Introduction
Pine forests have been damaged throughout the world by opportunistic pests, which generally occur after a fire, drought or fragmentation (Hernández, 2012). From the numerous pests, ones that stand out are the bark beetles and defoliating insects (Amri, Gargouri, Hamrouni, Hanana, Fezzani & Jamoussi, 2012). In Mexico, as in most countries, chemical products and entomopathogenic fungi have traditionally been used for control of forest pests in pines (Cibrián-Tovar, Méndez-Montiel, Campos-Bolaños, Yates-III & Flores-Lara, 1995). In the last decades, it has been suggested that biological resources should only be used to fight the contamination of water sources and the deterioration of environmental services caused by the use of chemicals (Fernández-Pavía, Rodríguez-Alvarado, Gómez-Dorantes, Gregorio-Cipriano & Fernández-Pavía, 2013).
In the northern and western parts of Mexico, pine sawfly (Zadiprion vallicola Rohwer) has caused a loss of up to 15000 ha per zone in a single year (Hernández, 2012; Velez & Salas, 2012; Ordaz-Silva et al., 2017). It has been suggested during recent years, that biological control should be carried out with hyper parasite insects, mainly wasps, whose effect has been slow and inefficient (Hodge & Dvorak, 2000; García, 2012). A successful case has been the use of the entomopathogenic fungus Metarhizium anisopliae, which at 1 × 108 conidia per millimeter of concentration has been used as an effective measure against sawfly Monoctenus sanchezi (Ordaz-Silva et al., 2017). As an alternative, extracts of plants with insecticidal effects can be tested for the control of Zadiprion vallicola, an option not used to date for forest pests (Krishna, Prajapati, Bhasney, Tripathi & Kumar, 2005).
Tagetes erecta L., a plant endemic to Mexico and Central America, known as cempasúchil, clavel chino, african marigold or botón de oro, has been widely studied for its medicinal effects on humans. It is also known for being a biocide against fungi, bacteria, viruses, nematodes and insect pests from vegetable crops, has suitable characteristics to combat larval insect states and whose effectiveness has been tested for the control of Z. vallicola (Krishna et al., 2005; Oranday, Martínez, Nuñez, Rivas & Flores, 2008; Aldana-Llanos et al., 2012; Joshi, 2015; Padalia & Chanda, 2015). The main extracts obtained from this plant against insects are flavonoids and terpenes. These extracts have larvae repellent properties against Diptera and Lepidoptera orders, with leaves being the main source of bioinsecticidal substances (Wang, Hanhong & Shanhuan, 2001; Xu, Wang & Shi, 2007; Geris et al., 2012; Xu, Chen Qi & Shi, 2012; Palacios et al., 2015; Selvam, Devaraj & Rani, 2015; Shetty, Sakr, Al -Obaidy & Shareef, 2015). It is of great importance to investigate the possibility of using this plant as biological control of larvae of the sawfly Z. vallicola in Pinus oocarpa. Particularly important is to know if it has a success similar to that reported in the literature for M. anisopliae, since in other studies it had an effect on mortality similar to that caused by T. erecta, for example, against Spodoptera frugiperda (Lezama et al., 2005; Aldana-Llanos et al., 2012).
Objectives
To evaluate the bioinsecticidal effect of Tagetes erecta leaves on larvae of Zadiprion vallicola.
Materials and methods
In July 2015, in a temperate agricultural plot of the state of Guerrero located at 16 ° 56’ 23.7” N, 98 ° 31’ 22.5” W, seeds of T. erecta were thrown in parallel rows on a surface of 40 m × 40 m. The crop was maintained with direct sunlight and natural irrigation in the rainy season. Organic fertilizer (cow dung and pine leaf litter) was applied 20 days after cultivation of the plot. On November 15th, 2015, the mature plants were collected separating the leaves of the specimens. The leaves were allowed to dry in the shade for 10 days. Once dried and pulverized, 500 g were placed in three 4 L containers. In one of these containers water was added until the material was covered completely; in two others acetone and ethanol were added in a similar way and allowed to macerate for three days. At the end of the maceration, the vegetable remains were separated by filtration with filter paper no. 1 and the solvents were separated by a water bath. The filtered extracts were diluted in distilled water to a final concentration of 250 mg/L, 500 mg/L, 750 and 1000 mg/L and distilled water was used as the control (Krishna et al., 2005; Oranday et al., 2008; Aldana-Llanos et al., 2012).
The larvae of Z. vallicola were removed from the leaves of individuals of Pinus oocarpa in a random and manual form during November 20th to 25th, 2015 (Cibrián-Tovar et al., 1995). The site is a pine forest in the state of Guerrero located at 16° 56’ 23.3” N, 98° 31’21.2” W, between 1500 m and 1700 m a.s.l. 5200 larvae were collected, which were placed in plastic boxes of (10 × 10 × 10) cm in groups of 20 larvae per box, in a total of 260 boxes. The boxes were covered with mosquito netting to allow the larvae to breathe. For their feeding, leaves of the same individual of P. oocarpa from which they were fed were collected and placed covering 50% of the volume of each box, the leaves being renewed daily at 07:00 h. During the study, the boxes were placed under laboratory conditions at 28 °C ± 0.3 °C and 61% H.R. ± 2% H.R.
The study was carried out from November 26th to December 1st, 2015. In each box some of the extracts were applied in their various concentrations, by spraying for three seconds at a separation of 10 cm. Acetone extract at 250 mg/L was applied in 20 boxes, at 500 mg/L in another 20 boxes and at the same number of boxes for 750 mg/L and 1000 mg/L. The same criterion was applied for the application of ethanol extracts and water extracts. In the case of the control it was applied in 20 boxes. The number of live and dead larvae was recorded at 6 days and mortalities were recorded in corrected proportions relative to the control (Cibrián-Tovar et al., 1995; Ordaz-Silva et al., 2017). Lethal concentrations in which half of the larvae die (LC50) were calculated by applying the different extracts at their various concentrations using a probit regression (Aldana-Llanos et al., 2012; Joshi, 2015).
In order to analyze the dynamics of the effect of the different treatments of the extracts, a two-factor ANOVA test was performed using the Past 3.11 program (Hammer, Harper & Ryan, 2001), using solvent (ethanol, acetone, water) and the concentration (250, 500, 750 and 1000) mg/L as factors. For this test the units of mortality were previously transformed in arcsin. After the ANOVA test, Tukey’s tests were performed to determine if there were significant differences between treatments.
Results
At the end of the six days in the control boxes there was zero mortality. The highest mortality of 0.96 was caused by ethanol extracts, followed by acetone at 0.81 (Table 1 and Fig. 1). It is possible to observe that from the application of 500 mg/L or more, the mortality caused by the different extracts is stabilized (Fig. 1). The highest LC50 lethal effect was that of ethanol calculated at 63.1 mg/L, followed by acetone calculated at 64.5 mg/L (Table 2, Fig. 2). Among the treatments, there were significant differences (Table 3) and ethanol extracts of T. erecta could be considered as successful to control Z. vallicola larvae.
Table 1 Mean mortalities ± standard deviation caused by the application of different extracts of Tagetes erecta on larvae of Zadiprion vallicola
| Mortality (proportion) | |||
| Water extracts | Acetone extracts | Ethanol extracts | |
| 250 mg/L | 0.38 ± 0.04 | 0.67 ± 0.06 | 0.79 ± 0.05 |
| 500 mg/L | 0.55 ± 0.03 | 0.74 ± 0.05 | 0.93 ± 0.06 |
| 750 mg/L | 0.60 ± 0.04 | 0.78 ± 0.06 | 0.94 ± 0.05 |
| 1000 mg/L | 0.64 ± 0.05 | 0.81 ± 0.04 | 0.96 ± 0.03 |
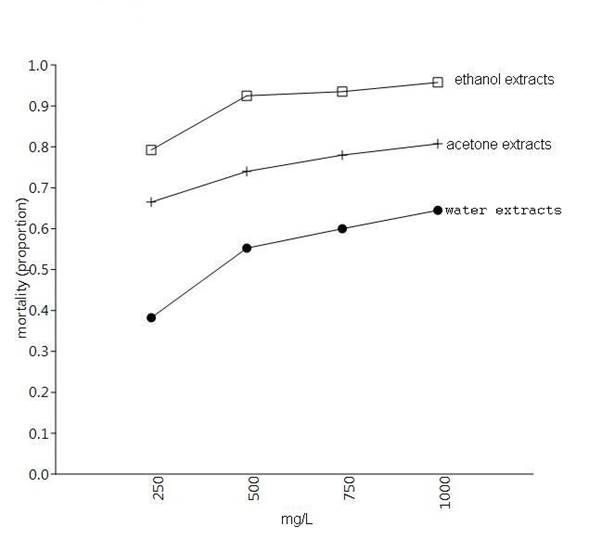
Figure 1 Graph of means for mortality caused on larvae of Zadiprion vallicola of three extracts of Tagetes erecta at four different concentrations
Table 2 Lethal Concentration of Different Extracts of Tagetes erecta Causing 50% Mortality of Zadiprion vallicola Larvae
| LC50 at 6 days | |||
| mg/L | X2 | p | |
| Water extracts | 398.1 | 0.0012 | >0.05 |
| Acetone extracts | 64.5 | 0.2318 | >0.05 |
| Ethanol extracts | 63.1 | 0.9938 | >0.05 |
Note: LC50 (lethal concentration with 50% of mortality)
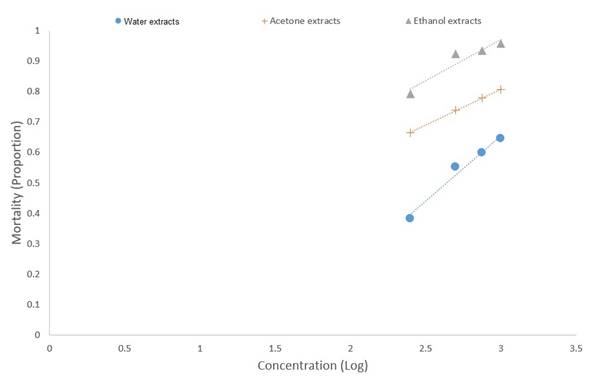
Figure 2 Mortality (in corrected proportion) caused by the application of three extracts of Tagetes erecta on larvae of Zadiprion vallicola in four different concentrations (Log).
Table 3 Results of Two-Way ANOVA about the effect of concentration and type of solvent of extracts of Tagetes erecta on mortality caused in larvae of Zadiprion vallicola
| Two Factors ANOVA | |||
| Factors | F | p | Treatments equal* |
| Concentration (ppm) | 171.4 | <0.001 | None |
| Solvent | 1060 | <0.001 | None |
| Interaction | 7.3 | <0.001 | Water extracts at 750 mg/L and 1000 mg/L Acetone extracts at 500, 750 mg/L and 1000 mg/L Ethanol extracts at 500 mg/L, 750 mg/L and 1000 mg/L |
Note: * If the probability of the Tukey´s Q value between pairs > 0.05
Discussion
Biological controls for pest control in agricultural crops can be extended to forest pests (Leonti, Sticher & Heinrich, 2003; Navarro, da Silva, da Silva, Napoleão and Paiva, 2013; Miller et al., 2015; Padalia and Chanda, 2015). The results of the present study show that it is possible to test the extracts of plants used in agricultural crop pests and try them in the forest field (Fernández-Pavia et al., 2013; and Selvam et al., 2015). Most studies on the allelopathic effects of various plant extracts on various pests focus on the application of laboratory extractions as the present research, which facilitates their interpretation and comparison (Singh, Batish & Kohli, 2001; Wang et al. , 2001; Xu et al., 2012; Shetty et al., 2015). Following these ideas, the success of the T. erecta ethanol extracts in this study are similar to those reported with M. anisopliae, in both cases causing mortality greater than 0.95 (in proportion) in sawflies (Ordaz-Silva et al. 2017). Similarly, 60 mg/L of leaves of Datura stramonium (known as toloache) cause a mortality of 52.2%, although unlike this, T. erecta does not have the inconvenience of being toxic to humans. (Flores-Villegas et al., 2019).
Ethanol extracts of T. erecta can be performed relatively easily and can be used effectively in places where Z. vallicola pests are present, which can be rapidly advanced (Sáenz-Romero, Guzmán-Reyna & Rehfeldt, 2006; Fernández-Pavia et al., 2013). This species in larval state has its highest activity at the end of November of each year, in a relevant way the same month in which T. erecta reaches its maturity (Cibrián-Tovar et al., 1995; Hernández, 2012; Shetty et al. 2015). This tuning allows using the macerated product of this plant, with effectiveness and without the need to add preservatives, to be able to apply almost immediately to its elaboration; in addition, it is notable that there is no deficiency of this plant, since it is a traditional crop in rural areas bordering forests (Leonti et al., 2003).
A number of plants with allelopathic effect against pests, such as viruses, bacteria, fungi and insects in humans and vegetable crops for human consumption, have been studied (Wang et al., 2001; Shetty et al. 2015). In the present study, it is demonstrated that they can be used in pests that afflict forests, using previous knowledge about the effects on similar groups of organisms (Velez & Salas, 2012; Fernández-Pavia et al., 2013). The case of T. erecta is outstanding because it has effects at the level of human disease, in vegetable crops and in this case in forest diseases, which can be presented as a pan-allelopathic plant (Kalemba & Kunicka, 2003; Narayanaswamy, Gleiser, Chalannavar & Odhay, 2014; Padalia & Chanda, 2015; Palacios et al., 2015; Selvam et al., 2015). Its distribution in the five continents has proved to be beneficial at the ornamental, medicinal and agronomic levels and the present study reinforces this view (Wang et al., 2001; Leonti et al., 2003; Xu et al., 2012; Fernández-Pavia et al., 2013; Shetty et al., 2015). (Flores-Villegas et al., 2019).
Conclusions
The ethanolic extracts of T. erecta at a concentration of 63.1 mg/L caused the death of 50% of the larvae of Z. vallicola, at a concentration of 1000 mg/ L, causing death of 96%.
Under controlled conditions, extracts of T. erecta are as effective as the biological controls traditionally used.
It is necessary to carry out field studies that demonstrate the real similarity between the application of extracts of T. erecta and other biological controls.











 text new page (beta)
text new page (beta)


