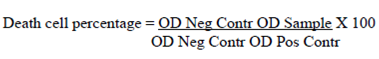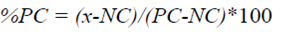Introduction
Breast cancer is a heterogeneous and complex disease, and it leads to most of the deaths from cancer among women throughout the world. In 2010, estimates showed that over 1.5 million women worldwide were diagnosed with breast cancer. this cancer can be classified into at least four subtypes: luminal a (MCF-7 cell line), luminal b, HER2 (HCC1954 cell line) and triple negative (HS578T cell line) (Holliday and Speirs, 2011). Each subtype has different prognoses and treatment responses (Perou et al., 2000). Different studies indicate the need for a new approach when screening potential new breast cancer therapies that use natural products. For over 40 years, natural products have remained important because they are established cancer chemotherapeutic agents. For example, antitumor compounds from microorganisms include anthracyclines, bleomycin, dactinomycin and mitomycin c. In turn, members of four types of plant-derived compounds are widely used as antitumor agents: bisindole alkaloids, camptothecins, epipodophyllotoxins and taxanes (Kinghorn et al., 2009).
During the drug discovery process, the pharmaceutical industry routinely assesses the P450 inhibition potential of drug candidates to exclude potent inhibitors from further development. Measuring the effect of new chemical entities on the activities of human cytochrome P450 marker using in vitro experimentation is an important experimental approach. In vitro drug interaction data can be used to guide the design of clinical drug interaction studies, or, when no effect is observed in vitro, the data can be used in place of an in vivo study to indicate that no interaction will occur in vivo. Therefore, determining the Ic50 values of drug candidates against major cYP450 activities is ideal for providing information to inform the decisions made during early drug discovery.
While searching for cytotoxic metabolites from the endemic flora of the Yucatan peninsula, we selected Lonchocarpus punctatus after using a bioassay-guided fractionation approach. Lonchocarpus punctatus is a common tree in the Yucatan peninsula. The Mayan names for this tree include “balché”, “x-balché”, and “saayab”. Although it is primarily an ornamental plant, its leaves are used as treatments for asthma and headache, as well as an antitussive; a mixture of its bark with honey is used as antiparasitic remedy, and the resin of this plant is used to treat stomachaches (Mendieta, 1981).
Previous phytochemical studies on L. punctatus have revealed the presence of stilbenes, longystiline A, B, C, and D in the bark and root extracts; these compounds have cytotoxic activity (Gonçalvez de Lima et al., 1975; Marta et al., 1979) recently, we found that the ethanolic extract of the L. punctatus inflorescence showed cytotoxic activity against McF-7, Hcc1954 and Hs578t cancer cell lines during an Mtt (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay. While considering the potential of L. punctatus as a source of bioactive compounds, the aim of this study was to isolate, purify and identify the cytotoxic constituents of this species. Of the five isolated compounds, trans 3,4,4’,5-tetramethoxystilbene (3) displayed the most interesting biological properties. this active compound was tested in three validated assays for human P450 marker substrate activities (diclofenac 4’α-hydroxylase for CYP2C9, dextromethorphan O-demethylase for CYP2D6 and testosterone 6 ß-hydroxylase for CYP3a4 and cYP3a5) while using the approaches described in the GLP (good laboratory practices) as per the code of U.S. Federal regulations. additionally, 3,4,4’,5-tetramethoxystilbene (3) was tested against immortalized hepatocyte cell line.
Methods
General
The 1H- and 13C-NMR spectra were recorded on a bruker avance 400 MHz spectrometer in cDcl3 while using tMS as an internal standard. Two microlitres of the samples were analysed via Lc-HrMS. The Lc analysis was performed on an agilent (Santa Clara, CA) 1200 while using a Zorbax Sb-c8 column (2.1 x 30 mm). Full diode array UV scans from 100 to 900 nm were collected in 4 nm steps at 0.25 sec/scan. The mass spectrometry data were acquired on a Bruker maXis Hr-QTOF mass spectrometer (Bruker Daltonics GmbH, Bremen, Germany) coupled to the previously described LC system. The eluting solvent was ionised using the standard maxis ESI source with the drying gas flowing at 111/min at 200°C and a nebulizer pressure of 40 psig. the capillary voltage was set to 4000 V. the mass spectra were collected from 150 m/z to 2000 m/z in positive mode. The LR-EIMS data for compounds 2 and 4 were determined on a Hewlett Packard GC Mass selective detector.
Plant material
The inflorescences of L. punctatus (2 Kg) were collected in October 2011 on the road in Libre Unión-Yaxcabá, Yucatán, México. The botanical identification was performed by doctora Martha Méndez from the Unidad de recursos Naturales. a voucher specimen was deposited in the herbarium “Roger Orellana” of the Unidad de recursos Naturales of CICY (Collection number 1787).
Extraction and fractionation of the plant material
The inflorescence (2 kg) was macerated with ethanol for three days (3×), to yield 99g of crude ethanolic extract. Partitioning the ethanolic extract (2.88 g) with hexane and mixtures of acetonitrile (CH3CN)/MeOH yielded 900 mg of polar extract. the CH3CN/MeOH extract was fractionated on a rediSep rf c18 flash silica column (20 g) attached to a Teledyne Isco flash chromatography system. Briefly, a linear gradient of MeOH/water starting from 10% MeOH to 100% MeOH over 35 minutes was established. the chromatogram was monitored by observing absorbance at 210 and 360 nm. Five major fractions were obtained. the fractions and the main extract were tested in an Mtt based bioassay to evaluate their cytotoxicity against tumour cell lines. Fraction five displayed cytotoxic activity and was further purified by HPLC.
HPLC purification
Bioactive fraction 5 (20 mg) was subjected to semipreparative HPLc. the HPLc system (Gilson 281 serie 100878) consisted of an auto-sampler, an injector (50 μl), a pump and a UV detector. A reverse-phase C18 column (250 x 9.4 mm Zorbax SB C18 agilent technologies) was employed. A gradient elution was performed with CH3CN/H2O mixtures from 10% CH3CN to 60% CH3CN over 10 minutes, followed by an isocratic elution at 60% CH3CN for 25 minutes and a final gradual ramp to 100% CH3CN over 10 minutes. The flow rate was 3.6 mL/min, and the ultraviolet absorbance data were observed at 210 and 305 nm (fig. 1). Repeated purification of fraction 5 allowed the isolation of compounds 1-5.
Tumour cell lines
The three breast cancer cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USa). The Hs578t and HCC1954 monolayer cells were maintained in RPMI medium supplemented with 10% foetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. the McF-7 cells were maintained in MEM supplemented with 0.01 mg/mL bovine insulin. For the control, normal cells Fa2N4 (Tebobio) were cultured in MFE Essential Support Medium F with MFE culture Medium Supplement a. all cell cultures were maintained at 37oc under a humidified atmosphere of 5% CO2.
Cytotoxicity assay
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide) assay test was used to evaluate the cytotoxic activity. The assay is based on the ability of drug-treated cells to reduce the yellow water-soluble MTT into a dark blue formazan product that is insoluble in water. The NADH, which is required for proper metabolic function, is provided directly by the cells. Therefore, the MTT reduction rate is an indicator of the functional integrity of the mitochondria, and, therefore, this assay measures the cellular viability. For the in vitro cytotoxic activity assay, 30 000 breast cancer and 20 000 Fa2N4 cells were used per well. The samples were incubated with 60-80% of confluent culture from each cell line for 24 h under 5% CO2 at 37oc.
In the first step, each extract was tested at 1 mg/mL in triplicate (data not shown). The active extracts were tested further at different concentrations ranging from 1 to 0.0625 mg/mL in triplicate (data not showed). The pure compounds were evaluated from a starting solution of 50 μM with 1:2 dilutions. After 24 h of incubation with the extracts/ fractions or 72 h with pure compounds, the optical density was measured at 570 nm with a Victor2TM Wallac spectrofluorometer.
The inhibition percentage against the tested cell line was determined by the following equation:
where + OD_Neg Contr is the optical density of the negative control at 570 nm (0% death cell); OD_Pos Contr is the optical density of the positive control at 570 nm (100% death cell) and OD_Sample is the optical density of the samples at 570 nm. The medium with 0.5% DMSO was used as negative control, and the positive control was the medium with 4 mM of methyl methane sulfonate (MMS). The internal control curves were those from paclitaxel, doxorubicin and vorinostat (50 μM; 1/2 dilution;10 points).
Data presentation and analysis
The MTT data were analysed using the Genedata Screener program (Genedata aG, Switzerland). This program uses the Levenberg-Marquardt algorithm to calculate CC50 values. DMSO was used to dissolve the compounds and was used as a negative control for all cells assayed; 2 mM MMS was the positive control. In all results the RZ’ factor was between 0.7-0.93.
CYP inhibition assay by HLM- LC/MS- MS method
The incubations used while evaluating the CYP inhibition were conducted in a 96-well plate format at 37°C. The final mixture (200 μL total volume) contained 0.25 mg/mL of HLM protein in 100 mM potassium phosphate buffer (pH 7.4), 1 mM NADPH, and the test compound was used in triplicates at 0.078, 0.313, 0.625, 1.25, 2.5, 5, 11, 22, 44 and 88 μM. The probe reaction for CYP3A4 was conducted with 50 μM testosterone and HLM protein for 15 min. the CYP2D6 probe reaction was conducted with 22 μM dextromethorphan for 15 min. the CYP2C9 probe reaction was conducted with 10 μM diclofenac for 15 min.
The test compound in DMSO/CH3CN 35/65 (v/v) (2 μL) was combined with 98 μL of NADPH solution, and the reactions were initiated by adding 100 μL of the enzyme substrate solution. The reactions were terminated by adding a quench solution (90 μL) of CH3CN containing the internal standards for the LC-MS/MS analysis (60 ng/ml cortisone, 100 ng/ml 4′-hydroxydiclofenac 13C6, 60 ng/ml levallorphan). Regarding the experimental design of incubation plates, eight positive controls (no inhibitor incubations) were included in each assay plate to determine the maximum amount of product formed, and, conversely, eight negative controls (no NADPH incubations) were included to assess the contribution of assay reagents to the signal readout in each assay plate. Control inhibitors, such as ketoconazole for CYP3a4, quinidine for CYP2D6, or sulfaphenazole for CYP2C9, were included in all incubation plates. The final DMSO content was established at 0.35% for all isoforms. the reaction supernatants, which were clarified by 10 min of centrifugation at 3717 G (4°c), were analysed by LC/MS-MS to obtain the relative quantities of the metabolites (6β-hydroxytestosterone, 4′-hydroxy- diclofenac, or dextrorphan) generated from the corresponding probe substrates.
LC/MS-MS analysis
The quantitative analyses of the probe substrate metabolites in quenched reaction supernatant was performed using an Agilent 1190 liquid chromatograph equipped with an aPI4000 (triple quadrupole) mass spectrometer (AB SCIEX). The chromatographic separation was achieved using a Discovery HS c18 (50 mm 2 mm, 5 μm) column (Supelco, torrance, ca) preceded by a Discovery HS C18 precolumn. The column temperature was 25oc. The mobile phase consisted of two solvents: (a) water/MeOH 90/10 (v/v) in 0.1% formic acid and (b) CH3CN/water 90/10 (v/v) with 0.1% formic acid. Mobile phase b was held at 10% for 0.5 minutes before being increased to 45% b for 1.50 minutes. This solvent composition was held for 0.30 minutes before being increased again to 95% b for 2.30 minutes, held for 0.50 minutes, and returned to 10% b for 0.10 minutes for re-equilibration, which occurred for 0.61 minutes. The total run time was 3.51 minutes, and the flow rate was 1 mL/min. the mass spectrometer was operated with APCI ionization probe in positive mode using the multiple reaction-monitoring scanning mode. The reaction product and internal standard peak areas were integrated using the analyst software.
Data analysis
All of the IC50 values and solubility limits were calculated using the GeneData Screener application software. The equation used for normalizing the CYP inhibition data is as follows:
Where %PC is the percentage of activity remaining at a particular concentration after normalization, NC is the median of the measured signal values for the negative control wells on a plate, PC is the median of the measured signal values for the positive control wells on a plate, and x is the measured raw signal value of a well. The normalized data were fitted with a smart engine embedded in the core of the Gene-Data Screener while using a four-parameter logistic fit to determine the IC50 values.
Results
The ethanolic extract (EtOH) of the inflorescence from L. punctatus showed cytotoxic activity against McF-7 (CC50 = 0.22 mg/ mL), Hcc1954 (CC50 = 0.14 mg/mL) and Hs578t (CC50 = 0.06 mg/mL) breast cancer cell lines during a preliminary evaluation. A separation of the EtOH extract by C18 reversed-phase chromatography afforded five main fractions. A bioassay-guided isolation led to the purification of the five major components present in the active fraction (Fig. 2). Metabolite 1, obtained as a pale yellow oil, was isolated together with metabolite 2. Both showed a pink colour upon spraying with a solution of 20% phosphomolibdic acid and UV spectrum characteristic of stilbenes. Metabolite 1 showed a molecular formula of C16H1602 which was deduced by its LC-HRMS (m/z 241.1223 [M + 1+]); Calcd 240.1141). the 1H NMR spectrum of 1 presented two methines at δ 7.07 (d, J = 16.3 Hz) and δ 7.09 (d, J = 16.3 Hz) indicatory of a trans-double bond. Three aromatic protons at δ 6.68(2H,d,J=2.2Hz) and δ 6.41 (1H, t, J = 2.2 Hz), two methoxyl signals at δ 3.84 (s, 6H), and five aromatic protons between δ 7.27 and δ 7.52 corresponding to a monosubstituted aromatic ring.
Metabolite 2, isolated as a pale yellow oil, appeared very similar to metabolite 1, but all the signals were somewhat upfield shifted with respect to those of 1. The only difference between the two metabolites was the coupling constant measured for the vinyl protons of 2 (J = 12.0 Hz). These spectroscopic data are characteristic of unsymmetrical trans (J = 16.0 Hz) and cis (J = 12.0 Hz) isomers of 3,5-dimethoxys- tilbene. The structures of metabolites 1 and 2 were confirmed by comparison with the stilbenes reported from the bark of some Pinus species and in commercial soy proteins (Boatright et al., 1998).
Metabolite 3, isolated as a yellow oil, showed a molecular ion peak at m/z 301.1443 [M + 1+] (Calcd 300.1363) from its LC-HRMS, corresponding to a molecular formula of C18H20O4. It was characterized by the presence of signals corresponding to a disubstituted trans double bond (doublets at δ 6.56 and δ 6.63 with J=16.1Hz) in its 1H NMR (fig. 2). The UV absorption suggested the presence of a skeleton of a stilbene in the molecule, whose substi- tuents were four methoxyl groups as seen by 1H NMR. Other signals present in the 1H NMR spectrum and their multiplicities support the identification of three as trans 3,4,4’,5-tetramethoxystilbene (fig. 3). This metabolite has been previously isolated from leaves of Piper caninum (Farediah et al., 1997). Metabolites 4 and 5 were isolated as yellow needles and were identified as 5-hydroxy-7,4’-dimethoxyflavone and 5,7,4’-trimethoxychalcone, respectively. The former was previously isolated from Biota orientalis and the latter was reported from Crotalaria ramossisima (Hyun et al., 1995; Lai et al., 2010).

Source: Authors, 2017.
Fig. 3 1HNMR spectrum of trans-3,4, 4’,5-tetramethoxystilbene (3) (400 MHz, CDCL3).
The isolates (1-5) were tested for cytotoxicity against the three human breast cancer cell lines while using paclitaxel, vorinostat and doxorubicin as positive controls. Additionally, a healthy hepatocyte cell line (Fa2N4) was included to evaluate the hepatotoxicity of the actives compounds. The results are summarized in table 1.
Table 1 Cytotoxic activity of the constituents of L. punctatus inflorescencea
| Compounda | MCF-7 | Hcc1954 | Hs578t | Fa2N4 |
|---|---|---|---|---|
| 1 | >50.00 | >50.00 | >50.00 | >50.00 |
| 2 | >50.00 | >50.00 | >50.00 | 48.20±3.25 |
| 3 | 2.20±0.11 | 1.10±0.07 | 1.80±0.26 | >50.00 |
| 4 | 4.80±0.75 | 0.80±0.09 | 1.13±0.32 | 4.60±0.81 |
| 5 | >50.00 | >50.00 | >50.00 | 31.20±2.50 |
| Paclitaxel | -- | < 0.10 | 40.02±2.60 | <0.10 |
| Vorinostat | 6.80±1.20 | 5.90±0.95 | 1.67±0.42 | 4.30±0.98 |
| Doxorubicin | 0.86±0.12 | 0.96±0.07 | 0.16±0.07 | 0.47±0.09 |
aResults are expressed as CC50 values (µM)
No cytotoxic effect was observed with compounds 1, 2 and 5; however 3,4,4’,5- tetramethoxystilbene (3) and 4,4’,6’-trime-thoxychalcone (4) were the active principles present in the EtOH extract of L. punctatus. In addition, 3,4,4’,5-tetramethoxystilbene (3) had the highest cytotoxic activity (cc50 values between 1 and 2.2 μM) and no cytotoxicity against the Fa2N4 cell lines versus 4,4’,6’-trimethoxychalcone (4). In fact, 3,4,4’,5-tetramethoxystilbene (3) showed CC50 values lower than those of Paclitaxel when tested against the Hs5787 cell line, similar to Vorinostat in all tumoural cell lines, but it has no effect against a healthy cell line (Fa2N4). Due to the different behaviours of this compound against tumoural and non-tumoural cell lines, we will study 3,4,4’,5-tetramethoxystilbene in greater detail.
The in vitro inhibition data for trans 3,4,4’,5-tetramethoxystilbene in specific human CYP enzymes (CYP, 2C9, 2D6, AND 3A4) was obtained using the previously described validated assays; this compound displayed medium inhibitory properties on the CYP3a4 (Ic50 = 2.95±0.53 μM) and CYP2C9 (IC50 = 6.48±0.04 μM) isoforms and a low inhibition on the CYP2D6 (Ic50 > 34.00 μM) isoform. because the inhibitory potency (IC50) was >1 μM in all cases, an in vivo drug-drug interaction would not be observed; For CYP3A4 and CYP2C9, however, the inhibitory potency was between 1 μM and 10 μM, indicating that the trans-3,4,4’,5-tetramethoxystilbene (3) might interact with other drugs metabolized via CYP3A4 and CYP2C9. Fortunately, these results indicate that multiple metabolic pathways are available to trans-3,4,4’,5- tetramethoxystilbene, decreasing the risk of DDI.
Stilbenes are a group of metabolites commonly found in higher plants, especially in the leguminose family. These compounds have demonstrated multiple chemopreventive properties including the inhibition of the three major stages of tumour formation: the initiation, promotion and progression phases (Jang et al., 1997). Methoxystilbenes have demonstrated cytotoxic activities. In fact, 3,4,4’,5-tetramethoxystilbene and trans 2,3’,4,5’-tetrametoxystilbene are potent apoptosis-inducing agents with clinical potential (Robeti et al., 2003). Among the other methoxystilbenes, 2,3’,4,4’,5’-penta- methoxystilbene is a potently induces apoptosis in colon cancer cells by targeting the microtubules (Li et al., 2009). This report is the first to describe the cytotoxic activities of inflorescence extracts of L. punctatus.
Conclusions
A bioactivity-guided approach identified trans-3,4,4’,5-tetramethoxystilbene (3) and 4,4’,6’-trimethoxychalcone (4) as the active principles of the inflorescence extract of L. punctatus. These compounds could account for most of the cytotoxic activity founded in this species. tetramethoxystilbene (3) exhibited potent cytotoxic activity against breast cancer cell lines, but its lack of cytotoxic effects against immortalized hepatocyte cell lines (CC50 > 50 μM), as well as its medium (CYP3A4, CYP2C9) and low (CYP2D6) inhibitory properties on P450 isoforms, make it a good candidate for further studies.











 nueva página del texto (beta)
nueva página del texto (beta)