Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.44 no.7 Texcoco oct./nov. 2010
Fitociencia
Application of low concentrations of salicilyc acid increases the number of flowers in Petunia hibrida
Bajas concentraciones de ácido salicílico incrementa el número de flores en Petunia híbrida
Rodolfo Martín–Mex, Silvia Vergara–Yoisura, Ángel Nexticapán–Garcés, Alfonso Larqué–Saavedra*
Centro de Investigación Científica de Yucatán A.C. Calle 43 No. 130, Chuburná de Hidalgo, 97200, Mérida, Yucatán, México. * Autor responsable: (larque@cicy.mx)
Received: October, 2009.
Approved: June, 2010.
Abstract
The effect of applications of low concentrations of salicylic acid (SA) on the number of flowers and the date of flower initiation in Petunia (Petunia híbrida) in reported in this paper. Concentrations of 1 μM to lpM of SA were spread on three occasions to the shoot of plantlets cultivated in greenhouse conditions. Analysis of the results showed that all the concentrations of SA tested increased the number of open flowers per plant. Concentrations as low as lpM or 0.lnM induced positive riesponses by 33 % and 37 %, as compared with that of the control. The highest concentration of 1 μM increased not only the number of flowers by 72 % but also induced early flowering by six days.
Key words: flowering, low concentrations, petunia, salicylic acid.
Resumen
Se reporta el efecto de aplicaciones de bajas concentraciones de ácido salicílico (AS) en el número de flores y la fecha del inicio de floración en petunia (Petunia híbrida). Se asperjaron concentraciones de 1 μM a lpM de AS en tres ocasiones, a plántulas cultivadas en condiciones de invernadero. Los análisis de los resultados mostraron que todas las concentraciones probadas de AS incrementaron el número de flores abiertas por planta. Concentraciones tan bajas como de lpM o 0.lnM indujeron respuestas positivas en 33 % y 37 %, en comparación con el testigo. La concentración más alta, de 1 μM, aumentó no sólo el número de flores en 72 %, sino también indujo la floración seis días antes.
Palabras clave: floración, bajas concentraciones, petunia, ácido salicílico.
INTRODUCTION
Since 1975, it was reported that applications of salicylates induce physiological responses in plants, such as stomata closure (Larqué–Saavedra and Martin–Mex, 2007). Further work demonstrated that salicylic acid (SA) should be considered as a growth regulator (Raskin, 1992). Among other plant responses, SA has been reported to affect various physiological processes such as photosynthesis, plant growth, nitrate metabolism, ethylene production, mineral nutrients, heat production (Hayat et al. 2007); increase the biomass of soya (Glycine max) and pine (Pinus patula) (Gutiérrez et al. 1998; San Miguel et al. 2003); increase, the somatic embryogenesis in tissue cultures (Luo et al. 2001; Quiroz–Figueroa et al. 2001); induction of abiotic stress tolerance in potato (Solatium tuberosum), bean and tomato (Licopersicum esculentum) plants (López–Delgado et al. 2004; Senaratna et al. 2000); and UV protection (Mahdavian et al. 2008). Besides, it is important in the activation of stress defense genes and in oxidative and calcium signaling (Holuigue et al. 2007; Kawano and Furuichi, 2007). High levels of endogenous SA have been identified as an important factor in the acquired systemic resistance (ASR) in several species (Shah, 2003).
Agronomists and ecologists know that stress conditions induce early flowering in certain species of plants described as avoiders. Stress stimulates the accumulation of fitohormones such as abscisic acid, ethylene and other metabolites such as salicylic acid that have been involved in the flowering process (Larqué–Saavedra and Wain, 1974; Raskin, 1992). Martinez et al. (2003) in Arabidopsis have began to explain at the molecular level how UV–C light stress activates via SA the flowering in Arabidopsis thaliana.
The effect of application of SA in flowering was reported by Cleland and Tanaka (1979) who found that this hormone could substitute the photoperiod stimulus in Lemma gibba, a long day plant. This finding was considered important in our line of research on salicylates. In a previous works it was found that African violet (Saintpaulia ionantha) a long day plant treated with 0.1 nM of SA increased the number of flowers by 75 % (Martin–Mex et al., 2005). The present report was carried out to test the hypothesis that application of low concentrations of SA affects the flowering of the long day ornamental plant petunia (Petunia híbrida).
MATERIALS AND METHODS
Seeds of Petunia plants (Petunia híbrida) cv "Madness White" (Ball Seed, Inc.) were sown into plug–trays (volume of each cell 40 mL) containing peat moss. These were watered and held for seed germination. At emergence, uniform seedlings were transferred and cultivated in 370 mL pots, under greenhouse conditions with average day and night temperatures of 30 °C and 19 °C, and under natural conditions of light (800 mmol m–2 s–1) with a 11/13 h day/ night photoperiod. A mixture of soil was used as a substrate (Sunshine Mix # 1®, Sun Gro Horticulture, Bellevue, Wash, and Redi earth ®Scotts–Sierra Horticultural Products Company). The plants were kept in well water conditions and fertilized weekly via the irrigation system with 130 mg L–1 of nitrogen, phosphorous and potassium (Haifa Chemicals Ltd.)– The experiment was carried out at the Scientific Research Center of Yucatán in Mérida, México, from August to December, following the regular cultural techniques and methods for ornamental plants.
The salicylic acid solutions (Merck, Co.) were applied as treatments at concentrations of 1.0, 0.01, 0.0001 and 0.000001 μM, and water as a control. Tween–20 was added to the solution as surfactant. All treatments were sprayed simultaneously to the shoot of plants until runoff at 6 a.m. The applications were carried out at 27, 34 and 41 d of plant age. The initiation of flowering was evaluated over the subsequent 18 weeks after the first applications of the solution by counting the number of fully open flowers (corolla fully open) exposed.
The experimental design was completely randomized with five replicates per treatment. Data from the experiment were analyzed with ANOVA (Tukey, p≤0.05) using SAS (2003).
RESULTS AND DISCUSSION
Results showed that applications of salicylic acid (SA) have a significant effect on the number of flowers per plant at the concentrations evaluated (Figure 1). The curve pattern registered indicates that the best treatment was 1 μM which increased the number of flowers by 72 %, in comparison with that of the control, while the treatments of 0.01 μM, 0.lnM, and lpM SA did by 58 %, 37 % and 33 %. Flowering initiated the third week after the first spraying and by the fifth week, the treatment of 1 μMSA presented 20 flowers per plant, while the control had only 9 (Figure 2).

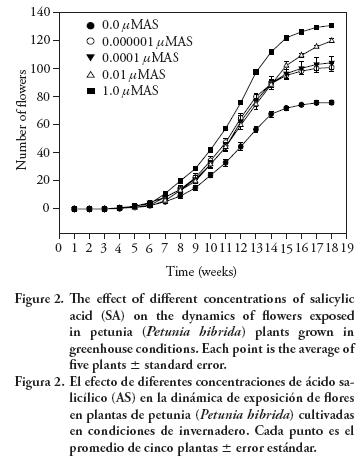
The data of this experiment confirm that applications of low concentrations of SA to the shoot of seedlings affect the flowering of ornamental plants. However the sensitivity of plants to SA concentrations when compared with data recorded on similar experiments with two other ornamental plants is different. African violet required only 0.lnM to increase the number of flowers per plant, gloxinia 0.01 μMof SA and Petunia 1 μMof SA. It must be said, however, that in the three species studied SA induce early flowering (Martin–Mex et al., 2005).
The results of the present experiment agree with our previous reports that there is a great sensitivity of plant tissues to applications of low concentrations of SA. Transformed roots as well as somatic embryogenesis bioassay systems and the data of the present report suggest that it might be a complex mechanism of action of SA to explain such responses (Quiroz–Figueroa et al. 2001; Martin–Mex et al., 2005; Echevarria–Machado et al. 2007).
The results of the present report with petunia, a long day plant, correlate with the work reported by Cleland and Tanaka (1979) with Lemna gibba, where application of SA overcome the demand of photoperiod to flower. Photoperiods as well as thermo period have been described as critical for the expression of genes in the flowering process. The data of the present report might be linked to the seasonal flowering locus (SFL), a regulatory molecular mechanism that switches photoperiod and thermo period to flowering (Mouhu et al. 2009).
Martinez et al. (2003) report that stress activates flowering in Arabidopsis thaliana via SA. Using a SA deficient mutant they show the importance of this molecule, that seems to induce flowering without the activations of well known genes, reported in the process such as CONSTANTS (CO) or FLOWERING LOCUS (FC). The present report could be linked with the proposal of these authors in the sense that SA induces flowering, following a novel and independent metabolic way. Besides, the use of SA might be a new tool for the molecular biologists as to elucidate the genes and mechanisms that regulate flowering, since this process is not fully understood.
The findings of the present report might be of importance for practical use in ornamental horticulture.
CONCLUSIONS
A dose response curve to estimate the effect of low concentrations of SA in the number of flowers in petunia plants was established. SA at 1 μMincreases up to 72 % the number of flowers per plant in comparison with the control. SA induced earliness in the flowering of petunia by six days.
ACKNOWLEDGEMENTS
To CONACYT Grant No. 33647–B; to E. Balám Uc and G. Briceño for their technical support.
LITERATURE CITED
Cleland, C. R, and O. Tanaka. 1979. Effect of day length on the ability of salicylic acid to induce flowering in the long–day plant Lemna gibba G3 and the short–day plant Lemna paucicostata 6746. Plant Physiol. 64: 421–424. [ Links ]
Echeverría–Machado I., R. M. Escobedo–G.M., and A. Larqué–Saavedra. 2007. Responses of transformed Catharanthus roseus roots to femtomolar concentrations of salicylic acid. Plant Physiol. Biochem. 45:501–507. [ Links ]
Gutiérrez C., M, L.C. Trejo, and A. Larqué–Saavedra. 1998. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 36:(8) 563–565. [ Links ]
Hayat, S., B. Ali, and A. Ahmad. 2007. Salicylic acid: Biosynthesis, metabolism and physiological role in plants (Chapter 1). In: Hayat, S., and A. Ahmad (eds). Salicylic Acid–A Plant Hormone. Springer, Dordrecht. The Netherlands, pp: 1–14. [ Links ]
Holuigue, L., P. Salinas, F. Blanco, and V. Garretón. 2007. Salicylic acid and reactive oxygen species in the activation of stress defense genes (Chapter 8). In: Hayat, S., and A. Ahmad (eds). Salicylic Acid–A Plant Hormone. Springer, Dordrecht. The Netherlands, pp: 197–246. [ Links ]
Kawano, T., and T. Furuichi. 2007. Salicylic acid and reactive oxygen species in the activation of stress defense genes (Chapter 10). In: Hayat, S., and A. Ahmad (eds). Salicylic Acid–A Plant Hormone. Springer, Dordrecht. The Netherlands, pp: 277–321. [ Links ]
Larqué–Saavedra, A., and R. L. Wain. 1974. Abscisic acid levels in relation to drought tolerance in varieties of Zea mays L. Nature 251(5477):716–717. [ Links ]
Larqué–Saavedra, A., and R. Martin–Mex. 2007. Effects of salicylic acid on the bioproductivity of plants (Chapter 2). In: Hayat, S., and A. Ahmad (eds). Salicylic Acid–A Plant Hormone. Springer, Dordrecht. The Netherlands, pp: 15–23. [ Links ]
López–Delgado, H., M. E. Mora–Herrera, H. A. Zavaleta–Mancera, M. Cadena–Hinojosa, and I. M. Scott. 2004. Salicylic acid enhances heat tolerance and potato virus X (PVX) elimination during thermotherapy of potato microplants. Am. J. Potato Res. 81:171–176. [ Links ]
Luo J. P., S. T. Jiang, and L. J. Pan. 2001. Enhanced somatic embryogenesis by salicylic acid of Astragalus adsurgens Pall.: relationship with H2O2 production and H2O2–metabolizing enzyme activities. Plant Sci. 161:125–132. [ Links ]
Mahdavian K., K.M. Kalantari, M. Ghorbanli, and M. Torkzade, 2008. The effects of salicylic acid on pigment contents in ultraviolet radiation stressed pepper plants. Biol. Plant. 52 (1): 170–172. [ Links ]
Martin–Mex, R., E. Villanueva–Couoh, T. Herrera–Campos, and A. Larqué–Saavedra. 2005. Possitive effect of salicylates on the flowering of African violet. Scientia Horticulturae; 103 499–502. [ Links ]
Martinez, C, E. Pons, G. Prats, and J. León. 2003. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 37:2 209–217. [ Links ]
Mouhu, K., T. Hytónen, K. Folta, M. Rantanen, L. Paulin, P. Auvinen, and P. Elomaa. 2009. Identification of flowering genes in strawberry, a perennial SD plant. BMC Plant Biol. 9:122. [ Links ]
Quiroz–Figueroa F, M. Méndez–Zel, A. Larqué–Saavedra, and V.M. Loyola–Vargas. 2001. Picomolar concentrations of salicylates induce cellular growth and enhance somatic embryiogenesis in Coffea arábica tissue culture. Plant Cell Rep. 20:679–684. [ Links ]
Raskin, I. 1992. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. and Plant Mol. Biol. 43:439–463. [ Links ]
San Miguel R., M. Gutiérrez and A. Larqué–Saavedra. 2003. Salicylic acid increases the biomass accumulation of Pinus patula. Appl. Forestry, 27:52–54. [ Links ]
SAS Institute Inc. 2003. The Analyst Application. Second Edition. Cary, NC, USA. 500 p. [ Links ]
Shah, J. 2003. The salicylic acid loop in plant defense. Curr,Opin. Plant. Biol. 6:365–371. [ Links ]
Senaratna T., D. Touchell, E. Bunn, and K. Dixon. 2000. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Reg. 30:157–161. [ Links ]














