Introductión
The family Rutaceae Juss. is placed in order Sapindales primarily because of the pinnately compound leaves, absence of resin ducts in the bark, wood rays, leaf veins, the presence of triterpenoid compounds and flowers with a distinct nectar disk (Cronquist, 1992; AGP III, 2003; Judd et al., 2008) and includes species of great economic importance as ornamental plants, medicinal or culinary uses or perfume industry such as the orange, lemon, lime, kumquat, mandarine and grapefruit. The Rutaceae are represented in Argentina by seven genera and approximately 18 species (Seo and Xifreda, 2008).
They are shrubs, trees, or sometimes herbs, sometimes scrambling or scandent, sometimes armed, with aromatic volatile oils contained in glands visible at surface of, at least leaves, young branchlets, inflorescences, flower parts, fruit, or cotyledons in seed. The genus Zanthoxylum L. is one of the major genera of Rutaceae with at least 200 species in tropical (mainly) and temperate regions of the world (Figueiredo Melo & Zickel, 2004; Zhang & Hartley, 2008). It includes shrubs sometimes scrambling, trees, or woody climbers, evergreen or deciduous, usually armed. The leaves are alternate, odd-pinnately 3-to many foliolate or sometimes digitately 3-foliolate. Zanthoxylum is represented in Argentina by 10 species, two of them inhabiting in central region of the country, Zanthoxylum coco Gillies ex Hook. f. & Arn. and Zanthoxylum armatum DC var. armatum. These taxa are the only species of Zanthoxylum found in Pampean hills of central Argentina, which belong to the biogeographic province of Chaco (Morrone, 2001; 2004). This province is characterized by xerophytic deciduous forests of Vachellia caven (Molina) Seigler & Ebinger, Aspidosperma quebracho-blanco Schltdl., Celtis ehrembergiana (Klotzsch) Liebm., Lithraea molleoides (Vell.) Engl. and Zanthoxylum coco as predominant species of trees. Although these species are studied in floristic, taxonomic and ethnobotanic treatments, there are very scarce morphological and anatomical studies of vegetative organs of these species.
Anatomy describes the internal structure of plants and is considered as a source of correct identification of taxa. Anatomy centres on the spatial arrangement of the dermal, ground, and vascular tissue systems (Nancy & Dengler, 2002). Similarly foliar epidermal microscopic features like shape of epidermal cell, type of stomata, presence or absence of pubescence and cell wall thickness are also considered as useful tools for correct taxa identification and their phylogenetic relationship with other taxa (Stace, 1965; Babalola & Victoria, 2009).
Although Zanthoxylum coco is one of the most common species of trees in forests in central Argentina Hills and it is used as medicinal plant (Battista et al., 2007), their leaves and stems have not previously been studied. The shrub Zanthoxylum armatum is an important medicinal plant extensively used, in the countries which is native, for curing Pneumonia, tick infestation and gum diseases (Iqbal & Hamayun, 2005), fruit is used for toothache, dyspepsia, and seeds are used as condiment and flavoring agent (Arshad & Ahmad, 2005; Abbasi et al., 2010). Recently the leaves and fruits were investigated for various pharmacological activities and has showed good results including cytotoxic, phytotoxic, antipyretic activities and rationalise its local uses (Suksathan et al., 2009; Barkatullah et al., 2011) and in Argentina was found as naturalized species (Arana & Oggero, 2009). Keeping in view the significance of morphological and anatomical studies for taxonomy and ecology, a comparative morphological and anatomical study of the leaves and young stems of the species of Zanthoxylum from central Argentina is carried out.
Material and Methods
Plant material
The collections of the botanical material and the field observations were carried out in Sierra de Comechingones, located in the southwestern part of Cordoba province, Argentina (Fig. 1).
The comparative morphological description of the leaves and stem was carried out using fresh material and herbaria specimens and it is based in Escalante (1961) for Zanthoxylum coco and Arana & Oggero (2009) for Zanthoxylum armatum. The herbaria consulted were CORD, LIL, LP, SI, RCVC and RIOC, and digital images of the types (provided by G and K) were also seen. The selected samples of the specimens collected were deposited in the Herbarium of Departamento de Ciencias Naturales, Facultad de Ciencias Exactas, Físico-Químicas y Naturales, Universidad Nacional de Río Cuarto (RCVC) and registered as follows:
Zanthoxylum armatum:
Argentina. Córdoba. Depto. Calamuchita, Embalse cerro Pelado, A. J. Oggero & M .D. Arana s.n. (RCVC); Villa Amancay, 14-III-2009, M. D. Arana & a. J. Oggero s.n (RCVC); Depto. Río Cuarto, Alpa Corral, 30-III-2000, C. A. Bianco, s.n. (RCVC 3625).
Zanthoxylum coco:
Argentina. Córdoba. Depto. Calamuchita, Embalse Cerro Pelado, 14-III2009, A.J. Oggero & M.D. Arana s.n (RCVC); Depto. Río Cuarto, Sierra de Los Cóndores, 10-X-1997, M. Ceballos, s.n. (RCVC 3948).
Morphological studies
The analysis of the stem and leaf structure was done using freshly collected material or fixed material. The leaves located in the nodes of the middle part of the newest branchlets (there is one leaf per node along the stem) were used to carry out the anatomical study. The samples were taken from the central zone of the middle leaflet and the rachis. To anatomical studies of stem, the internodes (a portion of a plant stem between nodes) of the middle region of the branchlets were collected.
The samples were placed in FAA (95% ethanol: glacial acetic acid: 37-40% formaldehyde: water; 50:5:10:35, v/v). The dehydration of samples was done according to the procedures outlined in Johansen (1940) using graduated solutions of ethanol and xylene. Fully infiltrated tissues were embedded in Histowax (highly purified paraffin wax blended with polymer additives). A series of transverse sections 12 μm thick were obtained from the sample blocks using a Minot rotary microtome. The sections were triple-stained with hematoxylin, safranin O and fast green FCF as described by Johansen (1940). To verify the presence of starch, semi-permanent slides with Iodine dissolved in an aqueous solution of Potassium iodide (Lugol ́s Iodini solution) colored were made.
Epidermal studies were carried out using leaflet samples fixed in FAA which were submerged in a solution 40% of sodium hypochlorite and heated to epidermal separation. The epidermal samples were washed with water and safranin coloured and the semipermanent slides for microscope using water-glycerine (1:1) solution were made, according to the usual techniques.
The stomatal index (SI) of each taxon was calculated from five fields in the middle region on lower surface of the blade of six leaves taken from different plants, using the formula proposed by Cutter (1969).
The stomatal density, trichomes and (just in case of Zanthoxylum coco) glands were counted using ten microscope fields (0.25 mm2) on each epidermis of six leaflets.
A standard Zeiss Model 16 microscope was used to assess the histological preparations and photomicrographs were taken with a Zeiss Axiophot microscope with an equipment of image capture and digitalization AxioVision 4.3, with camera AxioCam HRc.
Results
Zanthoxylum armatum var. armatum
Young stem and leaves morphology
The plants of Zanthoxylum armatum are shrubs to 5 m tall, deciduous, with dark brown cilindrycal stems, alternate phyllotaxis and compound, imparipinnate leaves (Fig. 2b). Stems, branchlets and leaflet blades abaxially on midvein with dark brown, stout prickles with a broad flattened base. Young branchlets and inflorescence rachises are glabrous or rust-colored pubescent. The leaves are 3-5(7) foliolate with rachis and petioles glabrous or rust-colored pubescent, broadly winged, wings to 6 mm on each side. The leaflet blades are dark green coloured, subsessile, opposite, narrowly elliptic (5-15 × 1-3 cm), base attenuate to broadly cuneate, the terminal leaflet is the largest. The margin is crenate, finely serrulate or entire and revolute when dry, apex acute to acuminate, with the midribs impressed above, elevated beneath. The lateral veins are 6-7 on each side of the midrib with pellucid gland on margins.
Anatomy of leaves and young stems
The leaflets are dorsiventral. In transverse section the epidermis of adaxial surface present isodiametric cells bigger than the abaxial surface. The stomata are present in abaxial surface and are at the same level of the surrounding cells. The palisade tissue is unilayered, represents the 50% of the mesophyll and it is continuous, even in the midrib region (Fig. 3a, b). The secretory cavities are present in the margins of the leaflets only. The vascular bundles are collateral, small and are proximal each other and have bundle sheaths (Fig. 3c).

Fig. 3 Micrographs in transverse section of leaves and young stems of Zanthoxylum armatum (A) midrib region of leaflet (B) detail of mesophyll (C) margin of the leaflets with secretory cavities, (D) midrib region of rachis, (E) detail of wing of rachis with peltate tricome, (F) young stem (G) and (H) detail the different zone of showing the starch in cells. co, cortex; ep, epidermis; p, pith; pa, parenchyma; ph, phloem; pp, palisade parenchyma; pt, peltate trichome; s starch; sc, secretory cavity; sp, spongy parenchyma; xi xylem; ue, upper epidermis. Scale bars = 50 μm (a), (c), (e); 40 μm (b), (f);100 μm (d), (h), and 20 μm (g), (i), (j).
The midrib is surrounded by parenchyma and it is very prominent in the abaxial surface (Fig. 3a).
The rachis is winged (Fig. 3d). The epidermis of the wings is similar to the leaflets but it is observed a difference in the proportion of the palisade tissue, which represent less than the 50% of the mesophyll (Fig. 3d). The central zone of the rachis presents a pith surrounded by xylem and phloem, where secondary growth is observed. The secondary phloem is in contact with chlorenchyma in the abaxial surface, where a layer of collenchyma is also found (Fig. 3e).
The epidermis of both, the leaflets and rachis, present peltate trichomes, which are glandular and multicellular. The trichomes are sunken in the epidermis (Fig. 3e).
The epidermis of leaves is unilayered in both, the adaxial and abaxial surfaces and present a slight striated cuticle (Fig. 4a, c). The epidermal cells of the adaxial surface have straight walls and are pentagonal or quadrangular of different sizes, while the cells of the abaxial surface are irregular with sinuous anticlinal walls. There are multicellular peltate hairs in both epidermical faces. These trichomes present a multicellular peduncle and a head with four central cells with strong walls and numerous peripheric cells (Fig. 4b). The average density of the trichomes was 3 per mm2 in abaxial surface and lower than 1 per mm2 in adaxial surface. The leaves are hypostomatic, the stomata are anomocytic and present different sizes and a heterogeneous distributional patron, some of them are so closed that the occlusive cells of the neighbouring stomata are in contact (Fig. 4d). The average SI was 22.4 whereas the stomatal density was 252.3 mm2.
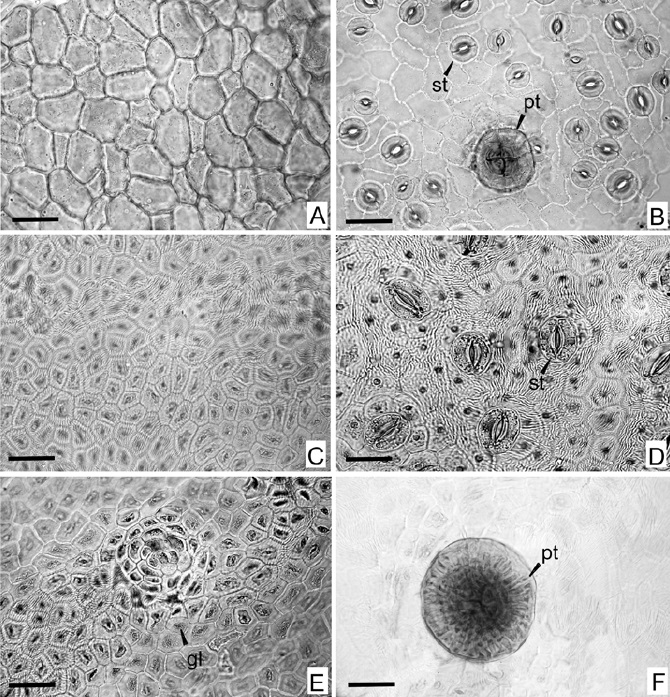
Fig. 4 Micrographs of leaf epidermis of Zanthoxylum armatum (A) adaxial epidermis, (B) abaxial epidermis withpeltate trichome; and Zanthoxylum coco (C) adaxial epidermis, (D) abaxial epidermis, (E) gland on adaxial epidermis, (F) peltate trichome. gl, gland; pt, tricome peltate; st, stoma. Scale bar = 50 μm.
The young stems present a dermal tissue that consists in a unilayered epidermis and a cortex formed by storage parenchyma subepidermic strata with abundant starch granules (Fig. 3g). The central zone of the stem presents a parenchymatous pith is surrounded by parenchyma with abundant starch granules (Fig. 3h) and secondary xylem forming a continuous cylinder with rays. a cambial zone separated the secondary xylem from the secondary phloem (Fig. 3f).
Zanthoxylum coco
Young stem and leaves morphology
The plant is a tree to 8 m tall, deciduous, with grey armed stems with short grey stout conic prickles when young and armed branchlets with straight pale brown prickles. The phyllotaxis is alternate with compound, imparipinnate or rarely paripinnate leaves (Fig. 2a). Young branchlets and inflorescence rachises are glabrous or minutely pilose, sulcate. The leaves are 3-7 foliolate with rachis and petiole glabrous, without wings. The rachis is sulcate, with two prominent glands in each node. The leaflet blades are membranaceous, glabrous, pale green coloured, with transparent oil glands dispersed on all surface. The leaflets are subsessile or pedicellate, opposite, narrowly obovate, base attenuate and obtuse apex, the terminal leaflet is similar to the lateral ones. The margin is crenate or serrulate and not revolute when dry. Rachises and petioles are armed with pale brown, straight or slightly curved prickles.
Anatomy of leaves and young stems
In transverse section of the leaflets blade, the cells of both epidermal faces tissue are similar in form and size and are protected by a thick cuticle. The stomata in abaxial face of the leaflet are at the same level of the surrounding epidermal cells. In the same face of the blade there is an important vascular bundle that shows a secondary growth, whereas to the adaxial face there is one little collateral vascular bundle that shows an inverted position. The vascular tissue is surrounded by parenchyma and it is prominent in both surfaces of the leaflet. The mechanical tissue is absent in this area (Fig. 5a). In the mesophyll the vascular bundles are collateral and surrounded by a bundle sheath. The palisade tissue constitutes the major part of the mesophyll and occurred on either side of the spongy mesophyll. The cells of the palisade tissue in the adaxial surface are long and narrow, while the cells of the abaxial surface are short and wide (Fig. 5b). In certain zones of the mesophyll the palisade parenchyma tends to dissapear and is replaced by the spongy one.

Scale bars = 50 um (a),(b),(c),(e) and 100 um (d),(f),(g).
Fig. 5 Micrographs in transverse section of leaves and young stems of Zanthoxylum coco (a) midrib region of leaflet (b) detail of mesophyll (c) margin of the leaflets with secretory cavity, (d) rachis, (e) detail of (d) showing peltate trichome, (f) young stem, (g) detail the (f) showing secretory cavity. c, carenae; ch, chlorenchyma; co, cortex; ep, epiderms; p, pith; pa, parenchyma; ph, phloem; pp, palisade parenchyma; pt, peltate trichome; sc, secretory cavity; sp, spongy parenchyma; xi xylem; ue, upper epidermis.
The secretory cavities are present in all parts of the leaflets, even in the margins (Fig. 5c). These secretory cavities are considered lysigenous because cellular remains in the borders of the cavities are observed. The secretory cavities are also found in all analyzed parts of the plant.
The Zanthoxylum coco epidermis of leaves is unilayered in both, the adaxial and abaxial surfaces and the cuticle is striated. The epidermal cells are similar in both surfaces, they are polygonal and present different sizes, with straight or slightly curved anticlinal cell walls (Fig. 4d, e). There are notorious multicellular peltate trichomes in both surfaces. These trichomes present a multicellular peduncle and a head with many central and peripheral cells (Fig. 4f). Also there are multicellular glands in both epidermis, the average density of these glands was 5 per mm2 in the adaxial surface and 3 per mm2 in the abaxial surface (Fig. 4e). The density of the trichomes was 4.5 per mm2 in abaxial surface and 1.5 per mm2 in adaxial surface. The leaves are hypostomatic, the stomata in the abaxial surface are separated by similar distances among them but they not showed a specific distributional pattern (Fig. 4e). The predominant type of stomata is anomocytic and there are some cyclocytic, both types of stomata are surrounded by 4-6 epidermic cells. The average SI was 8.03, and the stomatal density was 189.5 mm2.
The rachis is bicarenated and the carenae are in the adaxial surface. The epidermis is unilayered with stomata in the same level that the surrounding cells. The epidermis presents multicellular trichomes, which are peltate and glandular, located in cavities of the surface. Under the epidermis there are chlorenchyma interrupted by secretory cavities. There is a continuous cylinder of xylem and phloem surrounding a parenchymatous pith (Fig. 5d, e). The young stems (Fig. 5f) also present a unilayered epidermis covered by a thick cuticle protecting the parenchymatous cortex, which presents secretory cavities of relatively big size near to epidermis (Fig. 5g). The epidermis is interrupted by schlerenchyma, with cells highly lignified, in the areas where the p rickles are present. The vascular tissue has the same organization as the described for the rachis.
Discussions and conclusions
The studied Zanthoxylum species share morphological features, as armed stems, leaves alternate, odd-pinnately, 3-to many foliolate. The main differences are that Z. armatum is an evergreen (or sometimes deciduous) shrub with axillar inflorescences whereas Z. coco is usually a deciduous tree with terminal inflorescences.
Study of different types of tissues and other microscopic techniques like linear measurements, determination of leaf constants and quantitative microscopy are indispensable in the initial identification of plants materials (Jarald & Jarald, 2007). Some observed features in both species are characteristic of plants that inhabit places with xeric conditions as occurred in central hills of Argentina. Both species share the presence of secretory cavities, glandular trichomes and hypostomatic leaves. However each species presents particularities that could be important and related to the environment that the plants develop. These features are basically related to the leaves structure, owing to the fact that the leaf is the most sensitive organ and the first in showing responses to environmental conditions. The leaves reflect the morphological alterations as consequences of important environmental changes (Trewavas, 2003). Leaf epidermal studies are of immense significance in finding taxonomy of closely related species. taxonomists have given prime importance to leaf epidermal features to resolve taxonomic conflicts (Taia, 2005). Both species present thick epidermis being really striate in Z. coco. As well it has been informed that cuticle strias are under a strong genetic control (Ahmad, 1962). It is also considered that its development as the increase in thick of the epidermis, it can be influenced by environmental factors. In most of xerophytes, the epidermis presents different kind of strias while in species of mesophytes and hygrophytes the epidermis is plain (Dunn et al., 1965; Wilkinson, 1988). As well, it has been indicated that a thick epidermis contributes a protection of the photosynthetic tissues of a high sun radiation by an increase of the reflectance and striations can interfere in the absorption of the light by the mesophyll chloroplasts (Roth, 1990). In both species the walls of epidermic cells tend to be straight and thick, this characteristic could be associated to aridity conditions and/or to a bigger sunlight exposition (Wilkinson, 1988). by other hand the anticlinal walls of the cell of the abaxial epidermis always present more undulations than in the adaxial surface, this is coincident with what observed by Stace (1965) and agree with Roth (1990) who expresses that this characteristic is due to high shadow and humidity conditions. Both species present predominantly anomocytic stomata, this was also observed in an analysis of twelve species of the genus Zanthoxylum (Xochicale et al., 2010). Stomatal arrangement and types are considered best taxonomic criteria and provide efficient basis for relationship in taxonomic hierarchy (Hameed et al., 2008). Some workers like Sen & Hennipman (1981) had the idea that they may not be so an effective tool in taxonomy because of their inconsistent arrangement in epidermises, however stomatal values like stomatal number and stomatal index are of great value in the evaluation of leaf origin crude drugs (Evans, 2002; Barkatullah et al., 2014). Also, according to Wilkinson (1979) the values of SI are diagnostic features of importance in Systematics, because they not present alterations. However in this work, this affirmation is valid just for Z. coco, where the found value of SI (8.03) is similar to the SI (7.65) found in plants of Z. coco inhabiting the Yungas in northwestern Argentina (Arambarri et al., 2009). In Z. armatum we found a SI= 22.4, which is higher than the value of SI= 15, found in plants of Z. armatum from Thailand by Suksathan et al. (2009). Kürschner et al. (1998) and Salas et al. (2001) express that the value of SI is a parameter which is affected by stress conditions, specially nutritional and environmental ones. This allows us to determine that Z. armatum, which is exotic in Chaco biogeographical province, could be suffered remarked epidermal changes as result to adaptation to the xeric conditions of chacoan environment. Also we found that Z. armatum presents higher stomata density, that it would be possible, under hydric conditions, a higher gaseous exchange than Z. coco. Adaptation skills of plants depend on stomatal arrangement on the epidermises, as transpiration and photosynthesis are closely related to stomata. These are also useful in taxonomic categorization and detection of future clues for observing environmental factors. Micro and macro elements in plants are also closely related to stomatal density (Nabin et al., 2000; Brownlee, 2001). Very little work has been done on the stomatal study of family Rutaceae, Ogunkunle & Oladele (1997) reported paracytic, hemiparacytic, brachy paracytic, brachy paratetracytic and anomocytic stomatal complexes with uniform size from abaxial epidermises of various Citrus species (Rutaceae) and also proved that in spite of high stomatal density, they have relatively low transpiration rate as compared to species with low stomatal density. During a dry season, the margin of the leaves of Z. armatum are bended in direction to the abaxial area; this curving can also be suffered by the wings of the rachis. Therefore the appearance of the leaves changes, the stomata are protected, an attenuate diffusion gradient between chlorenchyma and the environment is generated and permits the reduction of stomatal transpiration.Tthis strategy allows the plants can use water efficiently so they are tolerant when this resource is limited (Fahn & Cutler, 1992).
As well the curving of the leaves is an especial answer to the hydric’s stress conditions that are observed in many species (Stevanovic, 1986; Stevanovic et al., 1992, Zivkovic et al., 2005), it is also related with a strategy for reducing the foliar area to an important sun radiation (Bocher, 1981). Z. coco does not present this behaviour and it is different from Z. armatum because shows a bigger development of the palisade parenchyma. This could indicate that it is better for high intensities of light of the region, due to a higher development of palisade parenchyma is related with habitats that are exposed to a drought and intense sun radiation (Bosabalidis & Kofidis, 2002; Fahn, 1964). It is necessary to highlight that Zanthoxylum coco is native of the northern and central areas of Argentina while Zanthoxylum armatum has recently been reported as naturalised (Arana and Oggero, 2009) and both species are found in the same habitat. Although the tissues organization in the stem is similar in both species, the stem of Zanthoxylum armatum shows a great capability to store starch in cells of the medulla, xylem and cortex as showed by the stained method used in this work.This species presents a small number of glandular trichomes and secretory cavities that are observed only on the margin of the leaves and the alate rachis, while in Z. coco the secretory conducts were very numerous in every analyzed organs and besides in this species it has been found glands in both foliar epidermis.
All these structures are common of observing in many species of Rutaceae (Judd et al., 2008) and it has considered that can contribute to the general regulation of transpiratory rate moderating any hydric unbalance. Kakic et al. (2009) studied Z. acanthopodium DC., and informed that the type of secretory structure and the secreted substances by this species and the members of this genus must be considered such as ecological meaningful systems of protection and defence that has a plant, since they are important for the taxonomic diagnostic (Metcalfe & Chalk, 1950) and also parameters of the hydric conditions.











 nova página do texto(beta)
nova página do texto(beta)




