Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.45 no.3 Texcoco abr./may. 2011
Biotecnología
Photosynthetic characteristics and growth of alginate-immobilized Scenedesmus obliquus
Características fotosintéticas y crecimiento de Scenedesmus obliquus inmovilizada en alginato
Alejandro Ruiz-Marín1*, Leopoldo G. Mendoza-Espinosa2, M. del P. Sánchez-Saavedra3
1 Universidad Autónoma de Ciudad del Carmen. 24180. Calle 56 #4. Avenida Concordia. Ciudad del Carmen, Campeche, México. (aruiz@pampano.unacar.mx). *Author for correspondence.
2 Oceanographic Research Institute, Universidad Autónoma de Baja California. 22800. Km. 107 Tijuana-Ensenada Road, Baja California, México. (lmendoza@uabc.mx).
3 Departamento de Acuicultura, Centro de Investigación Científica y de Educación Superior de Ensenada. Carretera Ensenada a Tijuana 3918, Zona Playitas, Ensenada, Baja California, México. (psanchez@cicese.mx).
Received: July, 2010.
Approved: February, 2011.
Abstract
The micfoalgae Scenedesmus obliquus was immobilized in Ca-alginate beads at two stocking cell densities (6.7 X105 and 1.5X 106 cell bead-1). The photosynthetic fate (P), the initial slope of the photosynthesis (α), and the threshold fot irradiance-saturated photosynthesis (Ek) were determined; later, the growth and protein content for S. obliquus immobilized in beads-alginate under two light intensities (135 and 200 μE m -2 s -1) was evaluated using stocking cell densities that had previously presented the largest photosynthetic rate. Results showed than photosynthetic rates (P) and α of cells immobilized in beads at low stocking density (0.14 μmol O2 h-1 10-6 cells and 0.00056) were greater than in beads with high stocking density (0.02 μmol O2 h-1 10-6 cells and 5X10-5). Therefore, the beads at low stocking density were selected to be cultured under two light intensities (135 and 200μE m-2 s-1). Both irradiances showed no significant differences on growth rates (0.157 d-1 and 0.172 d-1) and protein content (15-16 % of dry-weight biomass), which represents only around 5.14-4.73 mg L -1 as N-protein of the total nitrogen removed from medium. This suggests that the light intensity within the limitation area described in the waves P-I did not affect the growth and content of protein when low cells stocking density beads are used. Therefore, it was concluded that the light intensities selected in the present study not had significant effects in the growth and protein content in beads with low cells stocking.
Key words: Scenedesmus obliquus, photosynthesis, immobilized cells protein content.
Resumen
La microalga Scenedesmus obliquus fue inmovilizada en esferas de Ca-alginato en dos densidades celulares del cultivo (6.7X105 y 1.5X106 células esferas - 1). Se determinó la tasa fotosintetica (P), así como la pendiente inicial de la fotosíntesis (a), y el nivel de saturación de irradiación-fotosíntesis (Ek); después, el crecimiento y contenido de proteína para S. obliquus, inmovilizada en esferas de alginato bajo dos intensidades de luz (135 y 200 μE m -2 s -1), fue evaluado usando densidades celulares del cultivo que previamente presentaron una mayor tasa fotosintética. Los resultados muestran que las tasas fotosintéticas (P) y a de células inmovilizadas en esferas a una densidad celular baja (0.14 μmol O2 h - 1 10 - 6 células y 0.00056) fueron mayores que en esferas con alta densidad celular (0.02 μmol O2 h -1 10 -6 células y 5X 10 5). Por tanto, se seleccionaron las esferas con baja densidad celular para ser cultivadas en dos intensidades de luz (135 y 200 μE m -2 s - 1). Las dos irradiancias no mostraron diferencias significativas en las tasas de crecimiento (0.157 d- 1 y 0.172 d- 1) y contenido de proteína (15-16 % de la biomasa peso seco), lo que representa sólo alrededor de 5.14-4.73 mg L -1 como N-proteína del total de nitrógeno removido del medio. Esto sugiere que la intensidad de la luz dentro de la zona de limitación observada en curvas P-I no afectó el crecimiento y contenido de proteína cuando se usaron esferas de baja densidad celular. Por tanto, se concluyó que las intensidades de luz seleccionadas en el presente estudio no tuvieron efectos significativos en el crecimiento y contenido de proteína en las esferas de baja densidad celular.
Palabras clave: Scenedesmus obliquus, fotosíntesis, contenido de proteína de células inmovilizadas.
Introduction
Algal cultures have been extensively used for the tertiary treatment of wastewater (Lavoie and de la Noüe, 1985) and, under certain conditions, they may also be used for secondary treatment as an alternative to activated sludge (Tam and Wong, 2000). A topic of interest is the utilization of algal biomass, but this depends on its biochemical composition, which is affected by the nutrients concentration and culture medium composition, as well as, temperature, light intensity and wavelength. These can be manipulated during the stages of culture to improve the biomass production and biochemical composition (Sánchez-Saavedra and Voltolina, 2002). Changes in light intensity would result in variations in the pigment composition, concentrations of the components of electron transport chains, carboxylic enzyme activities, photosynthetic rates, dark respiration rates and biochemical composition (Bartual et al., 2002). Each species is usually characterized by a maximum growth rate under ideal conditions of growth (Bartual et al., 2002; Bouterfas et al., 2002). Therefore, it is important to determine and use optimized culture conditions for the correct interpretation of the experimental results.
Light is an important variable in the design and operation of microalgae culture systems and bioreactors (Andersen, 2005). The light limitation effect in immobilized microalgae increases inside the beads as the cellular density increases with time, suggesting that microalgae located in the center of the beads have limited access to light and, as a consequence, a normal physiological activity cannot be maintained (Chevalier and de la Noüe, 1985; Tam et al., 1994).
In immobilized cells cultures, light intensities commonly reported are within the range of 95-174 μE m -2 s -1, where approximately 90-95 % of nitrogen can be removed from artificial and urban wastewater (Kaya et al., 1996; Tam and Wong, 2000; Jiménez-Pérez et al., 2004). However, the optimum light intensity in immobilized cultures has not been reported, as growth and content of proteins within the matrix can exist, due to light availability. Light limitation by the self-shading effect is one of the main causes affecting algae growth and their protein content in immobilized systems (Pane et al., 1998). Therefore, in immobilized systems it is important to determine the appropriate amount of light in order to reach maximum levels of biomass, nutrients removal and protein content.
It is possible to reach high contents of chlorophyll and high photosynthetic rates for immobilized cells in alginate for species such as Chlorella sp. (Robinson et al., 1986), Botryococcus braunii (Bailliez et al., 1986) and Chlamydomonas reinhardtii (Vilchez and Vega, 1994). Jeanfils and Collar (1983) evaluated the oxygen evolution for the microalgae Scenedesmus obliquus alginate immobilized to one light intensity (60 W m -2), and reported a similar photosynthetic rate for free and immobilized cells (200 and 210 μmol O2 h -1 10 -9 cells), concluding that the chlorophyll-protein complexes were not affected by the immobilization.
However, there are few studies about the photosynthetic characteristics of S. obliquus and the relation between light intensity and growth, nitrogen removal and proteins content. Therefore, the purpose of this study was to evaluate the rate (P) and photosynthetic efficiency (α) in two types of cultures: 1) high and low stocking cell density in alginate beads; 2) the biomass production and protein content of S. obliquus immobilized in alginate in cultures under two light intensities within the range of light limitation.
Materials and Methods
Routine of culture
Stock suspension of S. obliquus was obtained from the culture collection of the Centro de Investigación y de Educación Superior de Ensenada (CICESE), Baja California, México. The cells were routinely cultured in artificial wastewater under non-axenic conditions in the Water Quality Laboratory of the Institute of Oceanographic Research (Laboratorio de Calidad del Agua del Instituto de Investigaciones Oceanológicas-IIO). The composition of the artificial wastewater (Aw) was 7 mg L - 1 NaCl, 4 mg L- 1 CaCl2, 2 mg L- 1 MgSO4.7H2O, 15 mg L- 1 KH2PO4, and 115 mg L- 1NH4Cl in purified water.
Artificial wastewater concentrations simulating the mean values of the secondary effluent from the Universidad Autónoma de Baja California (UABC) campus in Ensenada wastewater treatment plant prepared to reach the following concentrations: N-NH+4: 32.5 mg L- 1; N-NO - 3: 2.0 mg L- 1; P-PO - 3: 2.5 mg L -1. Trace metals and vitamins were added according to the guidelines for medium f/2 (Guillard and Ryther, 1962). Cultures were kept at 26 ±1 °C and illuminated continuously with white fluorescent tubes (135 μE m -2 s -1).
Immobilization method
Prior to immobilization, the stock suspension of microalgae was centrifuged at 2500 rpm for 15 min and the cell pellets were washed twice with distilled water to remove the residual nutrients that might adhere to the cell surface. The cells were re-suspended in 50 mL distilled water to form a concentrated algal suspension of 10 X107 cells mL -1 and mixed with 50 mL of sodium alginate to yield mixtures of 2 % (w/v) Na-alginate-algal suspension. About 6500 beads of calcium alginate (2.5 mm diameter) with concentration of 6.7X105 cell bead -1 and 1.5X106 cell bead -1 were formed after 100 mL of Na-alginate-algal suspension were titrated into a 2 % (w/v) CaCl2 solution. The calcium alginate beads were prepared with the method described by Tam and Wong (2000).
Photosynthetic rate estimation
Photosynthetic rates (P) of immobilized cell cultures of S. obliquus were estimated with the oxygen evolution method using a Clark-type oxygen electrode (Yellow Spring Instruments, 5221, Ohio, USA) in a 7 mL custom-made Plexiglas chamber at 28 °C and under 8 light intensities ranging from 30 to 2490 μE m2 s1. Two types of beads were prepared, one with stocking cell density of 6.7X105 cell bead -1 (Low density) and the other with stocking cell density of 1.5 X106 cell bead -1 (High density). For both experiments, beads were incubated and harvested during the exponential growth phase of S. obliquus for the photosynthetic rates estimation.
Ten beads and stock suspensions of S. obliquus were incubated by triplicate and independently placed in the Plexiglas chamber with artificial wastewater (Aw) after 15 min of pre-incubation in darkness. Maximum oxygenic photosynthesis (Pmax), the initial slope of the photosynthesis (α), and the threshold for irradiance-saturated photosynthesis (Ek) were determined by a non-linear direct fitting algorithm of the data to the exponential equation (Prioul and Chartier, 1977). The P vs. I curve indicated the specific irradiance that allows a maximum photosynthetic rate without reaching photo-inhibition. The changes in Pmax ratio in relation to the initial cellular density within beads allowed to reach the best conditions for the immobilized systems.
Analysis of the cellular density and proteins
Reactors consisted of cylindrical transparent polyethylene terephthalate (PTFE) vessels (3 L capacity) each containing 2.5 L of Aw and approximately 6500 beads with the initial cell density previously selected from the irradiance-photosynthesis curves. Cultures of immobilized S. obliquus were maintained under two light intensities within the range of light-limitation zone. Each reactor was operated with a beads concentration of approximately 2.6 beads mL -1 of wastewater maintained at 25 ±1 °C and continuous illumination. All experiments were run in triplicate. The beads in each reactor were kept in suspension and mixed by means of small air diffusers through which compressed air filtered through an activated carbon filter was introduced.
Every 6 h the numbers of cells in the beads were counted with a particle analyzer model Beckman Coulter Multisizer 3, after dissolving one bead in 5 mL of 0.25 M Na2HPO4.7H2O solution (pH 7.0) in triplicate. For the determination of ash-free dry-weight biomass in beads, five beads in triplicate were dissolved in 5 mL of 0.25 M Na2HPO4.7H2O solution (pH 7.0) and filtered through a Whatman GF/C glass fiber filter (2.5 cm diameter) previously rinsed with distilled water, and incinerated at 450 °C for 3 h to constant weight. The samples were dried in a conventional oven at 120 °C for 2 h to constant weight, and placed at 450 °C in a muffle furnace (Sorokin, 1973). The same procedure was used to measure the protein content of the algal biomass (Lowry et al., 1951). Proteins were analyzed using a standard of bovine albumin (98 %). Protein extraction was undertaken with a NaOH 1N solution at 100 °C and an extraction time of 90 min.
Statistical analyses
The photosynthetic rate data in the experiments with two cellular densities in alginate beads were analyzed by analysis of variance (ANOVA). ANOVA was also used to evaluate the growth and protein content in cultures under two light intensities (StatSoft Inc., Tulsa, OK, USA). The Tukey test (p≤0.05) was applied when results showed significant differences. As mentioned earlier, experiments were run in triplicate.
Results and Discussion
Activity of immobilized algae
The photosynthetic rates reached for high and low stocking density of immobilized S. obliquus were significantly different (p≤ 0.004). It was observed that the photosynthetic rates for immobilized S. obliquus at low density increased until 0.14 μmol O2 h -1 10 6 cells with respect to the irradiance followed by a short saturation zone after 300 μE m s and the decrease of the photosynthesis rate as a result of photo-inhibition over 500 μE m -2 s -1 (Figure 1). This may be caused by the previous adaptation of S. obliquus to low light intensities as a result of the light attenuation caused by the matrix or to the self-shadow effect (Platt and Jassby, 1976). Although fast light saturation was found at low stocking density, the initial slope (α) was higher (p≤ 0.05) than beads at high stocking density, suggesting that cells immobilized at low stocking density had higher physiological activity (Table 1). Jeanfils and Collar (1983) reported for S. obliquus alginate immobilized a low photosynthetic rate (210 μmol O2 h -1 10 -9 cells) compared to the present study. This difference is attributed to the fact that the photosynthetic rate was estimated to a light intensity (60 W m -2) and not for a maximum obtained to different intensities (photosynthetic curve) as reported in the present study.
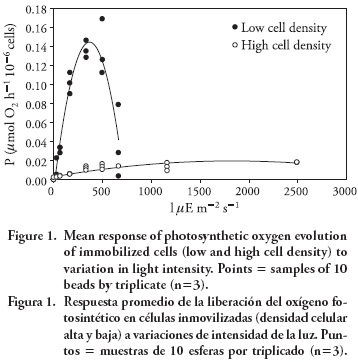
Beads with high cell density showed an increase of the photosynthetic rate with a maximum of 0.02 μmol O2 h - 1 10 - 6 cells at 500 μE m - 2 s - 1, which was followed by a longer saturation zone between 600-2500 μE m -2 s -1 without reaching photo-inhibition. This could be advantageous, because the cultures can work with a wide range of intensity of light in tropical regions. However, in immobilized cells this can represent a disadvantage, as the high cell density would cause light limitation within beads followed by lower growth and nutrients removal capacity. Chevalier and de la Noüe (1985) reported that a greater number of cells within the beads reduce the nutrient removal efficiency and algal growth due to the self-shading effect. It seems as if shading effects could be overcome by increasing light on the surface, yet high irradiance causes inhibition of photosynthesis, which is not desirable in immobilized cultures.
Results in the present study suggest that cellular activity of immobilized cells decreases as stocking density increases, as noted by the lower photosynthetic rate (P) and Ej obtained in cultures with high cell density beads (Table 1). Similar results were reported by Tam et al. (1994) and Robinson et al. (1985) for immobilized Chlorella vulgaris; both authors suggest that the cellular metabolic activity of immobilized Chlorella cells decreased as the cellular density increases.
In the analysis of P-I curves it is important to take into account the conditions of the culture, such as high cell density cultures, which may cause considerable light attenuation. The photosynthetic variables obtained for immobilized S. obliquus in the present study indicated a higher physiological activity for low stocking density beads than those reached in cultures with high cell density beads. On the basis of these considerations, the growth and protein content using beads with low cellular density cultivated under two intensities of light (135 and 200 μE m -2 s -1), presented the highest photosynthetic rates and, thus, were selected be further tested.
Growth
The growth of immobilized S. obliquus measured in terms of cell density by bead increased gradually with time from 3.5 X105 to 8X105 cells beads-1 at 200 μE m -2 s -1 in 2 d of culture. This cellular concentration was higher than that obtained at 135 UE m-2 s-1 from 3.5X 105 to 6.5 X105 cells beads-1. This suggests that after immobilization the microalga S. obliquus was still able to undergo cell division and carry out photosynthesis (Figure 2).
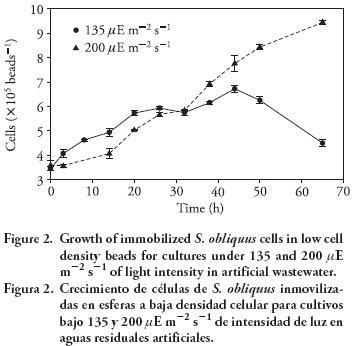
Both treatments showed immediate growth after the beads were added to the medium, unlike other studies where immobilized cells showed longer lag periods compared with free cells as reported by Chevalier and de la Noüe (1985) and Lau et al. (1997). The light intensity used in the present study (135 and 200 μE m -2 s -1) did not cause significant differences on growth rates (p≥0.05), within values of 0.157 d -1 and 0.172 d -1. A similar trend was reported by Bartual et al. (2002) for cultures of Rhodomonas saline under intensities between 15 to 320 μE m -2 s -1.
Both growth rates were lower than those reported for S. intermedius of 0.336 d -1 (Jimenez-Perez et al., 2004) and 1.08 d -1 (Chevalier and de la Noüe, 1985). This difference could probably be due to the different conditions of the cultures. Studies of Chlamydomonas reinhardtii have shown that in free cultures the growth rate and photosynthetic activity depend on the amount of light received by the culture (León and Galván, 1997). However, in the present study it was observed that the irradiance ranges matched the light levels used for the culture of this genus of microalgae (Kaya et al., 1996; Tam and Wong, 2000; Jimenez-Perez et al., 2004). A valid consideration is that if nutrients are not limiting and physiological conditions are optimal, then the photosynthetic activity is controlled only by light intensity. In the study of immobilized cells, the high activity associated to the high content of chlorophyll is related to the limitation of the light in the beads. However, it can be observed that in contrast to free-cell cultures, the appropriate light intensity obtained within the light limitation zone (P-I curves) does not guarantee a high biomass production and protein content. Other factors such as CO2 and temperature should be taken into consideration.
Protein content
The protein content (15-16 %) obtained in the cultures under two light intensities did not show significant differences (p> 0.125). The gradual proteins increase with time indicated that the growth and production of proteins began immediately after adding the beads to the culture medium, showing that the microalgae S. obliquus was maintained in exponential phase (Figure 3).
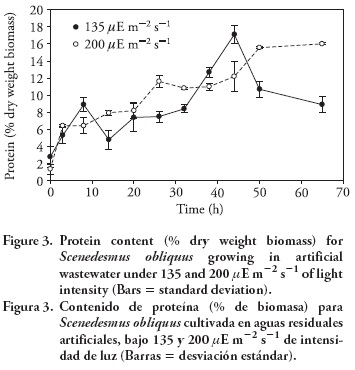
Ruiz-Marín and Mendoza-Espinosa (2008) showed that some ammonium can be removed by ammonia volatilization (17.6 mg L -1) as a result of the high pH (9.0-9.5) observed in similar systems. The pH did not seem to interfere with algal growth. Taking into consideration the amount of ammonium at the end of the 42 h of cultivation (6.56 and 7.03 mg L -1) it appears that only around 5.84 and 5.37 mg L -1 of the total nitrogen removed from the medium under light conditions 135 and 200 μE m-2 s-1 was incorporated as N-protein by the microalgae. For S. obliquus the amount of non-protein nitrogen (expressed as a portion of total nitrogen) is 12 % (Becker et al., 1976), yet the N-protein in the present study accounted for 5.14-4.73 mg L -1 which are lower than the values reported by Nuñez et al. (2001) for free S. obliquus cultures.
Under the light intensities selected in the present study there were no significant effects of light intensities on the growth and protein content of S. obliquus. In order to increase protein content, other factors such as CO2 level, temperature and pH should be tested if the aim is to use the biomass as food supplement.
Conclusions
The photosynthetic parameters obtained for immobilized S. obliquus in the present study indicated a higher physiological activity for low cell density beads than those reported for cultures with high cell density beads. The growth and protein content using beads with low cellular density cultivated under two intensities of light, which corresponded to the zone of the highest photosynthetic rate, showed a high response to light intensity at 200 μE m -2 s -1.
The high activity associated to the high content of chlorophyll is related to the limitation of the light in the beads. However, in contrast to free-cell cultures, the appropriate light intensity obtained within the light limitation zone (P-I curves) did not cause a high biomass production and protein content. Both irradiances showed that only around 5.14-4.73 mg L -1 as N-protein of the total nitrogen removed from the medium. Therefore, light intensities not have significant effects on the growth and protein content in beads with low cell density cultures.
Acknowledgements
This study was partially supported by the Universidad Autónoma de Baja California (Autonomous University of Baja California) internal project no. 568. A scholarship by the Mexican Department of Education (SEP) granted to Alejandro Ruiz Marin is gratefully acknowledged.
Literature cited
Andersen, R., A. 2005. Algal Culturing Techniques. Phycological Society of America. Elsevier Academic Press Amsterdam. pp: 578-600. [ Links ]
Bailliez, C., C, Largeau., C, Berkaloff, and E, Casadevall. 1986. Immobilization of Botryococcus braunii in alginate: influence on chlorophyll content, photosynthetic activity and degeneration during batch cultures. Appl. Microbiol. Biotechnol 23: 361-366. [ Links ]
Bartual. A., L. M, Lubián., J. A, Gálvez, and F. X, Niell. 2002. Effect of irradiance on growth, photosynthesis, pigment content and nutrient consumption in dense cultures of Rhodomonas salina (Wislouch) (Cryptophyceae). Ciencias Marinas 28: 381-392. [ Links ]
Becker, E. W., L. V, Venkataraman, and P. M, Khanum. 1976. Effects of different methods of processing on the protein efficiency ratio of the green alga Scenedesmus acutus. Nutr. Reports. Int. 14: 305-319. [ Links ]
Bouterfas, R., M. Belkoura, and A. Dauta. 2002. Light and temperature effects on the growth rate of three freshwater algae isolated from a eutrophic lake. Hydrobiology. 489: 207-217. [ Links ]
Chevalier, P., and J, de la Noüe. 1985. Efficiency of immobilized hyperconcentrated algae for ammonium and orthophosphorus removal from wastewater. Biotechnol Lett. 7: 395-400. [ Links ]
Guillard, R. L. L., and J. H, Ryther. 1962. Studies on marine plancktonic diatoms Cycloterlla nana Hustedt and Detonula Confervacea (Cleve). Gran Can. J. Microbiol. 8: 229-239. [ Links ]
Jeanfils, J., and F. Collar. 1983. Effect of immobilizing Scenedesmus obliquus cells in a matrix on oxygen evolution and fluorescence properties. Eur. J. Appl. Microbiol. Biotechnol. 17: 254-257. [ Links ]
Jiménez-Pérez, M. V., P. Sánchez-Castillo, O. Romera, D, Fernández-Moreno, and C. Pérez-Martínez. 2004. Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb. Technol. 34 : 392-398. [ Links ]
Kaya, V. M., J. Goulet., J. de la Noüe., and G. Picard. 1996. Effect of intermittent CO2 enrichment during nutrient starvation on tertiary treatment of wastewater by alginate-immobilized Scenedesmus bicellularis. Enzyme Microb. Technol. 18: 550-554. [ Links ]
Lau, P. S., N. F. Y. Tam, and Y. S. Wong. 1997. Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environ. Technol. 18: 945-951. [ Links ]
Lavoie, A., and J. de la Noüe. 1985. Hyperconcentrated cultures of Scenedesmus obliquus: a new approach for wastewater biological tertiary treatment. Wat. Res. 19: 1437-1442. [ Links ]
León, R., and F. Galván. 1997. Short communication: Analysis of effective light in different photobioreactors: its influence on growth, photosynthetic activity and glycerol production by the freshwater green alga Chlamydomonas reinhardtii. World J. Microbiol. Biotechnol. 13: 237-239. [ Links ]
Lowry, O. H., N. J. Rosebrough., A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275. [ Links ]
Nuñez, J. V., D. Voltolina, M. Nieves, P. Piña, A. Medina, and M. Guerrero. 2001. Nitrogen budget in Scenedesmus obliqqus cultures with artificial wastewater. Biores Technol. 78: 161-164. [ Links ]
Pane, L., M. Feletti, C. Bertino, and A. Carli. 1998. Viability of the marine microalgae Tetraselmis suecica grown free and immobilized in alginate beads. Aquaculture Int. 6: 411-420. [ Links ]
Platt, T., and A. D. Jassby. 1976. The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. J. Phycol. 12: 421-430. [ Links ]
Prioul, J. L., and P. Chartier. 1977. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: a critical analysis of the methods used. Ann. Bot. 41: 189-800. [ Links ]
Robinson, P. K., A. L. Dainty, K. H. Goulding., I. Simpkins., and M. D. Trevan. 1985. Physiology of alginate immobilized Chlorella. Enzyme Microb. Technol. 7: 212-216. [ Links ]
Robinson, P. K., K. H. Goulding, A. L. Mak, and M. D. Trevan. 1986. Factors affecting the growth characteristics of alginate-entrapped Chlorella. Enzyme Microb. Technol. 8: 729-733. [ Links ]
Ruiz-Marin, A., and L. Mendoza-Espinosa. 2008. Ammonia removal and biomass characteristic of alginate-immobilized Scenedesmus obliquus cultures treating real wastewater. Fresenius Environ. Bull. 17(9a): 1236-1241. [ Links ]
Sánchez-Saavedra, M. P., and D. Voltolina. 2002. Effect of photon fluence rates of white and blue-green light on growth efficiency and pigment content of three diatom species in batch cultures. Ciencias Marinas. 28(3): 273-279. [ Links ]
Sorokin, C. 1973. Dry weigh, packed cell volume and optical density. In: Stein, J. R. (ed). Handbook of Phycological Methods. Culture Methods and Growth Measurement. New York, Cambridge University Press: 440 p. [ Links ]
Tam, N. F. Y., P. S. Lau, and Y. S. Wong. 1994. Wastewater inorganic N and P removal by immobilized Chlorella vulgaris. Wat. Sci. Technol. 30: 369-374. [ Links ]
Tam, N. F. Y., and Y. S. Wong. 2000. Effect of immobilized microalgal bead concentrations on wastewater nutrient removal. Environ. Pollut. 107: 145-151. [ Links ]
Vilchez, C., and J. M. Vega. 1994. Nitrite uptake by Chlamydomonas reinhardtii cells immobilized in calcium alginate. Appl. Microbiol. Biotechnol. 41: 137-141. [ Links ]














