Introduction
The reduction and slow growth of native species, especially in tropical areas, have led to increased use of fast-growing species in order to satisfy the demands of the construction industry and for reforestation purposes. Exotic species, like teak (Tectona grandis), gmelina (Gmelina arborea) and black wattle or mange (Acacia spp.) have been selected for their fast growth, wood quality and high yields in Central America (Fonseca 2004; De Camino & Morales, 2013).
In order to mitigate the problems brought on by deforestation in Costa Rica, the Government established the National Forestry Fund in 1980 and promoted plantations of the above-mentioned species. Teak was one of the main species introduced in Costa Rica, and by 2010 there were 9291.12 ha (22963.72 acres) of teak from a total of 28337.73 ha (70038.88 acres) of plantations (Sage & Quirós 2001; Arias et al., 2005, Murillo & Guevara, 2013, de Camino & Morales, 2013) in the country.
The diseases that affect this exotic tree have been studied in Costa Rica (Arguedas et al. 2013); however, the effects of saprophytic fungi on construction wood have received little attention. The aim of this study was to determine the effects of a white rot fungus (Rigidoporus cfr. microporus) previously collected on teak on the chemical, mechanical and physical properties of wood from teak trees of different ages. Knowledge of the potential damage of teak wood by this fungus, which is commonly found on the field, could help to improve control strategies to preserve the wood from decay.
Material and methods
Wood samples and fungal strain
The wood samples and the fruiting bodies of Rigidoporus cfr. microporus were obtained from a teak plantation in Aguas Zarcas, San Carlos, Alajuela, Costa Rica (10°34 ́36 ́ ́ N, 84°23 ́17 ́ ́ y 84°21 ́38 ́ ́ W). A dikaryotic strain of R. cfr. microporus was isolated and maintained on 2% MEA. Mycelia discs were removed from the peripheral growth zone and used to inoculate the wood samples (blocks) within the decay chambers. Sapwood (S) and heartwood (H) blocks were used as wood sources as well as sawdust from 6 and 10 yr. old trees (age factor), all these materials were prepared by the company, following standard procedures (ASTM 2000a). All the experiments were carried out at the Forest Resources Laboratory, Institute of Engineering Research, and at the School of Biology, University of Costa Rica.
Assays
Decay chambers were prepared according to the ASTM D-2017-05 (ASTM 2005). Two hundred and sixty two decay chambers were prepared with 658 sterilized blocks (198 chambers with 3 blocks each for wood decay, and physical and chemical studies, and 64 with 1 block each for mechanical analysis). A set of uninoculated sterilized wood blocks were used as control. The chambers were incubated at 27°C in the dark and samples were removed monthly for the physical tests for a period of 6 months. Samples for chemical, mechanical and anatomical studies were removed after 3 and 6 months.
A total of 594 blocks were sampled for the decay, physical and chemical test. Before placing them into the decay chambers, their initial dry weight was determined. Three hundred sixty blocks were inoculated for physical analysis (2 wood sources x 2 age factors x 6 months x 15 inoculated blocks), and 176 blocks used as control. In all cases, the removed blocks were oven dried at 65 ± 1°C for a week until achieving constant weight. Wood biomass losses were determined on the basis of the initial and final dry weight.
Light and electron microscopy studies were carried out with 3 and 6 months decay wood blocks (2), following the methodology recommended by Carpio (1992) and Karnovsky (1965).
Wood density was obtained according to ASTM D-2395-02 (ASTM 2002). The mechanical test of maximum compressive strength parallel to the grain was carried out at the third and sixth month. A total of 64 blocks were used for this test (2 wood sources x 2 age factors x 2 sampling months x 8 inoculated blocks).The maximum stress sustained by the samples was determined following ASTM D-143-94 (ASTM 2000b).
Chemical quantification of wood components was performed in order to determine the fungal effect on the xylem cell walls (insoluble lignin on acid and 1% NaOH solubles). Chemical assays were carried out according to procedures of the Forest Resources Laboratory, University of Costa Rica (Blanco 1998). The tests were repeated 2 or 3 times with samples exposed to the fungus for 3 or 6 months (a total of 56 blocks). Control tests were carried out with sawdust from 6 and 10 yr. old trees. The Klason lignin chemical analysis and the 1% NaOH soluble analyses were conducted following LPF - QM - 04 y LPF - QM - 08 procedures of the Forest Resources Laboratory, University of Costa Rica (Blanco 1998).
Statistical analysis
Wood biomass loss was analyzed using the STATISTICA program (Anonymous 1995), and the Post-Hoc Comparison of Means test LSD (0.05 and 95% confidence levels) was used for comparisons among treatments and for statistical significance (Sokal & Rohlf 1981).
Results
Wood decay assay (anatomical studies)
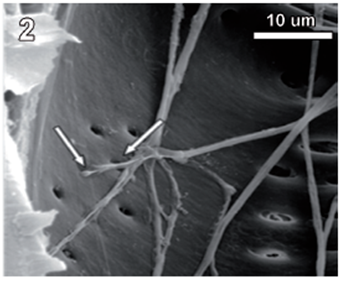
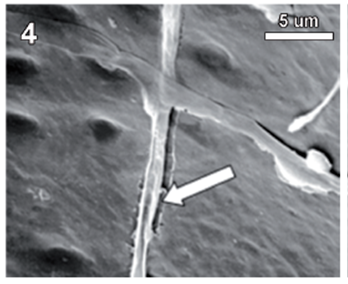
Rigidoporus cfr. microporus showed a rapid growth on MEA; hyphae with clamp connections were observed after a week and the mycelia covered the wood samples in 3-4 weeks. The infection process started on the multiseriate rays formed by parenchyma (fig. 1). After invading and degrading this tissue, hyphae penetrated the vessel elements through the bordered pits (fig. 2). During the first 3 months, a strong tissue disruption was observed as a loose arrangement of fibers, due to ray degradation and separation, but fibers did not show signs of deterioration (fig 3). After 6 months, severe damage was observed on 6 and 10 yr. old S blocks; 10 yr. old H samples were almost not degraded at all, but wall erosion furrows on the vessel elements were observed in all the samples (fig. 4).
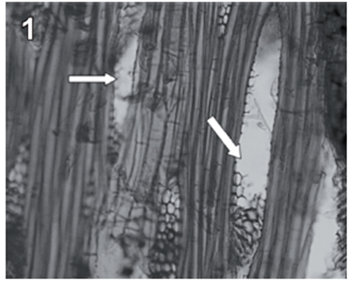
fig. 1 Tangential section (100 X) of teak wood block after 3 months of exposure. Arrows show degraded radial parenchyma.
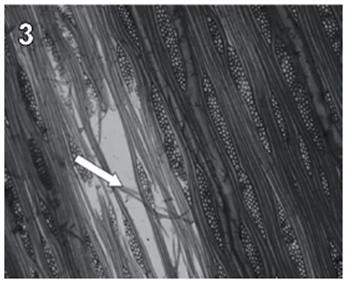
fig. 3 Tangential section (40 X) of teak wood block after 3 months of exposure. Arrows show tissue disruption observed as a loose arrangement of fibers.
Wood characteristics
Six and ten yr. old samples (HS) exhibited a moisture content of 10.0 %, and an average density of 0.54 g/cm3 with minimal differences between type of wood and age [lowest (0.49 g/cm3) and highest (0.61 g/cm3) in 6 and 10 yr. old (H), respectively].
Weight loss (on dry weight basis)
The greatest weight loss was obtained in 6 yr. old (S) and varied from 1.3 to 8.0%. Weight loss in 10 yr. old (S) and 6 yr. old (H) blocks was similar at the end of 6 months (5.8% and 6.0%, respectively). Minimal weight losses were obtained with 10 yr. old (H) (1.6% after 6 months of exposure). A comparison of mass weight losses in the different treatments after 3 and 6 months, revealed no statistically significant differences between 10 yr. old (S) and 6 yr. old (H) (tables 1-2).
table 1 Media comparison (DMS) of mass weight losses (%) of the different treatments after 3 months exposure. S-6 [sapwood 6 yr. old ], H-6 [heartwood 6 yr. old], S-10 [sapwood 10 yr. old ], H-10 [heartwood 10 yr. old ]
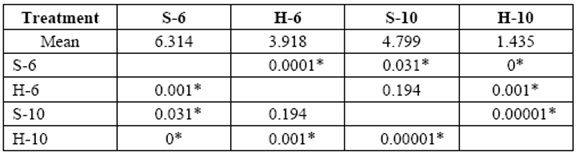
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
table 2 Media comparison (DMS) o mass weight losses (%) of the different treatments after 6 months exposure. S-6 [sapwood 6 yr. old ], H-6 [heartwood 6 yr. old], S-10 [sapwood 10 yr. old ], H-10 [heartwood 10 yr. old ].
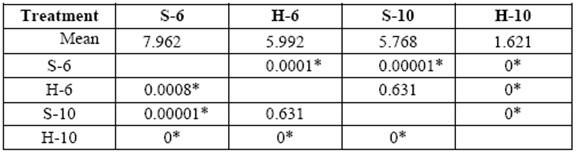
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
Maximum compressive strength parallel to the grain test
Previous tests carried out on control samples (6 and 10 yr. S and H blocks) showed that the maximum resistance to compressive strength was in the range of 600-814 kg/cm2.
This test was repeated with blocks exposed to fungal decay for 3 and 6 months. A loss of resistance was observed in all treatments over time due to the removal of the cell wall components. Greater losses were obtained in 6 yr. old S with values ranging from 544 kg/cm2 to 486 kg/cm2 in 3 and 6 month exposure, respectively (control: 600 kg/cm2) (table 3).
table 3 Compressive strength parallel to grain (kg/cm2) of 6 yr. old sapwood exposed to Rigidoporus cf. microporus: control (S-6-0), 3 months (S-6-3), 6 months (S-6-6).
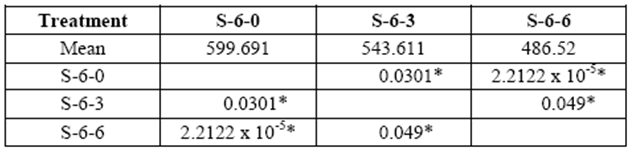
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
Loss of resistance of the wood exposed to decay was statistically significant compared to control samples at 3 and 6 month exposure.
Stress on 10 yr. old S varied from 752 kg/cm2 to 692 kg/cm2 in 3 and 6 month exposure respectively (control: 760 kg/cm2). Loss of resistance compared to control blocks was statistically significant after a sixth month decay (table 4).
table 4 Compressive strength parallel to grain (kg/cm2) of 10 yr. old sapwood exposed to Rigidoporus cfr. microporus: control (S-10-0), 3 months (S-10-3), and 6 months (S-10-6).
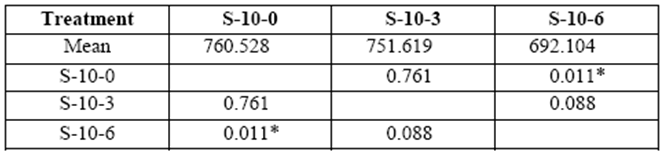
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
Six yr. old H blocks showed changes related to the applied stress that varied between 553 kg/cm2 and 557 kg/cm2 at 3 and 6 month exposures (control: 624kg/cm2). Loss of resistance was statistically significant at the sixth month of exposure (table 5).
table 5 Compressive strength parallel to grain (kg/cm2) of 6 yr. old heartwood exposed to Rigidoporus cfr. microporus: control (H-6-0), 3 months (H-6-3), 6 months (H-6-6).
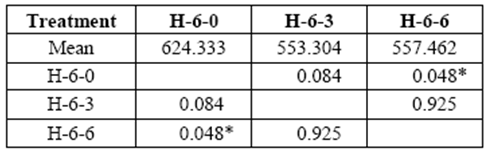
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
Variation in the applied stress on 10 yr. old H ranged from 749 kg/cm2 to 750 kg/cm2 at 3 and 6 months of exposure, respectively (control: 814 kg/cm2). Variations were not statistically significant compared to control samples (table 6).
table 6 Compressive strength parallel to grain (kg/cm2) of 10 yr. old heartwood exposed to Rigidoporus cfr. microporus: control (H-10-0), 3 months (H-10-3), and 6 months (H-10-6).
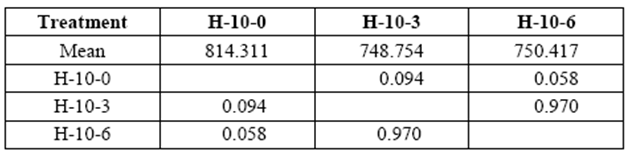
*Significant difference between treatments (STATISTICA Program, Pos-Hoc Comparison of Means test LSD, 0.05 and 95% confidence levels).
Chemical assay
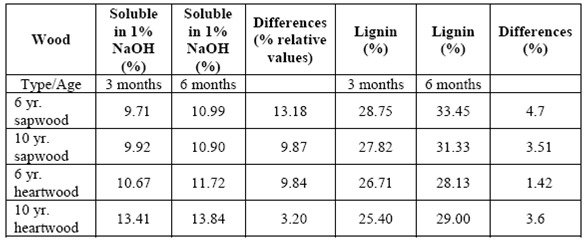
There was an increase in the quantity of soluble materials in 1% NaOH (relative values) and lignin content in all the samples analyzed at the end of the 3-month experiment, which continued increasing until the end of the 6 month period (table 7).
Discussion
The genus Rigidoporus is characterized by its monomitic hyphal system and simple septate generative hyphae (Gilbertson & Ryvarden 1987); nevertheless, Rigidoporus cfr. microporus developed clamp connections in culture, a peculiar character that also has been reported in Rigidoporus lineatus (Bakshi et al., 1963) but is not found on fruiting bodies.
The parenchyma tissue was first invaded during the initial stages of decay where the fungus utilized the starch and probably other components that can be degraded easily and provide the necessary energy for its establishment. The strong degradation of those cells but not of fibers observed in the early stages of decay has also been mentioned by Schwarze et al. (2000) and Fernandes et al. (2005). At the end of the experiment, some of the blocks showed the typical white-rot appearance described by other authors (Gilbertson & Ryvarden 1987, Ramsden et al., 2002; Schmidt, 2006; Schwarze et al., 2000).
Teak has been considered to be a very resistant wood to pathogens in plantations, natural forests or during storage (Bhat & Florence, 2003; Bhat et al., 2005, Kokutse et al., 2006; Thulasidas & Bhat, 2007). This resistance has been attributable to the high percentage of extract contents (organic compounds), especially that found in old heartwood walls (for example, napthoquinone and tectoquinone) (Chaves & Fonseca 1991; Fonseca, 2004; Rivero & Moya, 2006; Thulasidas & Bhat, 2007; Rowell, 2013).
Rivero and Moya (2006) and Thulasidas and Bhat (2007) mentioned that 6 and 8 yr. old teak showed less resistance to fungal or insect attacks due to reductions of organic compounds as compared with older trees. Kokutse et al. (2006) obtained < 20% mass loss in heartwood samples exposed to Antrodia sp. and T. versicolor and <7% when exposed to Pycnoporus sanguineus and Gloeophyllum trabeum. Another study by Moya et al. (2009) reported weight losses on heartwood of <11% in decay tests with Trametes versicolor and Pycnoporus sanguineus; however, weight losses in sapwood were up to 50% with T. versicolor and ≤ 35% with Pycnoporus sanguineus.
It is worth mentioning that none of the fungi used in those previous decay studies has been isolated from teak wood. Our study is the first one that utilized a fungus that grows on teak, and the overall small wood biomass lost (1.3-8%) confirmed the natural resistance of teak to fungal degradation.
Donoso and Veloso (1968) pointed out that there is a tendency in high-density woods to initially resist fungal infection, but during the decay process, the resistance decreases or disappears. In our study, the lack of a strong variation in wood density in the different treatments (control and decay samples) made it impossible to relate this factor to the resistance or susceptibility of teak wood to decay.
The results obtained from the compressive strength parallel to grain test and weight losses showed that age was an important factor determining resistance to compression and decay, probably due to the increase in lignified walls as mentioned by Rivero & Moya (2006). The loss of resistance over time was due to the removal of the cell wall components by white rot fungi, as pointed out by Schmidt (2006).
Variations in chemical contents revealed that the fungus has a preference for lowweight molecular carbohydrates, since the solubles in 1% NaOH increased in all the treatments. This suggests that low-weight molecular carbohydrates in wood increased due to fragmentation of polymer chains in wood walls (cellulose and hemicellulose). The increase in lignin content was related to modifications of the chemical structure of the wood. This could be interpreted as an increase in cellulose and hemicellulose degradation that caused a numerical increase in lignin content (Schmidt, 2006).
Conclusions
Rigidoporus cfr. microporus is a potential wood rotter of teak wood, and can cause undesirable changes in the chemical, mechanical and physical properties of teak wood. The decay risk is related to the type of wood and the age of the tree.
Silvicultural practices recommend that the first thinning in teak plantations should be done after four years (Fonseca, 2004). According to our results, the wood obtained from these first thinnings will be more vulnerable to decay, especially the sapwood. Therefore, it is highly recommended to protect it to prevent decay. Wood from 10 yr. harvest trees or older ones will be less vulnerable to decay and therefore more desirable for construction purposes.
Knowledge of the potential damages that this fungus can cause to teak wood might contribute to a better selection of wood and to the development of more effective protection measures against decay in the field or in construction wood.
Acknowledgments
The authors thank Flor y Fauna, S.A. for providing the teak wood samples and granting permission to collect them in the teak plantation. The Forest Resources Laboratory of the Engineering Research Institute, University of Costa Rica, provided the equipment to carry out the assays, and the Microscopical Structure Research Center, University of Costa Rica, provided their facilities for the electron microscopy studies. The authors thank Ingo Werhtmann, Paul Hanson and the peer reviewers for critical review of the manuscript. The first author thanks DAAD for financial support during her Master studies in Costa Rica.











 text new page (beta)
text new page (beta)