Introduction
Diabetes mellitus (DM) is a global health emergency. In 2019, about 463 million people were reported to have DM, and a yearly increase in the number of cases was estimated (IDF, 2019). Diabetes is a severe and long-term heterogeneous metabolic disorder that occurs because of hyperglycemia caused by impaired insulin secretion, defective insulin action, or both. Developing diabetes results from a combination of many environmental and genetic factors (Ekoé, 2019; IDF, 2019; Powers et al., 2018; Punthakee et al., 2018). Type 2 diabetes mellitus (T2DM) is the most common type of diabetes accounting for about 90% of the cases of diabetes worldwide (IDF, 2019).
T2DM is a complex metabolic disorder in which beta cells do not produce enough insulin and/or the body is resistant to insulin action. Hyperglycemia occurs because of reduced insulin secretion or insulin resistance and increased hepatic production of glucose (Jean-Marie, 2018; Powers et al., 2018; Punthakee et al., 2018). If diabetes is left untreated, it can lead to cardiovascular complications (e.g., cardiovascular disease), nerve injuries (e.g., neuropathy), kidney diseases (e.g., nephropathy), eye disorders (e.g., retinopathy, vision loss, and blindness), and amputations. These types of complications contribute to increased premature mortality and lower quality of life for the patient (Cristians et al., 2015; IDF, 2019; Hu & Jia, 2019; Surya et al., 2014).
According to the IDF (2019), one in seven adults has DM. The North America and the Caribbean region, which Mexico is a part of, has the highest prevalence of abnormal glucose tolerance (more than 12%), a condition that is commonly associated with prediabetes or the risk of developing T2DM. In addition, this region accounts for 43% of the world's healthcare expenditure. It is estimated that the expenditure for diabetes of one person per year in Mexico is US$1328.5 (IDF, 2019). In 2019, 438,393 new cases of T2DM were reported in Mexico with a higher incidence in the age group of 50-59 years (Secretaria de Salud, 2019).
Currently, oral antidiabetic drugs are used to treat diabetes. For example, sulfonylureas (e.g., glibenclamide), biguanides (e.g., metformin), glinides (e.g., repaglinide, nateglinide and mitiglinide), thiazolidinediones (pioglitazone and rosiglitazone ), α-glucosidase inhibitors (e.g., acarbose and miglitol), GLP-1 RAs and DPP-4 inhibitors, SGLT2 inhibitors (Hu & Jia, 2019; Surya et al., 2014). Although oral antidiabetic drugs are widely used in the control of hyperglycemia, they have undesirable side effects that reduce the quality of life of patients. Therefore, their use is limited (Gulmez & Kulak, 2021; Salehi et al., 2019; Seetaloo et al., 2019; Surya et al., 2014). For example sulfonylurea and glinides (glitinides) may cause weight gain and hypoglycemia; biguanides may induce weakness, and lactic acidosis; metformin may cause stomach problems; α-glucosidase inhibitors may cause diarrhea; the main adverse effects of SGLT2 inhibitors are urinary tract infection and genital infection and nasopharyngitis is the most frequently reported adverse event associated with DPP-4 inhibitors (Hu & Jia, 2019; Seetaloo et al., 2019; Surya et al., 2014).
Most of the world's population relies on herbal medicines for their health care needs (Gupta et al., 2018; Surya et al., 2014). Plant species produce secondary metabolites that are biologically active constituents with therapeutic and prophylactic applications in humans (Anwar et al., 2019; Durazzo et al., 2018). In plant research on T2DM treatment, the most mentioned secondary metabolites are alkaloids, flavonoids, tannins, saponins, phenolic compounds, glycosides, terpenoids, polyphenols, terpenes, quinones, coumarins, anthocyanin (Castro et al., 2014; Hu & Jia, 2019; Salehi et al., 2019; Shehadeh et al., 2021). The potential mechanisms of natural products against T2DM occur through several therapeutic targets and signaling pathways, either individually or synergistically (Castro et al., 2014; Gupta et al., 2018; Hu & Jia, 2019).
The utilization of herbs and spices that can act on carbohydrate and lipid metabolism, could contribute to the regulation of blood glucose has been reported (Hu & Jia, 2019; Seetaloo et al., 2019). Medicinal plants with antidiabetic properties may be a useful source for the development of safer and more effective oral hypoglycemic agents (Gulmez & Kulak, 2021; Surya et al., 2014). For example, in Mexico, the use of medicinal plants for the treatment of diabetes is frequent (Andrade-Cetto & Heinrich, 2005; Castro et al., 2014; Gupta et al., 2018). Traditional knowledge is a key source of information in the development of new plant-based drugs. For example, metformin, derived from the plant Galega officinalis L., is one of the most widely used antidiabetics (Seetaloo et al., 2019).
In Mexico diabetic patients, having or not having been prescribed antidiabetic drugs by their physicians, often use medicinal plants (Andrade-Cetto & Heinrich, 2005). Some patients even stop using allopathic medicine and opt for medicinal plants because of their low cost and easy accessibility. In this scenario, studies that support the use of medicinal plants for treating T2DM are crucial (Andrade-Cetto & Heinrich, 2005; Gulmez & Kulak, 2021; Mata-Torres et al., 2020; Mata et al., 2013).
Pteridophytes are not as widely used in traditional medicine as angiosperms (Ho et al., 2011). However, several types of pteridophytes are used in alternative medicine systems such as Ayurvedic, Unani, and homeopathic (Ho et al., 2011; Johnson et al., 2020). Some uses of pteridophytes are deeply rooted in cultural minorities or tribes such as those in India (Rao et al., 2007; Tanzin et al., 2013). Thanks to phytochemical and pharmacological research, the anti-inflammatory, diuretic, purgative, and antibacterial properties of some pteridophytes are better known (Ho et al., 2011; Johnson et al., 2020).
In traditional Mexican medicine a fern, Tectaria heracleifolia (Willd.) Underw. (Dryopteridaceae), is reported in ethnobotanical studies for the treatment of “urine sickness” and diabetes (Fig. 1) (Alonso-Castro et al., 2012; Avelino-Flores et al., 2019). Several plants originally used as antidiabetics are also used for renal complications (Andrade-Cetto & Heinrich, 2005). T. heracleifolia has other uses, for example, it is used to treat “air,” wounds, coughs, headache, pospartum pain, vertigo, toothache, heart attack infections, skin imperfections, among other uses (Alonso-Castro et al., 2012; Andrade-Cetto, 2009; Domínguez & Alcorn, 1985; Martinez, 1984; Zamora-Martínez & Nieto de Pascual, 1992). More recently its antioxidant and anti-inflammatory activity has been proven (Castrejón-Arroyo et al., 2016). There is a record of its average effect as molluscicidal and insecticide in bioassays of the aqueous extract of its rhizome (Domínguez & Alcorn, 1985). In Cazones de Herrera (Veracruz, Mexico) it is known as "hierba del sapo" or “mano de sapo” and it is recommended for cancer and diabetes; for the latter an infusion is prepared with three leaves in a liter of water (Romo, 2013). Because there is no pharmacological report on the hypoglycemic effect of T. heracleifolia exists this study aimed to evaluate the hypoglycemic effect in vivo of the aqueous extract using a NA/STZ-induced T2DM model, the safety of the extract using the Lorke protocol (Lorke, 1983) and qualitative tests to detect secondary metabolites.
Methods
Plant material
In September 2013, adult fronds of T. heracleifolia were collected in ejido “La Encantada”, Cazones de Herrera, Veracruz, Mexico. Herbarium specimens were submitted to the IMSS Herbarium (IMSSM, specimen number 16278) and to the Science Faculty Herbarium (FCME, specimen number 41537).
Obtention of the aqueous extract
After collection, the plant material was dried in an oven at 25 °C for one week. The fronds were separated from the petioles. They were fragmented using a manual mill and infused to obtain an aqueous extract. For the infusion, two liters of distilled water were boiled, 40 g of leaf fragments was added, and the heating source was turned off. Subsequently, two filtrations of the aqueous extract were conducted in a laminar flow hood using sterilized material. The first filtration and the second filtration were performed through two sterile gauzes and two discs of Whatman No. 1 filter paper, respectively. The filtrate was added into sterile glass bottles that were protected from light and frozen for subsequent lyophilization.
Colorimetric and precipitation techniques were performed using 30, 100, and 300 mg of the aqueous extract to determine the presence of main groups of secondary metabolites in the T. heracleifolia aqueous extract. The presence of terpenes was determined by the Libermann-Burchard test (positive result: presence of a green - bluish-green color); alkaloids was determined by the Dragendorff test (positive result: orange-brown precipitate); flavonoids by Shinoda test (positive result: effervescence); and glycosides by Mölish test (positive result: violet ring at the interphase) (Cristians et al., 2015).
Experimental animals
Male ICR mice with a body weight of 25-30 g were used (30 - 25 days old). The assays were performed at the Production and Experimentation Unit of Laboratory Animals of the Universidad Autónoma Metropolitana Unidad Xochimilco (UAM-X). The internal committee for the care and use of laboratory animals (CICUAL, UAM-X) approved the protocol. Animals were kept under the same conditions of temperature, humidity, and lighting during the treatments. The volume of administration of the substances used for the treatments was calculated based on NOM-062-ZOO-1999 (DOF, 2001).
Acute toxicity
The acute toxicity test was performed in two stages in which different doses of the aqueous extract were tested. Depending on the number of animal deaths observed in the first stage, the second stage was conducted, which was characterized by the administration of the highest doses of the aqueous extract (Lorke, 1983).
In the first stage, three mice were allocated to three groups for each dose evaluated (10, 100, 1000 mg/kg), and three mice were allocated to the control group (0.9% saline solution). Subsequently, all animals fasted for five hours, the aqueous extract was dissolved in a 0.9% saline solution and administered it orally to the mice using an orogastric tube. The total volume of the substance was administered according to NOM-062-ZOO-1999 (0.2 mL/10 g) (DOF, 2001).
Mice were observed at the time of administration and during 1, 2, 6, and 24 hours, as well as daily for a period of 15 days. After the administration of the treatments, the mice had ad libitum access to food and water.
The second toxicity test was performed based on the results obtained in the first acute toxicity test. The doses used were 1600, 2900, and 5000 mg/kg, and then were calculated according to the results of the first stage and based on the algorithm proposed by Lorke (1983). The second stage was conducted under the same conditions as those in the first stage and using the same observation schedule.
The calculation of the median lethal dose (LD50) was used as a parameter of the extract toxicity. The LD50 for these cases was calculated using the log-probit regression method (Lorke, 1983). The logistic model of grouped data was adjusted [log (π/ 1-π)]. At the end of both stages, the mice were placed in CO2 chambers to proceed with euthanasia according to the NOM-062-ZOO-1999 followed by the revision of the general state of their organs (DOF, 2001).
Nicotinamide/Streptozotocin-induced diabetic models in male mice
Forty male ICR mice weighing 25-30 g with a fasting period of 5 hours were used. Nicotinamide (NA 50 mg/kg SIGMA®) was administered by intraperitoneal (IP) injection to all the animals. Subsequently, the animals were IP administered with a dose (120 mg/kg) of streptozotocin (STZ, SIGMA®) to induce DM. This protocol is intended to protect pancreatic beta cells from excessive oxidative damage caused by STZ, thus ensuring the generation of a physiological response similar to that of T2DM (Masiello et al., 1998). The volume used for the IP administration (0.1 mL/10 g) was calculated based on NOM-062-ZOO-1999 (DOF, 2001).
STZ was prepared in a pH 4.5 citrate buffer (concentration: 7.5 mg/mL, dosage: 50 mg/kg mouse). The solution was kept cool and protected from light (Brosius, 2015). One week after NA/STZ administration, blood glucose levels of the mice were measured to ensure agreement with the T2DM conditions (≥200 mg/dL). Once the high glucose levels were determined in blood, the evaluation of the acute hypoglycemic activity of the T. heracleifolia aqueous extract was conducted (Mata et al., 2013).
Evaluation of the hypoglycemic effect
Doses of 30, 100, and 300 mg/kg of the lyophilized T. heracleifolia aqueous extract were evaluated. Saline (0.9%) and 10 mg/kg glibenclamide (SIGMA®) were used as negative and positive control, respectively. Ten groups of eight mice per group were formed. Five groups were induced to T2DM, while the other five groups were kept normal. All the mice fasted for 5 hours before the experiment. Subsequently, the aqueous extract dissolved in a 0.9% saline solution was orally administered to all mice using an orogastric tube. The volume of administration was calculated according to the NOM-062-ZOO-1999 (0.2 mL/10 g).
Blood glucose was measured with a glucometer and reagent strips (ACCU- CHECK Performa, Roche® Laboratories), through a single cut in the caudal apex, no larger than 1 mm. Glucose assessment was performed at the following time points: 0, 1.5, 3, 5, and 7 h after extract administration (Mata et al., 2013). The percentage of glucose variation was used as a variable to define the changes in the glucose levels. For glucose measurements after the time point 0, the dried blood was removed from the caudal apex using a moistened piece of paper so that a new drop of blood could be taken in the following measurements.
Statistical analysis
The results were analyzed using analysis of variance (ANOVA) with α = 0.05 and Dunnett post hoc tests using the statistical software Graph Path Prisma 5.0.
For the description of the acute hypoglycemic activity, the test results were plotted as a function of the percentage of glucose variation with respect to the time points 0, 1.5, 3, 5, and 7 h (Mata et al., 2013). The area under the curve (AUC) plots prepared to observe the overall glucose level difference between treatments.
Results
The yield of the aqueous extract was 13.5% (5.4 g). The tests for the identification of secondary metabolites produced positive results for alkaloids, flavonoids, and glycosides (Table 1).
Table 1 Qualitative tests for the identification of secondary metabolites in the T. heracleifolia aqueous extract (- negative result, + slightly positive, ++ positive, +++ strongly. positive).
| Concentration (mg/mL) | Terpenes | Alkaloids | Flavonoids | Glycosides |
|---|---|---|---|---|
| 30 | - | ++ | + | +++ |
| 100 | - | ++ | ++ | +++ |
| 300 | - | ++ | +++ | +++ |
In the acute toxicity test, no physiological or behavioral alterations were produced in the animals up to the dose of 5000 mg/kg where the death of the second individual administered occurred (Déciga-Campos et al., 2007). At necropsy, no alterations in the internal organs were observed either. Therefore, the LD50 of the T. heracleifolia aqueous extract was 5065.177 mg/kg (Table 2).
Table 2 Number of deaths of experimental subjects in the acute toxicity test of the T. heracleifolia aqueous extract.
| Dose (mg/kg) | Deaths |
|---|---|
| 10 | 0/3 |
| 100 | 0/3 |
| 1000 | 0/3 |
| 1600 | 0/3 |
| 2900 | 0/3 |
| 5000 | 1/3 |
In normoglycemic mice, significant differences were observed in all treatments with respect to the negative control (0.9% saline) (Fig. 2). Differences at 3, 5, and 7 h were observed for the 30 and 300 mg/kg doses, while differences at 7 h were observed for the 100 mg/kg dose (Fig. 3). Glibenclamide showed a significant difference in all the time points and doses evaluated (Fig. 3).

Fig. 2 Area Under Curve (AUC) reflecting the overall effect of treatment on glucose levels in normoglycemic mice. Ctrl. - = Negative control. * Indicates significant statistical differences. p < 0.05 Dunnett's post hoc test.
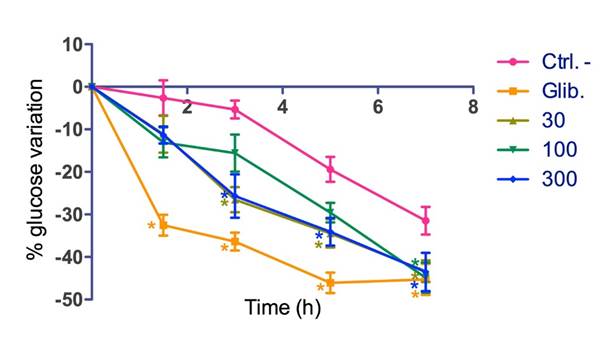
Fig. 3 Percentage of blood glucose variation over time in normoglycemic mice. Ctrl. - = Negative control. Each value is the mean ± SEM.*p < 0.05, n=8. Dunnett's post hoc test.
One week after inducing diabetes with STZ/NA, hyperglycemia, high blood glucose levels (≥200 mg/dL), a decrease in weight, frizzled hair, and increased water consumption were observed. Significant differences were observed for the 100 and 300 mg/kg doses of the T. heracleifolia aqueous extract and glibenclamide with respect to the control (Fig. 4). For the hypoglycemic effect of the extract and its relationship with the hours after oral administration, significant differences were observed with respect to the control (saline 0.9%). For the hypoglycemic effect of the extract and its relation to the hours after oral administration, significant differences were found with respect to the negative control at the 100 mg/kg dose at 3 and 5 h, as well as at the 300 mg/kg dose at hour 5 (Fig. 5).

Fig. 4 Area Under Curve (AUC) reflecting the overall effect of treatment on glucose levels in hyperglycemic mice. Ctrl. - = Negative control. * Indicates significant statistical differences. p < 0.05 Dunnett's post hoc test.
Discussion
In Mexico, pteridophytes most commonly used as medicinal plants are various species of the genus Equisetum, which is reported to have a hypoglycemic effect (Andrade-Cetto & Heinrich, 2005; Andrade et al., 2000). In the case of T. heracleifolia, this study is the first to report its hypoglycemic activity. Regarding the Tectariaceae family, Tsun-Thai et al. (2015) observed that of six fern aqueous extracts of Pleocnemia irregularis (Tectariaceae family), Christella dentata and Nephrolepis acutifolia, showed hypoglycemic activity, and their high flavonoid content could be key in their bioactive activity. Regarding the genus Tectaria, Rao et al. (2007) mentioned the ethnobotanical use of Tectaria macrodonta for stomach pain. In Tectaria paradoxaManivannan & Johnson, 2020 quoted the antidiabetic, anti-inflammatory, and cytotoxic activity, containing phenols, tannins, flavonoids, and triterpenes. The assay of antidiabetic activity with the methanolic extract by evaluating α-amylase inhibition in vitro was the most effective (Manivannan & Johnson, 2020).
The antioxidant and anti-inflammatory activity of T. heracleifolia has been reported as well as the presence of flavonoids and phenols in this species (Castrejón-Arroyo et al., 2016). The bioactive activity of ferns is mainly due to the presence of terpenes, such as ecdysteroids, flavonoids, alkaloids, and phenols (Ho et al., 2011; Manivannan & Johnson, 2020). In this study, qualitative phytochemical tests revealed the presence of alkaloids, flavonoids, and glycosides. Glycosides produced a strong positive reaction at all concentrations evaluated (Table 1). In the case of T. heracleifolia rhizome there is a report of the presence of β-sitosterol, terpenoids and tannins by phytochemical screening (Domínguez & Alcorn, 1985).
Flavonoids have diverse biological activities, including decrease of blood lipids, liver protection, hypoglycemic effect, and anti-inflammatory activity (Ho et al., 2011). Flavonoids such as kaempferol or catechins are natural antioxidants, and they may be beneficial in the management of diabetes because they could attenuate the oxidative stress that occurs in diabetes (Salehi et al., 2019; Vieira et al., 2020). Flavonoids such as naringenin and rutin are involved in decreasing renal, muscles and organs glucose absorption, helping in cases of insulin resistance (Salehi et al., 2019). Similarly, quercetin, fisetin, and morin are flavonoids that intervene in relevant molecular mechanisms (Salehi et al., 2019). Alkaloids such as lupanine, boldine, and berberine possibly intervene in insulin signaling pathways in different cells (Salehi et al., 2019; Vieira et al., 2020). Glycosides act on lipids and have antioxidant properties (Zhu et al., 2021). Therefore, we can assume that the hypoglycemic activity of T. heracleifolia could be mainly due to the presence of flavonoids and glycosides.
In this study, the antidiabetic evaluation of the T. heracleifolia aqueous extract compared with a commercial oral hypoglycemic drug of the sulfonylurea type, glibenclamide, was conducted in an in vivo model. Glibenclamide as a positive control showed the expected effects. In normoglycemic animals, a hypoglycemic effect was observed in the three evaluated doses of the aqueous extract (30, 100, and 300 mg/kg). The effect of the aqueous extract was observed after 3 h, while the effect of the drug was observed after 1.5 h. Furthermore, the aqueous extract had progressive effectiveness for up to 7 hours after administration without losing its effect. There were significant differences between glibenclamide and the doses of T. heracleifolia aqueous extract suggesting that despite the difference in their chemical nature, they both show a progressive hypoglycemic effect (Fig. 2).
Glibenclamide is a second-generation sulfonylurea, so its action is stronger and has a longer effect. It is a type of insulin secretagogue that acts on the pancreas stimulating insulin secretion in pancreatic β cells (Hu & Jia, 2019). The hypoglycemic effect observed in the T. heracleifolia aqueous extract, could suggest an insulin sensitizer or secretagogue activity. The post hoc tests revealed that there was no significant difference among the three concentrations of the aqueous extract. Therefore, the effectiveness of the treatment for the normoglycemic animals did not depend directly on the concentration of the extract.
In the hyperglycemic mice, glibenclamide began to have an effect after 3 h, while in the normoglycemic mice it had an effect at 1.5 h (Fig. 2). This result may indicate the relationship between the time of action of the drug and the physiological condition of the mice. However, the T. heracleifolia aqueous extract began to have an effect at 3 h at a dose of 100 mg/kg (Fig. 5). The 30 mg/kg dose was not significant compared with the rest of the treatments at any time after the administration of the extract (Fig. 3). Therefore, this dose was insufficient in producing the desired effect in hyperglycemic mice unlike in normoglycemic mice. An arising question is whether the concentration of secondary metabolites was insufficient to achieve the desired effect. For the 100 mg/kg dose, the effect was observed 3 h after the administration of the extract. Meanwhile, no significant differences were observed at 7 h, possibly because the hyperglycemia of the experimental model affects the time of action of the extract. Therefore, the activity of the T. heracleifolia aqueous extract is like the positive control, and the delay in action is a function of the condition of the animals. Furthermore, the fasting hours (12 h) should be considered because they denote the period in which the gluconeogenesis process could have been initiated, and thus the effect of the extract and the drug was diminished. The 300 mg/kg dose showed significant differences at 5 h and no significant differences at 7 h. Like the effect observed with the 100 mg/kg dose, the effect detected with the 300 mg/kg dose could be because the disease affected the duration of action of the extract. Only glibenclamide maintained the duration of treatment effect over time.
From the graph 5, it is observed that the aqueous extract treatments and glibenclamide exhibit the same trend; however, both cases exhibit a trend different from that of the negative control. Evaluations have shown that combining glibenclamide with an aqueous extract has a beneficial effect on glycemia (Gordillo et al., 2012). In Mexico, a study have demonstrated the hypoglycemic effect of the aqueous and butanolic extracts of a pteridophyte (Equisetum myriochaetum) with activity similar to sulfonylurea in mice with T2DM induced by STZ (Andrade et al., 2000). In these studies, a phytochemical analysis was performed and glycosides were found to be the main metabolites responsible for the hypoglycemic effect (Andrade et al., 2000). As shown in Table 1, glycosides were the most abundant metabolites found in this study.
The E. myriochaetum aqueous extract was evaluated clinically in 11 recently diagnosed diabetic patients. Hypoglycemic activity of the extract was reported. However, when insulin was measured in patients, it was observed that the mechanism of the pteridophyte was not similar to that of the secretagogue glibenclamide (Andrade-Cetto et al., 2002). It is possible that our plant, which displays activity similar to glibenclamide, does not exert the same mechanism of action. However, this information cannot be affirmed because E. myriochaetum and T. heracleifolia belong to different taxa, and their detailed chemical composition is unknown. It would be important to perform further research to elucidate the mechanism of action of medicinal T. heracleifolia.
Tanzin et al. (2013) evaluated the effect of the methanolic extract of Christella dentata in mice and showed that it has a hypoglycemic effect comparable to that of glibenclamide. The ethanolic and aqueous extracts of Adiantum philippense were evaluated in mice, and they showed a significant hypoglycemic effect with glibenclamide used as a positive control. The 2000 mg/kg dose was found to be safe in their toxicity protocol (Paul et al., 2012). This dose is like the acute toxicity of Tectaria heracleifolia evaluated in this study. Previously, Castrejón-Arroyo et al. (2016) demonstrated the antioxidant and anti-inflammatory capacity of the T. heracleifolia aqueous extract. Because diabetes is a multifactorial disease, free radicals are produced in the hyperglycemic state because of oxidative stress, which can be fatal (Paul et al., 2012). Antioxidants are beneficial to avoid complications such as those mentioned above (Vieira et al., 2020). In this study, nicotinamide was used to protect beta cells from the oxidative damage induced by STZ. According to Lorke (1983), when high doses such as 5000 mg/kg are administered to groups of three animals and only one out of three dies, the substance is practically harmless belonging to the category 5 of the OECD (2001) protocol 423 by the Global Harmonized System (GHS) (OECD, 2001). Therefore, the T. heracleifolia aqueous extract does not represent a health risk when ingested. Because it did not show toxicity, the plant can be considered for the development of herbal medicine. Herbal medicines are a remarkable option for the treatment of diabetes because their components have negligible or no toxicity (Salehi et al., 2019).
The use of oral hypoglycemic agents is currently being examined because of the risk of vascular accidents, resistance, toxicity, and the occurrence of death as cardiovascular disease continues to be the main cause of mortality in patients with T2DM. Available data suggest that sulfonylureas should be avoided in patients at risk of cardiovascular disease and weight gain because in 44% of patients the efficacy is lost after 6 years of treatment (Breite et al., 2020; Salehi et al., 2019; Seetaloo et al., 2019). In addition to the high cost, late adverse effects are still unknown. Longer evaluation times are required, but these times do not meet the demand for medication of T2DM patients. Much of the morbidity associated with chronic complications in T2DM can be reduced with therapeutic interventions that maintain blood glucose values close to the normal range (Cañigueral & Vila, 2005; Zárate et al., 2014). The use of herbal medicines has been increasing (Hu & Jia, 2019; Salehi et al., 2019; Surya et al., 2014). About 350 traditional plants used for diabetes have been reported to show no or few side effects (Salehi et al., 2019; Surya et al., 2014).
Like oral treatments, natural products act specifically on peptides and proteins of glucose metabolism. In vitro experiments have shown that flavonoids reduce serum DPP-4 levels and stimulate GLUT-4 translocation, modulating glucose levels in the body (Hu & Jia, 2019). The low cost of using plants, as opposed to conventional drugs, is one of the reasons why they are used in developing countries. Today, the use of herbal remedies is recommended because of their bioactive properties (Hu & Jia, 2019; Salehi et al., 2019; Surya et al., 2014). In Germany, at least two products made from Mexican medicinal plants, nopal (Opuntia spp.) and copalquin (Hintonia spp.), are available for the treatment of diabetes (Andrade-Cetto & Heinrich, 2005). This study demonstrates the antidiabetic properties of T. heracleifolia. However, further studies on the active principles of T. heracleifolia are required. Moreover, molecular studies should be performed to elucidate the mechanism of action of these active principles, which may represent an alternative source for drugs against T2DM.
Conclusions
Concentrations of 100 and 300 mg/kg of the T. heracleifolia aqueous extract showed a hypoglycemic effect like glibenclamide. The aqueous extract of the fronds of T. heracleifolia is safe for use according to the toxicity tests performed. The traditional use of the plant is supported; however, it is necessary to evaluate other types of extracts and other parts of the plant in chronic in vivo tests. Moreover, further investigations should be conducted on the hypoglycemic effect, the structure of the secondary metabolites or antioxidants with medicinal effects, and the mechanism of action of these metabolites. This is the first study on the hypoglycemic effect of T. heracleifolia showing potential for the development of herbal medicine against T2DM.











 nueva página del texto (beta)
nueva página del texto (beta)




