INTRODUCTION
The Solanaceae family is cultivated in Mexico since it has nutritional and medicinal properties conferred by its chemical composition (Gutiérrez et al., 2014; Benítez et al., 2018; Shajib et al., 2018). This group includes plants with antiinflammatory, antioxidant, anticancer and antithrombotic activities (Zhang et al., 2018; Silveyra et al., 2018; Rios et al., 2017; Zhuang et al., 2017). Although some medicinal properties are due to compounds derived from secondary metabolisms such as phenylpropanoids, flavonoids, alkaloids, acetogenins, polyketides, terpenes, steroids and carotenoids (Sultana and Asif., 2017; Paudel et al., 2017), others are due to compounds derived from primary metabolism such as bioactive proteins (Herbel et al., 2017; Moreno et al., 2015). The glucanases, chitinases, as well as, aspartic proteases with antimicrobial effect, are some examples of bioactive proteins isolated from plants (Silva et al., 2018; Ali et al., 2018).
As well, recent studies have reported the synthesis of proteins with different biological activity in some Solanum species (Shamsi et al., 2016). The proteases isolated from Solanum tuberosum is an example, since, some participate in plant-microbe interactions to mediate defense mechanisms (Frey et al., 2018). Therefore some of them have the antimicrobial effect (Muñoz et al., 2014). Another example is the dioscorine since it regulates the expression of some cytokines (Hsu et al., 2013), while lunasin from Solanum nigrum is effective against cancer (Alaswad and Krishnan, 2016).
Although some bioactive proteins of Solanaceae discovered, there is a large number of species that lack studies to validate their proper use. Accordingly, we observe the need to study new sources of bioactive proteins of Solanaceae, since they represent an alternative to help in the treatment of some diseases. Consequently, we proposed the study of the enzymatic, antioxidant, antibacterial and toxic activities of a protein fraction of S. marginatum L. f. previously characterized, to contribute to the study of Solanaceae species little studied. Since in the world only a few studies on the proper use of S. marginatum L. f. have been made. The infusion of S. mariginatum is used to treat abdominal pain, while the poultice of the fruit is used to treat respiratory problems in cattle. The seed is used for the treatment of external infection as a poultice, while on the other hand, the burnt and ground seed is consumed orally to treat a cough (Teklay et al., 2013).
In traditional Mexican medicine, S. marginatum L. f. is used as an antimicrobial and anticancer agent but lacks scientific validity (Villaseñor, 2016). In Mexico this plant is known as "sosa" and grows abundantly, so can be easily collected (Vidrio et al., 1988).
METHODOLOGY
Protein extraction, separation, and quantification
Protein extraction
Solanum marginatum leaves were collected at Tenosique Tabasco, Mexico. On the other hand, a sample of the plant was deposited and identified in the herbarium of the botanical department of the Autonomous University of Nuevo Leon (UANL), under accession number 027858. The identification of the plant material was carried out by PhD Marcela González Álvarez. Later, the plant was cut, lyophilized and, crushed in a food processor. Then, 10 g of crushed leaves were extracted with a sodium acetate buffer 50 mM and NaCl 5% under constant stirring (Nasr et al., 2016). Subsecuently, the proteins were separated by precipitation with ammonium sulfate (80% saturation index) for 24 hours at 4°C.
The separation was performed by centrifugation for 30 min at 10 000 rpm and 4°C. Finally, the pellet was resuspended in 50 mM sodium acetate buffer.
Protein fraction separation
The protein fraction was desalting out by fast protein liquid chromatography (PF-FPLC). In this technique, a gel filtration column Sephadex G-25 was used (Llorente et al., 2014).
The conditions used were a flow of 1 mL/min, an elution volume of 10 mL, a pressure of 1 mPa, and an injection volume of 500 µL. PF-FPLC was collected in 1 ml aliquots which were lyophilized and stored at -20°C until use in assays subsequent.
Protein quantification
Protein content in a 96 well plate by the bicinchoninic acid method (BCA) was measured. For this purpose, was done a calibration curve of bovine serum albumin (0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 ppm). In the assay 50 µL of the extract and 200 µL of BCA were mixed, and then this mixture was incubated at 37°C for 45 min and was read at 550 nm.
The protein content was measured in the crude extract as well as in PF-FPLC (Guevara et al., 1999).
Enzyme activity assay
The enzymatic activity was evaluated by the Kunitz method (Mazorra-Manzano et al., 2013). For this, a mixture of PF-FPLC with 1% casein for 30 min at 37°C was incubated. This reaction was stopped using the addition of trichloroacetic acid at 10%. The resulting solution centrifuged at 4 000 rpm for 10 min and the protein content of the supernatant was detected. Then, absorbance was measured at 280 nm using a microplate reader (GEN 5, Biotek, Intruments, Inc). The specific activity measured at different pH (1, 5.2, 6, 7 and 10), temperature (8, 25, 30, 40, 50, 60, 70 and 80°C) and reaction time (0 to 180 min). Additionally, the protein yield percent of every one purification step was calculated.
Protein separation by SDS-PAGE
The PF-FPLC was separated by the technique of polyacrylamide gel electrophoresis (SDS-PAGE), under reducing conditions (Rocha et al., 2015; Konozy et al., 2013).
The electrophoretic separation was carried out on a Bio-Rad Miniprotean III system, applying 100 V for 120 min. Protein fragments were revealed with Coomassie R-250 blue staining and a commercial marker was used for comparison.
DPPH assay
The DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay adapted to a 96-well microplate. This performed by mixing 100 µL of each sample with 100 µL of 0.1 mM DPPH. Then, the mixture was incubated for 30 min in the dark at room temperature. Glutathione (GSH) solution (1, 2, 3, 4 and 5 ppm) was used as a positive control for the sequencing effect of the protein extract and the effect was measured at 517 nm employing a microplate reader (TECAN, InfiniteM200PRO) (Yu et al., 2018).
ABTS assay
The ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) assay adapted to a 96-well microplate. This assay performed by means mixing 100 µL of 7 mM ABTS with 100 µL of potassium persulfate 2.4 mM. The mixture was incubated at 23°C in darkness for 16 h. This solution diluted with PBS (pH 7.2) (Karas et al., 2014). Additionally, a PF-FPLC solution at 10 ppm and GSH curve of 1, 2, 3, 4, and 5 ppm were tested. The readings at 734 nm in a microplate reader were performed.
Antibacterial effect evaluation
The antibacterial effect of PF-FPLC was carried out by microdilution method in a 96-well plate. Escherichia coli (ATCC 11229), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 15442) and Salmonella choleraesuis (ATCC 1070) strains were used. For each strain, a bacterial suspension at 1.5 × 108 CFU/mL was prepared using the McFarland scale of 0.5 (Cui et al., 2015; Bisi-Johnson et al., 2017). Concentrations of 0.01, 0.02, 0.04, 0.08, 0.16, 0.33, 0.63, 1.25, 2.50, 5.00, 10.00 µg/mL from PF-FPLC at 37°C for 24 h were tested. The absorbance at 625 nm in a Bioteck 800 XL microplate reader was read. Then, the bacterial inhibition percentage (%) was determined.
Toxicity evaluation
The Artemia salina immobilization assay was performed to evaluate the toxicity of proteins isolated from PF-FPLC and was carried out in a 96-well plate. A. salina cysts were hatched in artificial seawater at 37 g/L at 25 °C with aeration and constant light source for 24 h. Concentrations of 0.01, 0.02, 0.04, 0.08, 0.16, 0.33, 0.63, 1.25, 2.50, 5.00, 10.00 µg/mL from PF-FPLC for 24 h at 25°C were tested. A concentration-response curve with K2Cr2O7 at 5.00, 10.00, 15.00, 20.00 and 25.00 µg/mL, as well as a negative control with PBS (pH 7) (viability control) was performed (Salvador et al., 2015). LC50 with the percent of mortality M (%) by a linear regression analysis was determined (Hernández et al., 2017).
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Peripheral blood from healthy volunteers was obtained. The peripheral blood mononuclear cells were separated by Ficoll-Histopaque-1077 density gradient method (Kozachok et al., 2018). The mononuclear cells were washed three times with PBS and then centrifuged at 1250 rpm for 5 min. Finally, the cells were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO, Grand Island, N.Y. USA) and 0.04% ceftriaxone. After, 1 x 106 PBMC/mL were plated in a 96-well plate, subsequently, PF-FPLC concentrations of 0.01, 0.02, 0.04, 0.08, 0.16, 0.33, 0.63, 1.25, 2.50, 5.00, and 10.00 µg/mL were added. Finally, the plates were incubated for 24 hours at 37°C in a humidified atmosphere of 5% CO2, and the cell viability was measured by MTT method. The reading was carried out on a microplate reader (Bioteck 800 XL) at 450 nm (Wang et al., 2016).
Statistical analysis
The results of the statistical analysis are presented as the mean and the standard deviation (n = 3). Additionally, were analyzed data of enzymatic, antioxidant, antibacterial and cell viability activities by ANOVA and Tukey tests. The data to evaluate the specific activity of the extract against PF-FPLC were analyzed by the "t-student" test. Both tests were performed using the statistical program GraphPad Prism 5 ©. Statistical significance was accepted when p-values were ≤ 0.05.
RESULTS
Protein extraction, isolation, and quantification
The extraction procedure of S. marginatum L. f. showed a protein content of 315.8 ± 0.08 ppm, a yield of 71.05 % and a purification factor of 1.407.
The PF-FPLC isolation by FPLC showed a signal with a retention time (tr) of 2.13 min with a resolution of 2.81. As well, the equation allowed detecting protein content in the PF-FPLC of 9.259 ppm.
Enzyme activity assay
Assays to evaluate enzyme activity showed that the specific activity decreased in the purification steps, while the total activity increased. The precipitated extract presented a TA of 0.02 as well as a specific activity of 6.00 x 105 TA/mg. On the other hand, the PF-FPLC showed values of 0.13 and 1.78 x 10-3 TA/mg of total activity and specific activity respectively. Furthermore, enzymatic activity assays revealed a temperature of 37°C, a pH of 7 and 120 minutes as optimal conditions. In figure 1 the results are presented.
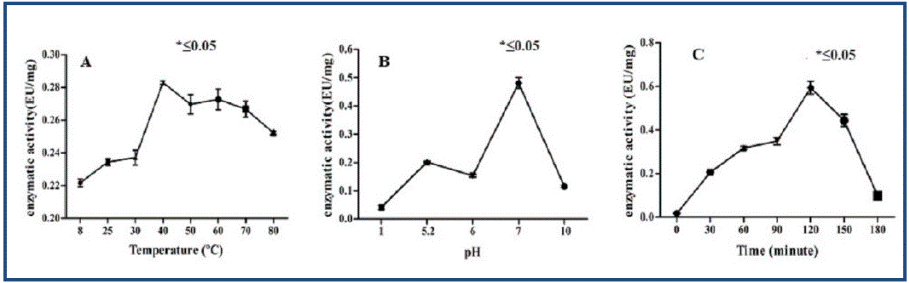
Fig. 1 Enzymatic activity evaluation of PF-FPLC against different conditions of temperature, pH and time. In A) temperature effect, B) pH effect and C) time effect.
The PF-FPLC presented a reversion of 71.05% and a purification factor of 0.152. Additionally, there was a decrease in the protein content in each purification step.
Protein separation by SDS-PAGE
The protein separation from PF-FPLC using SDS-PAGE showed seven bands. Although only seven bands were observed, there is a possibility that each band contains a group of proteins with different biochemical and biological properties. In figure 2 the results are presented.
Antioxidant effect evaluation
In PF-FPLC an antioxidant effect of 20.03 ± 0.09% and 62.08 ± 0.08% was found by the ABTS and DPPH methods, respectively. The results are shown in figure 3.
In PF-FPLC an antiradical effect of less than 25% was found; while the GSH used as a positive control (1, 2, 3, 4 and 5 ppm) showed an effect of 98%.
Toxicity evaluation
A. salina assay showed that PF-FPLC had no toxic effect at the concentrations tested. The positive control showed an LC50 of 13.90 ± 0.51 ppm.
Antibacterial effect evaluation
The PF-FPLC showed an antimicrobial effect on S. aureus and P. aeruginosa with a percent inhibition value of 30% to 5 ppm. This fraction showed an effect in E. coli and S. choleraesuis with a percentage of bacterial inhibition ≤ 8%, however, hormesis phenomenon between 0.312 and 0.635 ppm was observed. The concentrations tested on S. aureus and E. coli showed a significant difference (*p ≤ 0.05) by the Tukey test. The results showed that there is not a significant antimicrobial effect because the percentage of inhibition of S. choleraesius, S. aureus, and E. coli was ≤ 20% and in P. aeruginosa had no effect. The results are shown in table 1.
Table 1 Antibacterial effect evaluation from PF-FPLC
| ppm | Inhibition (%) | |||
|---|---|---|---|---|
| S. holeraesius | S. aureus | E. coli | P. aeruginosa | |
| 0.0025 | - | - | - | 20.164 ± 0.564 |
| 0.0050 | - | - | - | 21.387 ± 1.001 |
| 0.0100 | - | 5.362 ± 0.684 | - | 24.707 ± 0.799 |
| 0.0200 | - | 6.398 ± 0.228 | - | 24.759 ± 0.602 |
| 0.0400 | 5.647± 0.539 | 7.680 ± 0.630 | 5.156 ± 0.993 | 19.075 ± 0.578 |
| 0.0800 | 4.526 ± 0.458 | 8.097 ± 0.435 | 1.391 ± 0.876 | 13.374 ± 0.626 |
| 0.1600 | 3.243 ± 0.157 | 7.672 ± 0.321 | 5.631 ± 0.686 | 15.347 ± 0.293 |
| 0.3120 | 4.310 ± 0.575 | 9.440 ± 0.268 | 5.268 ± 0.557 | 10.196 ± 0.740 |
| 0.6250 | 7.356 ± 0.992 | 9.653 ± 0.862 | 5.837 ± 0.278 | 12.613 ± 0.605 |
| 1.2500 | 4.803 ± 0.826 | 11.020 ± 0.444 | 7.660 ± 0.721 | 10.665 ± 0.994 |
| 2.5000 | 2.695 ± 0.402 | 12.901 ± 0.151 | - | 14.029 ± 0.180 |
| 5.0000 | - | 17.198 ± 0.978 | - | 31.391 ± 0.694 |
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
The MTT assay was used to evaluate PBMC cell viability after treatment with PF-FPLC (fig. 4) showed that this fraction does not reduce cell viability to 0.019 µg/mL. In contrast, from 0.039 to 10 µg/mL, cell viability was significantly reduced.
DISCUSSION
The protein content in S. marginatum L. f. fruits is higher than that reported for other Solanaceae plants and similar to that recovered from protein extracts enriched with potato pulp (Rocha et al., 2015; Waglay and Karboune, 2017). The resolution ensured the effectiveness of chromatographic separation since it showed a value ≥ 1.5 (Harris, 2007).
Assays to evaluate enzyme activity showed that in purification steps specific activity was decreased while total activity was increased; because some lower molecular weight protein compounds lost during desalting (García et al., 2013).
According to specific activity results, in S. marginatum L. f., were found values below the values reported for the cysteine proteases (15 and 16 EU/mg) of the crude extract of the fruit of Solanum granuloso-leprosum (Vallés et al., 2011). On the other hand, enzymatic activity assays under optimal conditions showed that PF-FPLC contains more than one type of protein from the group of proteases; because they have activity at neutral, acid and basic pH (Errasti et al., 2018; Ahmed et al., 2009; Mohamed-Ahmed et al., 2009). However, when specific activity values compared, an increase in activity at neutral pH was observed; due to the ability of each protease to work at a specific pH. Some examples are cysteine protease isolated from S. granulosum having activity at pH 6, while other proteases isolated from the Solanum genus act at pH 11, 60°C and 25 h (Li et al., 2018; Mohamed Ahmed et al., 2009). Therefore, the enzyme activity of each protein depends on its catalytic mechanism (van der Hoorn and Rivas, 2018; Galaz et al., 2013). The percent yield determination showed that there is a decrease in protein content at each stage of purification.
The protein separation by SDS-PAGE assay showed that PF-FPLC contains proteins similar to the serine protease, identified in most plants (Kumari et al., 2012) because their molecular masses are of 19-110 kDa (Antão and Malcata, 2005; Li et al., 2018). Additionally, there is a possibility that PF-FPLC contains some reductases; such as s-nitrosoglutathione reductase isolated from Solanum lycopersicum, having a molecular weight of 45 kDa (Kubienová et al., 2013); as well as some oxidoreductases, since in Solanum tuberosum some have been identified that have molecular weights that oscillate of 47-68 kDa (Batista et al., 2014). Additionally, there is the possibility that the proteins contained in PF-FPLC are similar to those of Solanum dubium. Since they contain proteins with molecular weights of 10-95 kDa (Ahmed et al., 2009), as well as, S. tuberosum aspartic protease since it has a molecular weight of 40 kDa.
The antioxidant effect of PF-FPLC was below GSH, this behavior reported with other proteins (Chen et al., 2012; Ahn et al., 2014). This antiradical effect presented because the amino acids residues are capable of promoting radicals elimination (Li et al., 2017). However, the antiradical effect shown by the PF-FPLC is higher than that reported in another's species of Solanum genus. Some examples are patatin of Solanum tuberosum that has an antiradical effect of 50% to 582 ppm (Elahi and Mu, 2017), as well as proteins isolated from S. tuberosum and Solanum betaceum, which have an antiradical effect of 50% using 55 and 73 ppm, respectively (Ordóñez et al., 2011). These results suggest that PF-FPLC purification could increase its enzymatic activity and its biological effect. The antioxidant effect could occur by cysteine content in the amino acid sequence because it is known that this amino acid is a precursor of glutathione and has a powerful antioxidant effect (Aldini et al., 2018; Sah et al., 2016). Furthermore, the antioxidant effect of a protein compound is greater when it has residues of 5 to 20 amino acids, a molecular mass ˂ 5 kDa, 41% hydrophobic amino acids and 12% aromatic amino acids (Sarmadi and Ismail, 2010).
A. salina assay suggests that the proteins contained in PF-FPLC have a selective effect since also the antibacterial and lymphoproliferative effects in peripheral blood mononuclear cells were observed. Briefly, it should be noted that the antibacterial effect exhibited by PF-FPLC is related to molecular weight, amino acid residues (AA) and lysine or arginine content since these amino acids have antimicrobial effects. This effect is due to the structural conformation of the amino acids which includes properties such as net charge, amphipathicity, and hydrophobicity (Yeaman and Yount, 2003; Xie et al., 2013). Therefore, the PF-FPLC proteins might be able to destabilize bacterial membranes and kill some pathogens (Hu et al., 2015). The effect is similar to S. tuberosum aspartic protease since present an effect on Bacillus cereus, E. coli, and S. aureus of 0.24-4.24 M (Mendieta et al., 2006; Frey et al., 2018).
The reduction PBMC treated with different concentrations of PF-FPLC occurs by an interaction between cellular sensitivity and protein chemical structure. However, the reduction in viability caused by PF-FPLC is acceptable since there are compounds which present a viability reduction in small concentrations; paclitaxel is an example (Kasemwattanaroj et al., 2013). The PF-FPLC proteins showed an effect on the decreased viability of PBMC directly proportional to test concentrations. There are insufficient data on the mechanism of S. marginatum L. f. proteins action on normal blood mononuclear cells. Nevertheless, could be related to the ability of cells to adapt to the effects of proteins involved in chemotactic effects (Alfaro Leon et al., 2005), such as serine proteases capable of producing cytokines (IL-4, IL-10 and TGF-β) (de Matos Guedes et al., 2010; Øya et al., 2018).
Additionally, some compounds with antioxidant effect diminish PBMC viability since they give a rise oxidative change, induce apoptosis, and cause changes in the granularity and size of these cells. Therefore, the PF-FPLC antioxidant effect could be implicated in the viability decrease of PBMC (Bors et al., 2012). Some compounds isolated from plants have an anti-lymphoproliferative effect (Meng et al., 2013), but most have a proliferative effect (Yeap et al., 2007). On the other hand, some proteins possess multifunctional properties including the immunomodulatory effect in PBMC, lectin of Microgramma vacciniifolia is an example (de Siqueira Patriota et al., 2017). These results are related to effect shown by a protein isolated from Solanum tuberosum, which is capable of producing significant changes in hematological parameters; such as the reduction of neutrophils and the increase of lymphocytes in mice (Lynch et al., 2012).
CONCLUSIONS
In this work, a high yield protein was found in PF-FPLC that is comparable to that reported in other plants. The extract contains at least seven fragments with molecular masses of 18-112 kDa. Additionally, this fraction displays enzymatic activity and has antioxidant effects. On the other hand, we observed that PF-FPLC has no toxic effect in A. salina, but reduces the viability of PBMC. Additionally, this fraction shows a low antimicrobial effect against the microorganisms tested. Consequently, the antimicrobial effect is related to the use of the plant in Mexican traditional medicine. In addition, the antioxidant and enzymatic effects could be related to the antibacterial effect and the reduction in the viability of the PBMC cells. Since this type of compounds can permeabilize both prokaryotic and eukaryotic cell membranes.











 nova página do texto(beta)
nova página do texto(beta)





