Introduction
The fruit of blackberry belonging to the genus Rubus of the Rosaceae family, is a perennial shrub with aggregate fruit and is considered widely varied all over the world (Li et al., 2022; Li et al., 2012). In the zone of the mountain mesophilic forest of the central region of the state of Veracruz, it is one of the states in which blackberry is cultivated, as wild blackberries as introduced ones; and they are consumed mainly as fresh fruit, in jellies, and a liqueur known as “morita”. These fruits are obtained from the cultivars in the field of localities of the central region of the State of Veracruz. For example, Atecaxil, Ixhuacan de los Reyes, Veracruz, Mexico is a locality rich in culture and most of the population has been devote to the sale of goat cheese, breeding creole poultry, egg production and the elaboration of handmade products derived from bush berry, as an economic sustenance source. These products are made by a local family which is dedicated to producing jelly and handmade liqueur made of blackberry (Rubus fruticosus). Previous research has reported that the fruit of bush berry (Rubus ssp) has a great number of polyphenolic components (Robinson et al., 2020), and is a rich source of minerals, vitamins (such as vitamins E and C), calcium, flavonoids, anthocyanins, ellagic acid, ellagitannins, epi/catechin, and proanthocyanidins (Baby et al., 2018). In recent years, consumers have shown increasing interest in blackberries due to their rich nutrient content, which are considered nutraceutical to human health (Li et al., 2022). The anthocyanins are natural colorants (Morata et al., 2019) and some are considered as bioactive compounds (Huang et al., 2022). They could be functional products due to presence of these phenolic compounds, which are considered as chemopreventive, antiinflammatory agents, antioxidant capacity and neuroprotective effects (Gardener et al., 2021; Zannou & Koca, 2022). Also, diets higher in flavonoids appear nutritionally beneficial in the prevention of cardiovascular disease (Parmenter et al., 2020; Santacruz Cifuentes, 2011). However, it is unknown if antioxidants are still preserved in processed products. Several studies have investigated the effects of juice processing on blueberry polyphenolics, for example, in black mulberries jelly (Morus nigra), the content of antioxidants in the processed fruit it decreases (Tomas et al., 2017), but approximately, 60-70% of the fruit anthocyanins were retained in the final juice (Tomas et al., 2015); however, these variations should not be generalized for all processed foods, because mashing and pressing some times are effective for the recovery of fruit polyphenolics into the juice fraction. Thus, it is important to evaluate the presence of these phenolic compounds in processed products, in this case, handmade products, and we could contribute to the knowledge and relevant nutrimental information to producers and consumers. The handmade products do not have nutrimental information available for consumers and have had a huge demand in the central region of the State of Veracruz. The main objectives of this study were 1) to determine the presence of flavonoids and total anthocyanins in cultivated fruits “Rubus fruticosus”, as well as in derived products, liqueur and handmade blackberry jelly, and 2) to determine carbohydrates, lipids, proteins total and the energetic value measured in calories and number of portions by container of handmade blackberry products elaborated in Atecáxil, Ixhuacán de los Reyes, Veracruz, Mexico.
Materials and methods
Collection of handmade products
The locality of Atecaxil, geographically is located in Municipality of Ixhuacan de los Reyes of the state of Veracruz, Mexico, in the coordinates Length (dec) -97.076389 and Latitude (dec) 19.373611, with a height of 1580 meters above sea level (msnm by its abbreviation in Spanish). Jars of liqueur and blackberry jelly bottled of the community of Atecaxil, Ixhuacan de Los Reyes, Veracruz, were obtained in 2017, 2018 and 2019; each product by years were kept in freezer at -20 °C with thermostat (Black&Decker®) until their analysis at laboratory. Likewise, fruits of blackberry (Rubus fruticosus) cultivated in the town of Atecaxil, Ixhuacan de Los Reyes, Veracruz (Figura 1), were obtained during the months of March and May of each year. These were placed in a paper bag and stored in a container to be taken in the freezer at -20 °C (Black&Decker®) until their analysis at laboratory by each year, and to avoid degradation of the phytochemicals.
Sample ethanolic extraction
Extracts of cultivated blackberry fruits (Rubus fruticosus) and jelly (5 g) were made by sonication with 100 mL ethanol to 96% (Cole-Parmer® 8891 ultrasonic bath) for 30 minutes, until the sample was depleted. Obtained extracts were concentrated under reduced pressure in a rotary evaporator (Avante®, RE100-Pro) at a controlled temperature (45°C). The blackberry liqueur was filtered with Whatman paper number one, and it was kept, previously labeled, in freezer at -20°C (Black&Decker®) for their analysis and to avoid degradation of the phytochemicals (Carmona-Hernández et al., 2014).
Preliminary phytochemical screening
The identification of the secondary metabolites present in ethanolic extracts of fruits (Rubus fruticosus), jelly and blackberry liqueur, is carried out by qualitative tests for alkaloids, flavonoids, phenolic compounds, triterpenes/sterols, coumarins and saponins, using described method by (Domínguez, 1979) and modified by Carmona-Hernández et al., (2014). All samples were analyzed by each year, If there was no variation in preliminary phytochemical screening, it was considered to analyze the handmade blackberry product at the end of production, only in year 2019.
High-Performance Thin-Layer Chromatography (HPTLC)
The ethanolic extracts of fruit, liqueur and jelly of blackberry were applied on a glass plate Silica gel Merk 60 F254 (2 μm thick) of 10 by 10 cm. 3 μL of each sample were injected onto the plate, including 4 standards (routine, quercetin, chlorogenic acid and caffeic acid), bandwidth of 8 mm, first application on the X axis to 15.8 mm, first application on the Y axis 15 mm, distance between bands of 11.4 mm, application speed 10s/μL. The plates were developed using a mobile phase of n-butanol - acetic acid - water (50:10:20: V/V), drying of the plate for 30s, followed by a saturation of 20 min at room temperature; and after the development a drying of 10 min. The derivatization was performed with Anisaldehyde-sulfuric acid (a universal reagent for natural products) by heating the plate at 100°C for 10 min (Agatonovic-Kustrin et al., 2019; Wagner & Bladt, 1996). The derivatization was used for target-directed identification of biologically active molecules separated on chromatographic plates. The chromatographic plates were developed and visualized in visible light and at wavelengths at 254 and 366nm with the CAMAG AUTOMATIC TC SAMPLER 4 (CINVESTAV-IPN, Irapuato Unit, Guanajuato).
Total flavonoids content
The colorimetric method of aluminum chloride was used to determine total flavonoids (Martínez-Cruz et al., 2011; Zhishen et al., 1999). 200μL of fruit extracts, jelly and blackberry liqueur were taken and mixed with 800μL at 96% ethanol, plus 1mL of aluminum chloride at 2%. Subsequently, the mixture was incubated for 30 min at room temperature and in the absence of light. The absorbance was measured at 510nm in a spectrophotometer (JENWAY-GENOVA®). For the calibration curve, Quercetin solutions between 0-50μg were used (Sigma Aldrich®). Total flavonoid content was expressed as milligrams (mg) Quercetin (EQ) equivalents/100 g of sample. This procedure was carried out with each of the extracts obtained in triplicate.
Total anthocyanins content
Determination of total anthocyanins was carried out by the method described by (Di Stefano & Flamini, 2008) and method modified by (Ivanova et al., 2010). 200μL of fruit sample, jelly and blackberry liqueur were mixed with 1800μL of an ethanol solution/ H2O/HCl (70:30:1), pH 2 and immediately afterwards the absorbance at 540 nm was measured in a spectrophotometer (JENWAY-GENOVA®). The content of total anthocyanins was calculated using the following equation:
Where: A540nm is the absorbance and, d is the dilution and 16.7, molar extinction coefficient of malvidin-3-glucoside, using described method modified by Ivanova et al., (2010).
Statistical Analysis
Total flavonoids and anthocyanins content were reported as means ± standard deviation of five samples by years. The data were subjected to analysis of variance (ANOVA) to repeated samples and were compared with a Tukey test (P ≤ 0.05). All statistical analyses were performed using the STATISTICA® program for Windows.
Basic Nutrimental Profile of Products
Random containers were obtained of each product (10 containers), blackberry liqueur and jelly. To quantify carbohydrates, lipids and total proteins, from each sample of blackberry liqueur and jelly. First were obtained the constant weight to determine dry weight and percentage of humidity in the samples to be analyzed. Then, 5g of jelly and 5mL of liqueur were used to determine the amount of carbohydrates, lipids and total proteins following the protocol (Analytical Official Chemists Association guide was considered, “AQAO by its abbreviation in Spanish” 2015).
Total Carbohydrates Quantification
It was used the method described by (López-Legarda et al., 2017) which consisted of taking aliquots of 2mg of blackberry liqueur and blackberry jelly in which 1mL of phenol were added at 5% and 5mL of H2SO4 absolute concentration. The tubes were shaken for 30 seconds approximately, until to be mixed. The reaction of samples was stopped in cold bath during a period of 20 minutes, once the reaction was stopped, a reading in the spectrophotometer (JENWAY-GENOVA®) was carried out at 490nm. The values obtained of absorbance were helpful to plot a graph which is very crucial in estimating carbohydrate content. The calibration curve took place with a glucose standard (100mg/mL), repeating the same method used previously.
Total Lipids Quantification
It was used the method modified by (Lykke & Padonou, 2019), to determine the total Lipids Quantification, which consisted of homogenizing 5g of liqueur dry extract (extracts previously obtained to eliminate alcohol, humid and impurities) with 10mL of chloroform and 20mL of methanol; the same procedure was used with 5g of blackberry jelly (extracts previously obtained). Later, another 10mL of chloroform were added and homogenized for 1min. When the procedure ended, 10mL of water were added and it was homogenized for 1 more min. The mixture was filtered with Whatman paper number 1, the paper was set in a Buchner funnel in vacuum. The filtered mixture was transferred to a test tube of 50mL, once again the filter was washed with 10mL of chloroform, and it was filtered one more time. The filtered solution was left to rest until obtaining chloroform and methanolic phase, since separation was obtained, methanolic superior phase was withdrawn with a Pasteur pipette and the volume of chloroform phase was transferred to a flask previously weighed (first weighting of flask). Later, it was set in water bath to 50°C in order to evaporate chloroform phase and the remaining was dried over anhydrophosphoric in a desiccator in vacuum. After that, the flask was weighed for a second time, and 5mL of chloroform was added to extract lipids. This solution was poured into another flask, cleaned and dried previously. The flask containing lipids evaporated one more time and it was weighed for third time.
The next calculation was carried out.
Lipids weight = weight of flask that containing lipids evaporated X total volume of chloroform used (30mL) / evaporated chloroform volume.
Total Protein Quantification
It was used the Biuret method (Ceballos Luna et al., 2018; Coutiño et al., 2015), which consisted of adding 0.5mL of liqueur and 0.5g of blackberry jelly in test tubes. In each test tube containing the samples, 1mL of NaCl were added in 0.9% and 3mL of Biuret reactive, subsequently, it was mixed till homogenize. The obtained mixtures were heated to 50°C in water bath for 10min, when it finished a reading was carried out in the spectrophotometer at 540nm a calibration curve was taken in albumin standard (100mg/mL), repeating the procedure used before.
Elaborating Nutritional Information
To elaborate the nutritional information of homemade products of berry and to obtain the energy calculation from conversion factors, it was used the method described by (Salvador Badui Dergal, 2006) and (Pérez Grana, 2013) while consisted in used accuracy of food composition tables in the determination of nutrients. As well, it was determined the amount of carbohydrates, lipids and total proteins by each portion of blackberry liqueur and jelly, 15mL of liqueur and 10g of homemade blackberry jelly. The reported values were converted into the same units and synthetized in a table. The Mexican official standard NOM-051-SCFI-1995 was used to reference the table of homemade products.
Results and discussion
Preliminary phytochemical analysis
The qualitative analysis for secondary metabolites in the fruit of blackberry, jelly and handmade liqueur in three years showed a similar pattern. Mainly displayed presence of alkaloids and flavonoids; and minor presence of saponins, terpenes and steroids. However, in all samples, coumarins had a low presence (Table 1, only showed results in 2019). These results were similar during the three years and are therefore an important parameter to evaluate the phenolic contents in Rubus fruticosus of Atecaxil, Ver.; mainly the anthocyanins and total flavonoids content in fruit, jelly and handmade blackberry liqueur was carried in the final year of production on 2019. (Mulero et al., 2011) reported antioxidant activity in all 3 types of wine elaborated and found no differences in the concentrations of the different types of phenolic compounds in wine made with the 3 different methods, and during 3 months of storage showed a similar pattern. Thus, we still found the prevalence of alkaloids and flavonoids in the fruit of blackberry (Rubus fruticosus) and in processed blackberry products of Atecaxil, Ver. These qualitative findings, depends, not only on the quantity of sample, but also on the bond and/or interaction of these compounds with other molecules, on the location in the food matrix, and the presence of other bioactive compounds into samples (Minatel et al., 2017). During the cooking process, the temperature thermal may to destruction of cell wall and other subcellular components, stimulating the release of these compounds (alkaloids, flavonoids, saponins, terpenes and steroids) and were stable during the manufacturing process. Several research studies have shown increase in the phenolic compound levels, as well as in the antioxidant activity after cooking (Leong & Oey, 2012; Murador et al., 2016). It's important pointing, there are indications that the retention of phytochemicals and the antioxidant properties are present after the cooking and vary considerably between the different vegetables and methods used in their preparation ((Jiménez-Monreal et al., 2009; Minatel et al., 2017; Pellegrini et al., 2009). However, is important to remember that the exact composition of blackberries is highly dependent on the cultivars, cultural practices and numerous preharvest factors, especially climatic and soil conditions (Vergara et al., 2016). Mainly the flavonoids are a group of natural substances with variable phenolic structures and are widely distributed in plants; recently, 26 phenolics compounds were identified and quercetin and isoquercitrin were the predominant phenolic compounds in the fruit in Rubus ulmifolius (Schulz et al., 2019). In year previous, have been reported thousands of phenolic antioxidant compounds exist and are classified into several categories based on structural similarities (Craft et al., 2012), in the maturation stages (Schulz et al., 2019). For example, separated phenolics into seven categories: phenolic acids, coumarins, flavonoids, isoflavonoids, stilbenes, lignans, and phenolic polymers (tannins), hydroxybenzoic acids, flavan-3-ols and anthocyanin. These groups differ from each other in functional group placement, or the addition of a chemical moiety as in glycosylation. These results reinforce the prevalence potential of flavonoids alkaloids, saponins, terpenes and steroids in blackberry fruit (Rubus fruticosus) and liqueur and handmade jelly from Atecaxil, Ixhuacan de Los Reyes, Veracruz (Table 1). On the other hand, there are limited information is available on how different processing methods in processed products, impact on the polyphenolic content of the fruit (Brownmiller et al., 2009), which is implied by an increase of its bioavailability in the human body when they consume it.
Table 1 Qualitative result of preliminary phytochemical analysis of samples from blackberry fruit, liqueur and handmade jelly from Atecaxil, Ixhuacan de Los Reyes, Veracruz, on year 2019.
| Compound | Alkaloids | Flavonoids | Saponins | Coumarins | Terpenes/ steroids | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Products | Qualitative Tests | |||||||||
| M | D | W | A | C | S | F | SL | Co | LB | |
| Blackberry fruit | ++ | +++ | ++ | + | +++ | +++ | ++ | n/d | + | + |
| Jelly | - | ++ | ++ | ++ | +++ | ++ | - | n/d | + | - |
| Liqueur | - | ++ | +++ | - | +++ | ++ | ++ | n/d | + | ++ |
Alkaloids: M= Mayer Test, D= Drangendorff y Test, W= Wagner Test. Flavonoids: A= H2SO4 Test, C= FeCl2 Test, S= Shinoda Test. Saponins: F= Foam Test, SL= Lieberman Test. Coumarins: Co= Fluorescence Test. Triterpenes and/or Sterols: LB= Lieberman-Bouchard Test. Intensity: major +++, medium ++, low +, null - and n/d non-determined.
High-Performance Thin-Layer Chromatography
The plate observed in visible light (RT-White), was found in the sample of fruit and liqueur of blackberry a band of brown to red light brick color with a Rf of retention 0.25 and another thin band with a Rf 0.4. While, in the jelly sample, a band from purple to light purple was observed with a Rf 0.3. Also, a wavelength of 254 nm, light bands were observed in the three samples, with a Rf 0.25, they were observed in visible light; but with a single variation of bands observed with a Rf 0.8 (Fig. 2). Finally, at a wavelength of 366nm, bands with a similar Rf 0.4, 0.6 and 0.85 were observed both in fruit and blackberry liqueur. On the other hand, a Rf 0.15 and 0.45 were observed in liqueur (Fig. 2). These similarities and differences indicate that there are compounds shared, at least, some similar chemical structures or they are similar compounds. Observed coloration in visible light between fruit, liqueur and blackberry jelly, can indicate that both in fruit and products, there are compounds that are maintained after the elaboration process, bottled and shelf life. Also. the pigments continue to be observed. Similar studies in blackberry fruits of the species Rubus adenotrichus Schltdl and other wild blackberries (Rubus ssp) from the central region of Veracruz, have reported presence of these pigments (Aguilera-Otíz et al., 2011; Morata et al., 2019), known as anthocyanins (Martínez-Cruz et al., 2011). Aguilera-Otíz et al., (2011) reported that intensification of blue coloration is due to increase of phenolic ring hydroxyls, while introduction of methoxyls cause the formation of red color. Also, (Morata et al., 2019) reported that the color of natural anthocyanins covers most of the visual spectra from yellow - orange to blue-violet. The stability of anthocyanins depends on several parameters, such as pH, temperature, and oxidative conditions, but they are normally quite stable in acidic media. Likewise, it has been reported that in aqueous solutions, pigments are basically stable, and they have an intense red color, and at pH above 7, quinoidal shapes are present (A, A-) purple color that is degraded rapidly because of oxidation with air; sometimes when oxygen is excluded from the system, color deterioration is not observed (Garzón, 2008; Morata et al., 2019). Thus, during the elaboration and conservation of handmade products from the locality Atecaxil; these chemical factors mentioned before, had not effect on pigments, since purple color or light purple or blue and brown tones to light brick red are still observed in both products; aversely, temperature applied in the elaboration of blackberry jelly did not affect, and the cane alcohol added to the blackberry liqueur, could detonate particular features in each product. Therefore, during the elaboration process of these products, it is possible to detect pigments light brick brown-reddish coloration in blackberry handmade liqueur, meanwhile in blackberry jelly from purple to light purple color can be observed (Fig. 2-C and 3). In this study, we found pigments still when submitted to thermal processing, in water or ethanol (Fig. 2). (Kalt et al., 2020), reported two blueberry polyphenolic compounds, including both flavonoid and nonflavonoid types. There are abundant nonflavonoid polyphenolic compounds in blueberries are the hydroxycinnamic acid esters (especially chlorogenic acid). In our results, we found bands con Rf similarities to standard chlorogenic acid (Fig. 3 A).
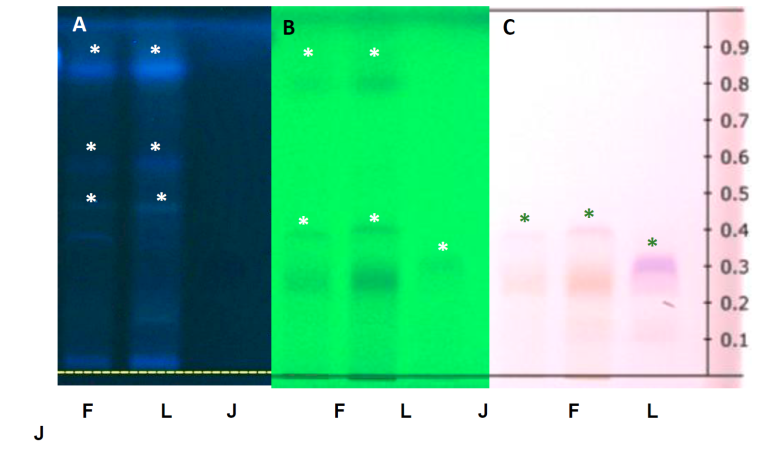
Fig. 2 High performance thin layer chromatography of Fruit (F), Liqueur (L) and blackberry Jelly (J) observed at wavelengths of 366nm (A), 254 nm (B) and RT White (C); observing the separation of compounds reflected in bands, with similarity and difference between Rf’s each sample [Indicated with an asterisk in white (A and B) and green (C)].
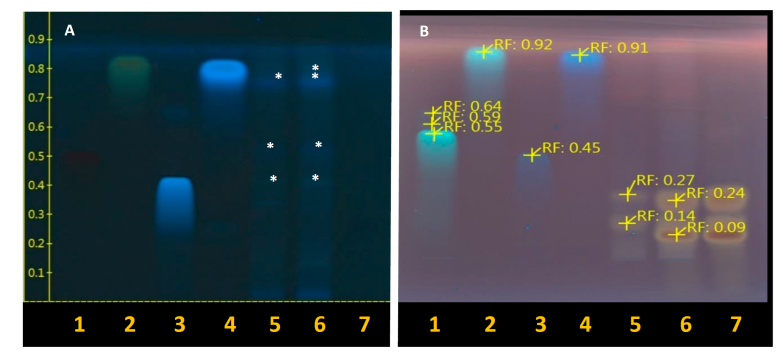
Fig. 3 High performance thin layer chromatography of Fruit (F), Liqueur (L) and blackberry Jelly (J) observed at wavelengths of 366nm. A) Not derivatized and B) Derivatized with Anisaldehyde-Sulfuric Acid (AS) reagent. 1: Rutin, 2: Quercetin, 3: chlorogenic acid; 4: Caffeic Acid, 5: blackberry fruit, 6: blackberry liqueur, 7: blackberry jelly. It is observing the separation of compounds reflected in bands, with similarity and difference between Rf’s each sample [Indicated with an asterisk in white (A)].
It can be observed, bands Rf similar to the standards used (Rutin, Quercetin, Chlorogenic Acid and Caffeic Acid) whit the technique not derivatized (Fig. 3-A), between fruits and liqueur showed similar results. While that derivatized with Anisaldehyde-Sulfuric Acid (AS) reagent, it is reflected the separation of others compounds in Rf’s each sample (Fig. 3-B), i.e., all samples revealed additional bands in the upper part of the chromatograms, showed similar results (Rf 0.09 to 0.27) between fruits, liqueur and handmade blackberry Jelly. Among the factors that affect the leaching of matrix compounds, we can include the polarity of medium used; such as water, allow changes in the phenolic compound levels. In contrast, if the medium is nonpolar (use of oil for frying, in both deep frying and pan frying), the loss of compounds is lower due to the lack of diffusion or migration to the medium or, may be due to the amount of fruit used to make the homemade jelly and the fruit, flavonoids were retained more in the jelly that liqueur. Blackberry contain abundant phenolic compounds, including anthocyanins (Cyanidin Delphinidin, Malvidin, Peonidin), flavonols and chlorogenic acids (Sandoval et al., 2020). Some of these compounds are pigments that impart pleasant and characteristic colours to the fruits. Another hand, (Li et al., 2022) found phenolic acids such as p-hydroxybenzoic acid, chlorogenic acid, coumarin, and syringic acid are the most widely available polyphenols in fruit juices (samples of blueberry, cherry and mulberry). The Rutin in mulberry juice is the highest polyphenol (420.87 μg/g). Therefore, the composition and content of phenolic substances are different in fruit juice. This authors, descripted in previous research that polyphenolic compounds are the material basis for the flavor, color, and nutritional properties of fruits, which directly affect the taste and quality of fruits and fruit-based processed foods (Maragò et al., 2016), or depending on the type of used solvent, it can interfere in the number of compounds present in fruits (Lee et al., 2012; Li et al., 2022). While Minatel et al., (2017) mentioned that flavonols improved the color stability of mulberry juice during storage. In addition, blueberries are one of the richest sources of anthocyanins among common fruits (Wu et al., 2006) and anthocyanins are the pigments that confer red, blue, and purple coloration. During berry ripening, anthocyanin content rises dramatically to provide a visual cue to distinguish between early to fully ripe fruit (Kalt et al., 2020). Finally, in the three samples, indicate that there is presence of flavonoids and anthocyanins in blackberry jelly and liqueur (Fig. 3).
In the Table 2, showed contend similar in total flavonoids and anthocyanins in fruit samples, handmade blackberry jam and liqueur, do not show significant year-by-year differences (Table 2). When comparing anthocyanin concentrations between handmade products, there are a higher concentration of anthocyanins in blackberry jelly and lower concentration in liqueur and fruit (Table 2), the fruit anthocyanins were retained in the final jelly, the temperature did not affect the presence of anthocyanins. The anthocyanins quantity found in handmade products may be due, firstly, to the amount of fruit used to make both products and state of maturity of the fruit as has been reported in Zarzamora (Rubus ulmifolius) by Borrego Corchado, (2018). Similarly, previous studies by (Li et al., 2022) reported an increment of anthocyanin content whit the increased gradual of the ripening stages of fruits. Also, indicated anthocyanins content in blackberry does not decrease with respect to the thermal processing of fruit, so they propose the idea that they are preserved and can be a good source of antioxidants (Bernal-Roa, 2012; Garzón, 2008). There is evidence that in the blackberry pulp have a concentration of monomeric anthocyanins between 6.05 to 7.37 mg/L (Rodríguez-Pérez et al., 2010) or ranged from 70.3 to 201 mg/100 g FW (fresh weight). In other studies have reported an average of 12.3 mg of anthocyanins (equivalent of malvidin-3-glucoside) for each gram of dried fruit of Rubus adenotrichus species (Martínez-Cruz et al., 2011). Previous reports indicated that blueberries sample is quite different from those published for blueberries from other locations, being the major anthocyanins found peonidin-3-O-arabinoside and delphinidin-3-O-arabinoside (37.43 ± 4.76 and 34.43 ± 3.28 mg/100 g fresh weight, respectively) followed by malvidin-3-O-glucoside and petunidin-3-O-rutinoside (Johnson et al., 2020; Yousef et al., 2013). Mustafa et al., (2022) also found anthocyanins were the main phenolic constituents in blueberry and strawberry. Furthermore, the higher total phenolic content in the blueberry fruit and jam justified their greater antioxidant capacity measured by DPPH free radical assay, compared to strawberry. Elez Garofulić et al., (2012) reported that anthocyanins are the predominant wine pigments, transferred to wine from both fruit skin and pulp during the maceration process and the concentration oscillated in an extremely wide range, from 5.07 mg/L (CBW 2) to 217 mg/L (CBW 7), but there is difference between the conventional wines (76.2 mg/L) and organic wines, was considerably lower (53.5 mg/L) (Amidžić Klarić et al., 2020). This is in accordance with the study of Johnson & Gonzalez de Mejia, (2012), that reported concentrations of total anthocyanins in blackberry wines in the range from 10.71 mg/L to 191.95 mg/L (expressed as cyaniding-3-glucoside equivalents) and an average concentration of 75.56 mg/L. Mudnic et al., (2010) also reported an extremely wide range of total anthocyanin values for blackberry wines, i.e., from 13.4 ± 3 to 164 ± 3 mg/L (expressed as malvidin 3-glucoside equivalents) and found it to be comparable to total anthocyanins in red grape wine. In contrast, the results of the present study did not indicate a significant difference in total anthocyanins between the years, but there is significant difference between products. These variations may be due to studied species and the amount of used sample or location, cultivar, and time of harvest; as is reported by other authors (Bunea et al., 2011; Fia et al., 2018). However, the anthocyanin, cyanidin-3-O-glucoside (C3G) are consistently the predominant phenolic antioxidant found in blackberries and have been shown to have prominent bioactivity (Schulz et al., 2019). Among the factors that affect the prevalence of compounds, is the polarity of medium used. Polar mediums, such as water and ethanol, allow changes in the phenolic compound levels (Gardener et al., 2021; Pellegrini et al., 2009).
Table 2 Total flavonoids and anthocyanins in fruit samples, handmade blackberry jelly and liqueur from Atecaxil, Ixhuacan de Los Reyes, Veracruz, during three years of production 2017 to 2019.
| Sample | Total Flavonoids | Total Anthocyanins | ||||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | |
| Blackberry fruit | 20.5 ± 2.8 | 26.1 ± 3.6 | 24.3 ± 1.0 | 12.37 ± 1.8 | 16.19 ± 0.7 | 13.26 ± 5.7 |
| Jelly | 38.8 ± 6.8 | 43.6 ± 1.3 | 38.0 ± 4.06 | 59.3 ± 4.4 | 54.6 ± 3.8 | 52.1 ± 2.6 |
| Liqueur | 16.0 ± 3.6 | 24.0 ± 3.4 | 20.0 ± 1.7 | 20.5 ± 3.8 | 23.6 ± 0.2 | 22.1 ± 3.2 |
Values are represented in miligramos (mg)and standard deviation from 3 replicates by years (ANOVA-Tukey test, P≤0.5).
Total Flavonoids expressed in milligram (mg) EQ/100g
Total Anthocyanins expressed in milligram (mg) of malvidin-3-glucoside/100g
On the other hand, when comparing total flavonoids concentrations between handmade products, there is a higher concentration in blackberry jelly and lower concentration in liqueur and fruit (Table 2). In blackberry jelly there is more fruits incorporated, while in blackberry liqueur has less fruit used for its preparation, according to the comments of the producing family. Similarly, other authors mention that black mulberry juice is rich in phenolic acids compared to the fruit and anthocyanins are highly retained during juice processing, approximately 60-70% (Tomas et al., 2015, 2017), effectively, recover polyphenolics into the juice. Other authors have found (+)-Catechin and its isomer (−)-epicatechin, are consistently the most abundant flavan-3-ols (class of flavonoids) in blackberries (Robinson et al., 2020). (Li et al., 2022) analyzed polyphenols in fruit juice samples of blueberry, cherry and mulberry, they found that the yields of polyphenols extracted by ethanol from fruit juice samples were obviously higher than those by water and acetone. They determined how canning, puréeing and juicing of blueberries, as well as storage of processed products at 25°C, influenced the retention of chlorogenic acid, total flavonols, total anthocyanins, and total procyanidins. The retention of flavonols (57- 99%) and chlorogenic acid (64-100%) was greater than that of anthocyanins (42- 72%) and procyanidins (19-78%). In non-clarified juices retained higher levels of chlorogenic acid, total flavonols, and total anthocyanins than clarified juices, but clarified juices contained higher levels of total procyanidins. The 97% retention of chlorogenic acid in non-clarified juices was much higher than the previously reported value (53%) for non-clarified blueberry juices (Skrede et al., 2000). It was also found in organic blackberry wines (BW) than the concentration of caffeic acid and p-coumaric acid, both being higher in the organic BW samples than in conventional vines. While than the concentration of total flavonoids in the analyzed samples of blackberry wine ranged from 161 to 774 mg/L, and the mean concentration between the organic and conventional group of samples did not differ (Amidžić Klarić et al., 2020). Another factor that affects the prevalence of compounds is the polarity of medium used, such as water and ethanol, allow changes in the phenolic compound levels (Gardener et al., 2021; Pellegrini et al., 2009). In any case, processing the fruit facilitated its subsequent extraction in our studies, we are recovering total flavonoids and anthocyanins after liqueur and jelly processing of the blackberry fruit (see Table 2). In this study, we confirmed that it is conserve the total flavonoids and anthocyanins and the elaboration process does not affect the presence of flavonoids; the antioxidant properties have been associated with their phenolic composition and particularly with the high content in anthocyanins and flavonoids. Genova et al., (2016), mentioned that an appropriate management of fruit harvesting date, postharvest and processing may lead to an improvement in nutraceutical quality of juices or another product. The phytochemical quantity retained in fruits and vegetables, after the processing, depends on the stability of these compounds during the different food preparations. Molecular modifications induced by processing and the transformations that occur before the consumption are mainly related to the sensibility of the compounds to oxidation and/or isomerization (Leong & Oey, 2012). Theses researchers corroborating our results phytochemical, total flavonoids were maintained in fruit samples, handmade blackberry jam and liqueur From Atecaxil, Ver. Mex. Generally, it has been reported that blackberry (red fruits) are primary sources of flavonoids, and they can be associated with antioxidant activity (Bunea et al., 2011; Geleijnse & Hollman, 2008; Lillo et al., 2016; Martínez-Cruz et al., 2011). The antioxidant content of blackberries and in particular their phenolic content ought to be considered as an important trait for breeding programmes (Clark et al., 2011; Milošević et al., 2012), as well as rural or urban areas. Minatel et al., (2017) reported that even though there are innumerous studies comparing the biological actions and in vitro antioxidant activity of phenolics, and the function of its content in vegetables and consequently in human, there is no consensus about the best way of preparing/consuming fruits and vegetables intending preservation or to increase their antioxidant activity.
Total Carbohydrates, Lipids and Protein Quantification
Total carbohydrates concentration in homemade liqueur of blackberry was of 0.84 ± 0.5 mg/mL and in blackberry jelly was of 0.74 ± 0.5 mg/mL, studies carried out in raspberry wine (Autumn bliss var), have found a concentration of carbohydrates of 1.8 ± 0.3 mg/mL and, in red fruit wine, 2.2 ± 0.47 mg/mL (Sánchez Trujillo, 2013). Total lipids concentration in blackberry liqueur and jelly were of 0.0009 ± 0.2 µg/mL (9 mg/mL) and 0.0013 ± 0.15 µg/mL (1.3mg/mL), respectively. This variation in quantities can be due to two important factors, the amount of fruit used or the extraction only of the fruit juice during the manufacturing of blackberry liqueur, because, in the jelly was used all fruit. For instance, during the elaboration of blackberry jelly, producers used all drupe of the fruit, including seeds, meanwhile in blackberry liqueur, it was used only juice of fruit, therefore, it is reflected a higher amount of total lipids in jelly than in juice. Preceding studies such as, Cerón et al., (2012) and Pantoja-Chamorro et al., (2017) reported that content of fatty acids presents in the fruit of castle blackberry (Rubus glaucus), they have bigger numbers of unsaturated fatty acids within blackberry pips, such as, linoleic acid (61.6%) and linolenic acid (25%), attributing beneficial properties to human being. However, Zafra Rojas, (2019) reported low presence of lipids in subproducts of Rubus fruticosus. For example, in Zarzamora sauces (Rubus fruticosus), a low amount of fat was found for each sample of 100 gr (Ceballos Luna et al., 2018). Lastly, the amount of proteins present in blackberry liqueur was 0.001 ± 0.047 mg/mL and in blackberry jelly, 0.004 ± 0.031 mg/mL of proteins were found. The seeds, fruits and leaves are important sources for both protein and fat. Fruits generally have lower content of protein, fat and minerals, but these components are still present. Studies done in red wine, found 0.001 mg/mL of proteins, while in blackberry jelly and strawberry jelly from well-known brands report in the label 0.002 mg/mL and 0.003 mg/mL of proteins, respectively (De Rosso et al., 2009; Kassara et al., 2022). In blackberry sauces, proteins totals ranged from 7 to 9% for each sample of 100 gr (Ceballos Luna et al., 2018).
In blackberry liqueur had an energetic value of 18.9 kcal/15 mL, having in total 33 portions each container, meanwhile, in blackberry jelly, it was found an energetic value of 27.1 kcal/10 g, having around 34 portions by container (Table 3). Information on the nutrient composition of food is essential to estimate adequate nutrient intake both at individual and group levels (Joyanes & Lema, 2006). Vergara et al., (2016) described that blackberry plays an important role in human nutrition, due to the elevated content of certain bioactive compounds including ascorbate, anthocyanins, phenolic acids, carbohydrates and proteins. In this present research, we corroborating that the handmade products could be highly competitive in the market. Likewise, there is not a significant difference when we comparing the two handmade products in the three years (data not relevant), while we can explain the color and unique flavor of the products. In addition, we provide the consumer, the amount of macronutrients ingested in each serving of both handmade products from Atecaxil, Ver., Mex, important knowledge for your health.
Table 3 Total macronutrients in handmade products of blackberry (Rubus fruticosus) from Atecaxil, Veracruz, Mexico in years 2019.
| Blackberry Liqueur | Blackberry jelly | |
|---|---|---|
| 33 servings per-container/652.6 kcal | 34 servings per-container/894.3 kcal | |
| Serving Size 1 cup (15 mL) | Serving Size 1 tablespoon (10 g) | |
| Energetic value 82.48kJ/19.7 kcal per 15 mL | Energetic value 110.11kJ/26.3 kcal per 10 g | |
| Total Carbohydrate 3.5 ± 0.48 g | Total Carbohydrate 6 ± 0.51g | |
| Lipids 0.1 ± 0.2 g | Lipids 0.1 ± 0.15 g | |
| Protein 0.1 ± 0.05 g | Protein 0.1 ± 0.039g |
Conclusions
In handmade blackberry products, there are presence qualitative of flavonoids and alkaloids; and minor presence of saponins, terpenes and steroids. In both products, is possible to observe pigments of purple color or light purple or blue and brown tones to light brick red. This color is plant pigments, and the anthocyanins are biologically active, water soluble and are responsible for blue, purple, and red colors, especially in fruits and blooms. Therefore, handmade blackberry products of Atecaxil, Ixhuacan de los Reyes, Veracruz., continued preserving the flavonoids and anthocyanins after of its manufacture, there was no difference in the flavonoid and anthocyanin contains between years, but there was difference in type products. These products could have antioxidant potential, as has been reported in blackberry fruit by other authors. Furthermore, we provide to consumer relevant information about metabolites source, flavonoids and total anthocyanins, which are important in the food field for human beings. Total macronutrients in handmade Blackberry Liqueur and jelly found, are relevant to new sources of information about total carbohydrate, lipids and protein for the Consumers.











 nueva página del texto (beta)
nueva página del texto (beta)



