatic embryos
Embryogenic calluses with somatic embryos that germinating were observed at 60 d of culture and the percentages were as follows: 20 % at 60 d and 50 % at 150 d for 100 /µM BA; 1 to 10 % at 60 d and about 20 % at 150 d for 25 /µM BA, 200/µM BA and control (Figure 3A). The amount of somatic embryos germinating per EC was as follows: for 100 µM BA, responding calluses had 5 embryos germinating at 60 d (Figure 3B), increasing to 10 at 150 d; for 25 µM BA and the control there were less than 2 embryos germinating at 150 d (Figure 3B); for 100 /µM BA the amount was three–fold that of the other treatments. The total yield of somatic embryos germinating obtained from 100 non treated explants (control) was 19 at 150 d; in contrast, with 100 /µM BA the yield increased to 249 (12 times) (Table 1).
These results differ from reports by Chan et al. (1998) who indicate that the formation of embryogenic calluses required a medium supplemented with 2,4–D alone, because the addition of BA decreased EC formation, and similarly, other cytokinins reduced EC formation (Azpeitia, 2003). Those authors used plumule from the original coconut embryo, whereas in the present study the explants were embryogenic structures excised from calluses obtained after three callus multiplication cycles ín vítro. These contrasting results might be explained by the fact that the original endogenous cytokinin content in the plumule explants would be higher than that of embryogenic–structures. Similarly, a response to BA dependant on the type of explant was observed during the regeneration of Stenotaphrum secundatum; where different concentrations of this cytokinin did not affect the formation of callus in different tissues (early immature embryo, immature embryo and shoot base of young seedlings) used as initial explants; however, the ability to regenerate was dependent of the type of explant used and the initial concentration of BA added to the culture medium (Li et al., 2006).
Although usually 2,4–D alone induces somatic embryogenesis in monocot species (Bhaskaran and Smith, 1990; Krishnaraj and Vasil, 1995), in some species such as Cynodon dactylonX Cynodon transvaalensís (Li and Qu, 2002), Ophíorrhíza prostrata (Martin et al., 2007), and Stenotaphrum secundatum (Li et al., 2006), the use of a low (44 nM–0.22 µM) concentration of BA in the callus induction medium enhances the formation of somatic embryos, but higher (2.22–4.22 µM) concentrations of BA inhibite the embryogenic response (Debeaujon and Branchard, 1993; Martin et al., 2007).
In our study 100 µM of BA did not change the percentage of explants forming callus, but it produced a late effect, improving the efficiency of embryo formation and germination. Such an improvement might be used for the propagation scheme proposed by Pérez–Núñez et al. (2006) to increase yields cumulatively. This is relevant because the focus of research by Chan et al. (1998), Sáenz et al. (2006) and Pérez–Núñez et al. (2006) is the development of an efficient technique to support replanting programs in México, as planned by the National Association of Coconut Farmers (CONACOCO), and based on the use of MPT2 coconut palms.
CONCLUSIONS
The addition of BA (100 µM) in medium I increased the formation and germination of somatic embryos when they are transplanted to medium II containing 6 µM 2,4–D and 300 µM BA, based on the protocol of EC multiplication. These results improved the overall efficiency of the system for the regeneration of coconut palm, which could have a practical application.
ACKNOWLEDGMENTS
The authors would like to thank CONACYT, México, for partial funding for the research reported here (Program grant 43834–Z) and for an scholarship for M. Montero (Grant 183253).
LITERATURE CITED
Azpeitia M, A. 2003. Diferentes estrategias para promover la embriogénesis somática en cocotero (Cocos nucífera L.) a partir de explantes de plúmula. Tesis de Doctorado. Centro de Investigación Científica de Yucatán, México. 130 p. [ Links ]
Azpeitia, A., J. L. Chan, L. Sáenz, and C. Oropeza. 2003. Effect of 22(S), 23(S)–homobrassinolide on somatic embryogenesis in plumule explants of Cocos nucífera (L.) cultured ín vítro. J. Hort. Sci. Biotechnol. 78: 591–596. [ Links ]
Bhaskaran, S., and R. H. Smith. 1990. Regeneration in cereal tissue culture: a review. Crop Sci. 30: 1328–1336. [ Links ]
Chan, J. L., L. Sáenz, C. Talavera, R. Hornung, M. Robert, and C. Oropeza. 1998. Regeneration of coconut (Cocos nucífera L.) from plumule explant through somatic embryogenesis. Plant Cell Rep. 17: 515–521. [ Links ]
Debeaujon, I., and M. Branchard. 1993. Somatic embryogenesis in Cucurbitaceae. Plant Cell Tissue Organ Cult. 34: 91–100. [ Links ]
Eeuwens, C. J. 1976. Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucífera L.) and cultured ín vítro. Physiol. Plant. 36: 23–28. [ Links ]
Gaj, M. D. 2004. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabídopsís thalíana (L.) Heynh. Plant Growth Regul. 43: 27–47. [ Links ]
Harrison, N. A., and C. Oropeza. 2008. Phytoplasmas associated with coconut lethal yellowing. In: Harrison N. A., G. P. Rao, and C. Marcone (eds). Characterization, Diagnosis and Management of Phytoplasmas. Studium Press LLC, Houston, USA pp: 219–248. [ Links ]
Krishnaraj, R. S., and I. K. Vasil. 1995. Somatic embryogenesis in herbaceous monocots. In: Thorpe T. A. (ed). In vítro Embryogenesis in Plants. Dordrecht, The Netherlands, Kluwer Academic Publishers pp: 471–540. [ Links ]
Li, L., and R. Qu. 2002. In vítro somatic embryogenesis in turf–type bermudagrass: roles of abscisic acid and gibberellic acid, and occurrence of secondary somatic embryogenesis. Plant Breed. 121: 155–158. [ Links ]
Li, R., A. H. Bruneau, and R. Qu. 2006. Improved plant regeneration and ín vítro somatic embryogenesis of St Augustinegrass [Stenotaphrum secundatum (Walt.) Kuntze]. Plant Breed. 125: 52–56. [ Links ]
Martin, K. P., S. Beegum, C. L. Zhang, A. Slater, and P. V. Madhusoodanan. 2007. In vítro propagation of Ophíorrhíza prostrata through somatic embryogenesis. Biol. Plant. 51: 769–772. [ Links ]
Michaux–Ferriere, N., and M. P. Carron. 1989. Histology of early somatic embryogenesis in Hevea brasiliensís: The importance of the timing of subculturing. Plant Cell Tissue Organ Cult. 19: 243–256. [ Links ]
Perera, P. I. P., V. Hocher, J. L. Verdeil, S. Doulbeau, D. M. D. Yakandawala, and L. K. Weerakoon. 2006. Unfertilized ovary: a novel explant for coconut (Cocos nucífera L.) somatic embryogenesis. Plant Cell Rep. 26: 21–28. [ Links ]
Pérez–Nuñez, M. T., J. L. Chan, L. Sáenz, T. González, J. L. Verdeil, and C. Oropeza. 2006. Improved somatic embryogenesis from Cocos nucífera (L.) Plumule explants. In Vítro Cell Dev. Biol. Plant 42: 37–43. [ Links ]
Raju, C. R., P. Kumar, M. Chandramohan, and R. D. Lyer. 1984. Coconut plantlets from leaf tissue cultures. Plant Crop 12: 75–81. [ Links ]
Sáenz, L., A. Azpeitia, B. Chuc–Armendariz, J. L. Chan, J. L. Verdeil, V. Hocher, and C. Oropeza. 2006. Morphological and histological changes during somatic embryo formation from coconut plumule explants. In Vitro Cell. Dev. Biol. Plant 42: 19–25. [ Links ]
Schwendiman, J., C. Pannetier, and N. Michaux–Ferriere. 1990. Histology of embryogenic formation during ín vítro culture of oil palm Elaeís guíneensís Jacq. Oléagineux 45: 409–415. [ Links ]
Tan, R. R., A. B. Culaba, and M. R. I Purvis. 2004. Carbon balance implications of coconut biodiesel utilization in the Philippine automotive transport sector. Biomass & Bioenergy 26: 579–585. [ Links ]
Verdeil, J. L., C. Huet, F. Grosdemange, and J. Buffard–Morel. 1994. Plant regeneration from cultured immature inflorescence of coconut (Cocos nucífera L.): evidence for somatic embryogenesis. Plant Cell Rep. 3: 218–221. [ Links ]
Zizumbo, D., M. Fernandez, N. Torres, and R. Cardeña. 1999. Lethal yellowing resistance in coconut germplasm from México. In: Oropeza C., J. L. Verdeil, R. Asburner, R. Cardeña, and J. M. Santamaría (eds). Current Advances in Coconut Biotechnology. Kluwer Academic Publishers, Dordrecht, The Netherlands pp: 131–143. [ Links ]














