Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Madera y bosques
versão On-line ISSN 2448-7597versão impressa ISSN 1405-0471
Madera bosques vol.21 no.1 Xalapa Mar./Jun. 2015
Artículos de investigación
Drought stress in provenances of Lupinus elegans from different altitudes
Estrés por sequía en Lupinus elegans procedentes de diferentes altitudes
José Carmen Soto-Correa1, Cuauhtémoc Sáenz-Romero1, Horacio Paz2 y Roberto Lindig-Cisneros2,*
1 Instituto de Investigaciones Agropecuarias y Forestales. Universidad Michoacana de San Nicolás de Hidalgo (IIAF-UMSNH). Michoacán, México.
2 Instituto de Investigaciones en Ecosistemas y Sustentabilidad. Universidad Nacional Autónoma de México. Michoacán, México. * Corresponding author: rlindig@cieco.unam.mx
Manuscript received on September 13th 2013.
Aceppted on July 1st 2014.
ABSTRACT
The response of plants to altitudinal gradients depends on several factors and might differ among life strategies. Understanding these responses is highly relevant for management of forest species, particularly under climate change scenarios. We explored the response to drought of different provenances of Lupinus elegans, obtained from an altitudinal gradient. This species is a shrub that acts as a nurse plant in temperate forests in its geographical range. Seeds were collected from five natural provenances across an altitudinal gradient (2312 m to 2885 m a.s.l.). A common-garden experiment was conducted with four drought treatments (irrigation at every 3, 7, 15 and 21 days) in a shade-house located at 1972 m a.s.l. All provenances presented reduced heights and numbers of leaves with increased drought intensity, regardless of site of origin. Survival among provenances presented an altitudinal pattern, where those belonging to higher sites exhibited greater survival. Provenances from lower altitudes, coming from drier and warmer sites, exhibited poorer survival against drought stress. Overall, our results indicate that there are differences among provenances, but since this species is a short lived perennial (five years on average), it is more sensitive to microclimate than to conditions determined for large scale patterns such as altitudinal gradients. This should be considered for management practices such as ecological restoration.
Key words: altitudinal gradient, climate change, Fabaceae, pine forest, restoration.
RESUMEN
La respuesta de las plantas a los gradientes altitudinales depende de varios factores y puede variar entre estrategias de vida. Entender esta respuesta es relevante para el manejo de especies forestales, en particular ante los efectos esperados del cambio climático. En este trabajo se exploró la respuesta a la sequía de diferentes procedencias de Lupinus elegans, obtenidas de un gradiente altitudinal. Esta especie es un arbusto que actúa como planta nodriza en bosques templados a lo largo de su área de distribución geográfica. Se colectaron semillas de cinco procedencias a los largo de un gradiente altitudinal (2312 m a 2885 m snm). Se llevó a cabo un experimento de jardín común con cuatro tratamientos de sequía (riego cada 3, 7, 15 y 21 días) en una casa de sombra localizada a 1972 m snm. Las plantas de todas las procedencias mostraron un menor tamaño y número de hojas conforme aumentó el grado de sequía, independientemente de la procedencia.
La supervivencia entre las procedencias mostró una relación con el gradiente altitudinal de origen, pues aquellas procedentes de sitios a mayor altitud mostraron mayor supervivencia. Las procedencias de altitudes menores, que en principio son de lugares más secos y cálidos, mostraron baja supervivencia en respuesta a la sequía. Los resultados indican que hay una diferenciación entre procedencias, pero que siendo esta especie perenne de vida corta (5 años), es más sensible a las condiciones microclimáticas que a las condiciones determinadas por patrones a escalas mayores como son los gradientes altitudinales. Esto debe de ser considerado para prácticas de manejo como la restauración ecológica.
Palabras clave: gradiente altitudinal, cambio climático, Fabaceae, bosque de pino, restauración.
INTRODUCTION
Plant species distribution and abundance at large scales are largely determined by climatic variables (i.e. precipitation and temperature) and these patterns can therefore be altered by climate change (Parmesan 2006; Fitzpatrick et al., 2008; Rehfeldt et al., 2009; Vitasse et al., 2009). Faced with altered environmental conditions caused by climate change, plants can respond by modifying their size, reducing their growth rates, or at the population level, by presenting high rates of mortality (Vitasse et al., 2009). Latitudinal and altitudinal displacement can also be caused in the long term (Lenoir et al., 2008), therefore, it is necessary to understand the relationship between altitudinal gradients and plant growth responses.
With climate change, reductions are expected in the current distribution ranges of almost all the pine-oak forest species in Mexico (Gómez-Mendoza and Arriaga, 2007). This is because aridity is expected to increase in the country as the result of increase of temperatures, reduced total annual precipitation (Sáenz-Romero et al., 2010), and altered seasonal distribution patterns, with torrential and irregular rains that will tend to increase during winter, followed by long periods of drought that will increase in summer (Rambal and Debussche 1995; Reichstein et al., 2002). In addition, high temperatures can themselves increase water stress in the forests, regardless of precipitation patterns (Barber et al., 2000; Angert et al., 2005).
The relationship between altitudinal gradients and stress has been studied mostly for tree species (i.e. van der Maaten-Theunissen et al., 2013; Chen et al., 2011; Jump et al., 2007; Yu et al., 2006; Zhang et al., 2012), and few studies have been done with shrubs or other herbaceous species (Li et al., 2006). More research is needed for non-tree species, particularly for pioneer shrub species, because of their role in succession.
Shrubs from the genus Lupinus (Fabaceae) are short lived species (ca. 5 years) common in many temperate forests of North America. In general these are pioneer species with a considerable capacity for tolerating stress and fixing nitrogen. Some species of the genus have been shown to facilitate the establishment of native trees in disturbed lands (Blanco-García et al., 2011; Gómez-Ruiz et al., 2013). This is the case of Lupinus elegans (Fabaceae), an endemic species to Mexico that is found within the pine-oak and pine forests of the central eastern region of the country, distributed between 1800 m and 3000 m a.s.l. (Dunn, 2001). Recent tests of ecological restoration demonstrated that this species has a notable capacity for improving soil conditions and facilitating the establishment of native trees and understory plants (Blanco-García et al., 2011; Díaz-Rodríguez et al., 2012; Díaz-Rodríguez et al., 2013).
OBJECTIVES
The objective of this study was to determine whether plants of Lupinus elegans respond differently to drought, depending on the altitudinal origin of each provenance because this information is needed for adaptive management of restoration under climate change scenarios. By means of a common-garden experiment, as well as climatic information of the altitudinal gradient derived from spline climatic models (Crookston, 2010; Sáenz-Romero et al., 2010), we examined the possible effects of temperature stress caused by differences between the temperatures that occur at the experimental site and the ones at the sites where provenances originated to generate quantitative data on possible altitudinal migration ranges.
METHODS
Seeds were collected through open pollination of 11 individuals from each of five natural provenances of Lupinus elegans across an altitudinal gradient of 2312 m to 2885 m a.s.l. (Table 1) in the Mil Cumbres area, in the central-eastern region of the state of Michoacán, Central-Western Mexico. The gradient covers the range of the species distribution in the area. Seed collection was conducted between December 2008 and February 2009. The location at which seeds were collected will henceforth be referred to as the origin, and the group of individuals belonging to the same location will be referred as provenance.
Geographical coordinates were taken from the experimental and seed collection sites and used to interrogate spline climatic models, in order to obtain the contemporary climate (average 1961-1990) (Crookston, 2010; Sáenz-Romero et al., 2010). Mean annual temperature and mean annual precipitation were estimated for the contemporary climate for each seed origin and for the experimental site. Contemporary climate was used to estimate the drought stress index that occurred due to transfer of the seeds between the site of origin and the site at which they were subsequently grown.
Because L. elegans seeds require a pregerminative treatment (Robles-Díaz et al., 2014), all collected seeds were cleaned and scarified in the laboratory by immersion in 97% H2SO4 (Fermont®) for 30 minutes. This process increased the permeability of the seed coat (Medina-Sánchez and Lindig-Cisneros 2005). The assay was conducted in a shade house belonging to the Instituto de Investigaciones en Ecosistemas y Sustentabilidad of the Universidad Nacional Autónoma de México (Cieco-UNAM), in Morelia, Michoacán (Table 1). The shade house was covered with translucent plastic at 5 m of height and the sides were left sufficiently open to avoid overheating the plants while still sheltering them from the rain.
Seeds were planted in 380 ml containers with a substrate composed of two parts of a commercial substrate (Creciroot®, Uruapan, Michoacán, México) to one part sand, sowing one seed per container at a depth of 2.5 cm. The experimental design was a randomized complete block, comprising three blocks. Each block included four irrigation treatments (treatment 1 = irrigation every three days, treatment 2 = irrigation every seven days, treatment 3 = irrigation every 14 days, treatment 4 = irrigation every 21 days), and the five provenances were represented within each treatment in groups of nine plants in a row. Survival and growth (plant height and number of leaves) were monitored for plants of all provenances and treatments.
The experiment began in August 2009, during the beginning of the rainy season in the field, and the plants were grown under frequent irrigation for 57 days and then subjected to the different irrigation treatments for 85 days. After this, irrigation was then applied every three days to all treatments for 74 days in order to ensure that plants identified as dead really were so. Once the experiment was in progress, percentage of survival and growth were evaluated every 15 days. Number of leaves lost and total number of leaves were recorded, and relative growth rate in height was calculated.
Absolute growth (final size — initial size) and relative growth rate were evaluated:
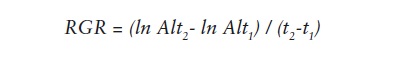
Where:
RGR = relative growth rate
ln Alt2 = natural logarithm of final height
ln Alt1 = natural logarithm of initial height
t2 = value of final time
t1 = value of initial time.
Experimental plants were subjected to two sources of drought stress that operated together. First, the irrigation treatment caused different levels of soil water stress. Second, the higher temperatures at the experimental site (Morelia) compared to all the altitudinal origins, imposed atmospheric drought stress with intensity increasing towards those provenances of high altitude (Table 1). To capture the joint effects of drought on plant performance we derived an index of drought stress (DSI) as follows:

Where:
DSI = index of drought stress
TTD = difference in temperature between where they were planted experimentally and the site of origin (temperature transfer distance)
R = percentage of days in which the plants were irrigated.

Where:
TES = average annual temperature at the experimental site according to the spline climatic model (Crookston, 2010)
TPS = average annual temperature at the site of origin of the provenance according to the spline climatic model
r = number of days with irrigation
d = duration of the experiment.
In our study DSI varied from 0.18 to 1.55 depending on the combination of irrigation treatment and provenance, and these values showed a wide overlapping among provenances (Table 1). We then explored the effects of potential drought stress on growth and survival of lupine plants by fitting regressions. For every provenance we used the value of DSI at which the 50% of mortality occurs (EL50%) as an indicator of resistance to drought stress. In order to evaluate differences between treatments and provenances, an analysis of variance was conducted using Proc GLM of SAS (SAS, 2004), with the following statistical model:
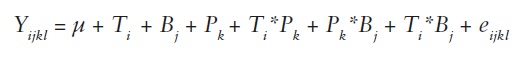
Where:
Yijkl = observation
µ = effect of the general mean
Ti = effect of the i-th treatment (fixed effect)
Bj = effect of the j-th block
Pk = effect of the k-th provenance
Ti*Pk = effect of ik-th interaction treatment*provenance
Pk*Bj = effect of kj-th interaction provenance*block
Ti*Bj = effect of ij-th interaction treatment*block
eijkl = error.
To evaluate the relationship between altitude of seed origin and drought stress index and relate these to response variables such as, total leaves, leaves lost, growth in height, relative growth rate and provenance survival, linear regression analysis and/or quadratic regression analysis were conducted using Proc REG of SAS (SAS, 2004), with the following statistical models:
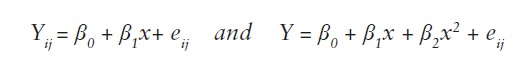
Where:
Yij = provenance mean
β0 = intercept
β1 = slope
x = altitude of
eij = error.
RESULTS
Analysis of variance revealed highly significant differences among irrigation treatments for all variables (P ≤ 0.0201; Table 2). There were significant differences between provenances for height (P = 0.0309), but not for survival, total leaves, lost leaves, absolute and relative growth rates (Table 2). The interaction between the treatment levels and the provenance was significant for three variables: leaf loss, number of leaves and survival. Nevertheless, the contribution to the total variance of these interactions was low (leaf loss 3.1%, number of leaves 11.6% and survival 2.5%) when compared with the contribution of the provenances (leaf loss 34%, number of leaves 21% and survival 57%).
It was observed that provenances with higher relative growth in height had more leaves, while those with lower growth in height showed more variation for this correlation. This pattern was observed across all the drought treatments (r2 = 0.5758, P = 0.0007; Fig. 1 A). There was also a highly significant relationship between provenances in terms of the number of leaves lost and the relative growth in height (r2 = 0.7757, P = 0.0001; Fig. 1 B), in which provenances had more leaf loss when relative growth in height was low and less leaf loss when growth was higher.
Plant growth expressed as the increase in height and the total number of leaves produced showed a significant negative relationship with the drought stress index (r2= 0.8314, P = 0.0001; fig. 2 A). To interpret this result, it is necessary to recall that the five provenances were subjected to different levels of drought stress as a consequence of the different irrigation treatments, and additionally, we assumed that each provenance in each treatment exhibited different levels of drought stress caused by the change in temperature between the provenance and the experimental site. Provenances with higher values of drought stress index (those from higher altitudes) had lower heights and leaf numbers, while provenances with lower drought stress index values (those from lower altitudes) presented higher growth and leaf numbers (r2=0.5758, P=0.0007, Fig. 2).
DISCUSSION
Plants collected as seed from different provenances of L. elegans were exposed to drought stress and, regardless of their site of origin, the increased stress reduced their growth in height and leaf production and increased leaf loss, which is a typical response to drought (Levitt 1980; Martínez-Vilalta y Pockman, 2002; Tenopala et al., 2012). The same response in height growth occurs in other wild species, such as Pinus leiophylla (Martínez-Trinidad et al., 2002) as well as in cultivated plants such as cotton, where number of leaves and growth in height is strongly affected by drought stress (Méndez-Natera et al., 2007). The experimental site was located at a lower altitude (1972 m) than all the sites of origin of the five provenances tested. This is important because water availability in this experiment (four irrigation treatments) was controlled, while temperature was not. Provenances closer in elevation to the experimental site were subjected to lower drought stress (that is reflected in the DSI values), while provenances originating far from the experimental site were subjected to greater drought stress. Differences in plant height between provenances of Lupinus elegans across the altitudinal gradient reflect quantitative genetic differentiation. Genetic differentiation between populations has been detected for this species (Lara-Cabrera et al., 2009; Soto-Correa et al., 2013) and represents a response to local environments (Rehfeldt et al., 2009; Vitasse et al., 2009).
According to the climate estimates used for the sites where seeds were originally collected for each provenance, those from lower altitudes are subjected to higher temperatures and less rain, while those of the higher elevations experience lower temperatures and more rain. This pattern has also been reported in other studies (Vitasse et al., 2009; Vitt et al., 2010). These climatic differences cause plants of the same species to modify their morphology across the altitudinal gradient as a strategy to reduce the negative effects of limiting climatic conditions (Filella and Peñuelas, 1999; Rundel et al., 1994; Körner 2003). For this reason, provenances from lower altitudes could be expected to be more resistant to drought stress than those from higher altitudes, as has been found in other species (Rehfeldt et al., 2009). For the provenances of L. elegans tested in this study, however, the opposite occurred, and the provenances from higher elevations survived the drought stress better than those from lower altitudes.
In another experiment, it was found that provenances of L. elegans also presented an altitudinal pattern in which the foliar tissues of plants from higher elevations showed more resistance to freezing temperatures (Soto-Correa et al., 2013). This led us to believe that the possible reason why provenances from higher altitudes show more resistance to drought is the known relationship between drought tolerance and resistance to low temperatures. Both drought and low temperatures, cause a similar response in plants, increasing solute concentration in the leaves, making them more resistant to stress (Medeiros and Pockman, 2011; Charra-Vaskou et al., 2011).
CONCLUSIONS
Temperatures are expected to increase in the coming decades, and it is known that high temperatures can, by themselves and regardless of precipitation, increase drought stress in the forest (Barber et al., 2000; Angert et al., 2005). This may lead to a reduction in the current distribution ranges of almost all the pine-oak forest species in Mexico (Gómez-Mendoza and Arriaga 2007; Rehfeldt et al., 2009). Based on the results of this study, a reduction in growth could be expected in all the provenances tested, with greater mortality in the provenances from lower altitudes, producing an altitudinal contraction in this species towards higher elevations.
ACKNOWLEDGEMENTS
We want to thank DGAPA-UNAM for funding through grant PAPIIT IN202112, and two anonymous reviewers who improved the manuscript considerably by their comments.
REFERENCES
Angert, A., S. Biraud, C. Bonfils, C.C. Henning, W. Buermann, J. Pinzon, C.J. Tucker and I. Fung. 2005. Drier summers cancel out the CO2 uptake enhancement induced by warmer springs. Proceedings of the National Academy of Sciences of the United States of America 102:10823-10827. [ Links ]
Barber, V.A., G.P. Juday and B.P. Finney. 2000. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405:668-673. [ Links ]
Blanco-García, A., C. Sáenz-Romero, C. Martorell, P. Alvarado-Sosa and R. Lindig-Cisneros. 2011. Nurse-plant and mulching effects on three conifer species in a Mexican temperate forest. Ecological Engineering 37(6):994-998. [ Links ]
Charra-Vaskou, K., G. Charrier, R. Wortemam, B. Beikiecher, H. Cochard, T. Ameglio and S. Mayr. 2011. Drought and frost resistance of trees: a comparison of four species at different sites and altitudes. Annals of Forest Science 69(3): 325-333. [ Links ]
Chen, L., S. Wu and T. Pan. 2011. Variability of climate-growth relationships along an elevation gradient in the Changbi mountain, northeastern China. Trees-Structure and function 25(6):1133-1139. [ Links ]
Crookston, N.L. 2010. Research on Forest Climate Change: Potential Effects of Global Warming on Forests and Plant Climate Relationships in Western North America and Mexico http://forest.moscowfsl.wsu.edu/climate/. Visited 24/July/2010. [ Links ]
Díaz-Rodríguez, B., A. Blanco-García, M. Gómez-Romero and R. Lindig-Cisneros. 2012. Filling the gap: restoration of biodiversity for conservation in productive forest landscapes. Ecological Engineering 40:88-94. [ Links ]
Díaz-Rodríguez, B., E. del-Val, M. Gómez-Romero, P. A. Gómez-Ruiz, and R. Lindig-Cisneros. 2013. Conditions for establishment of a key restoration species, Lupinus elegans Kunth, in a Mexican temperate forest. Botanical Sciences 91(2):225-232. [ Links ]
Dunn, D.D. 2001. Lupinus. In: G. Calderón de R., J. Rzedowski. Flora fanerogámica del Valle de México. Instituto de Ecología, A.C. - Conabio. Pátzcuaro, Michoacán, México. p:290-300. [ Links ]
Filella, I. and J. Peñuelas. 1999. Altitudinal differences in UV absorbance, UV reflectance and related morphological traits of Quercus ilex and Rhododendron ferrugineum in the Mediterranean region. Plant Ecology 145:157-165. [ Links ]
Fitzpatrick, C. M., D.A. Gove, J.N. Sanders and R.R. Dunn. 2008. Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia. Global Change Biology 14(6):1337-1352. [ Links ]
Gómez-Mendoza, L. and L. Arriaga. 2007. Modeling the effect of climate change on the distribution of oak and pine species of Mexico. Conservation Biology 21(6):1545-1555. [ Links ]
Gómez-Ruiz, P.A., R. Lindig-Cisneros and O. Vargas-Ríos. 2013. Facilitation among plants: a strategy for the ecological restoration of the high-andean forest (Bogotá D.C. - Colombia). Ecological Engineering 57:267-275. [ Links ]
Jump, A.S., J.M. Hunt and J. Peñuelas. 2007. Climate relationships of growth and establishment across the altitudinal range of Fagus sylvatica in the Montseny mountains, northeast Spain. Ecoscience 14(4):507-518. [ Links ]
Körner, C. 2003. Plant Alpine Life. Springer-Verlag, Berlin. [ Links ]
Lara-Cabrera S., N. Alejandre-Melena, E. Medina-Sánchez and R. Lindig-Cisneros. 2009. Genetic Diversity in populations of Lupinus elegans Kunth. Implications for ecological restoration. Revista Fitotecnia Mexicana 32(2):79-86. [ Links ]
Lenoir, J., J.C. Gégout, P.A. Marquet, P. de Ruffray and H. Brisse. 2008. A significant upward shift in plant optimum elevation during the 20th Century. Science 320:1768-1770. [ Links ]
Levitt, J. 1980. Responses of plants to environmental stresses. Academic Press. New York. [ Links ]
Li, C., X. Zhang, X. Liu, O. Luukkanen and F. Berninger. 2006. Leaf morphological and physiological responses of Quercus aquifolioides along an altitudinal gradient. Silva Fennica 40(1):5-13. [ Links ]
Martínez-Trinidad, T., J.J. Vargas-Hernández, A. Muños-Orozco, and J. López-Upton. 2002. Respuesta al déficit hídrico de Pinus leiophylla: consumo de agua y crecimiento en plántulas de diferentes poblaciones. Agrociencia 36(3):365-376. [ Links ]
Martínez-Vilalta, J. and W. T. Pockman. 2002. The vulnerability to freezing-induced xylem cavitation of Larrea tridentata in the Chihuahuan desert. American Journal of Botany 89(12):1916-1924. [ Links ]
Medeiros, S. J. and W.T. Pockman. 2011. Drought increases freezing tolerance of both leaves and xylem of Larrea tridentata. Plant, Cell and Environment 34(1):43-51. [ Links ]
Medina-Sánchez, E. and R. Lindig-Cisneros. 2005. Effect of scarification and growing media on seed germination of Lupinus elegans. H. B. K. Seed Science and Technology 33(1):237-241. [ Links ]
Méndez-Natera, J.R., L. Lara and J.A. Gil-Marin. 2007. Efecto del riego por goteo en el crecimiento inicial de tres cultivares de algodón (Gossypium hirsutum L.). Idesia 25(2):7-15. [ Links ]
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Reviews in Ecology, Evolution and Systematics 37:637-669. [ Links ]
Rambal, S. and G. Debussche. 1995. Water balance of Mediterranean ecosystems under a changing climate. In: J.M. Moreno and W.C. Oechel, eds. Global change and Mediterranean-type ecosystems. Springer Verlag, New York. p: 386-407 [ Links ]
Rehfeldt, G. E., D.E. Ferguson and N.L. Crookston. 2009. Aspen, climate and sudden decline in western USA. Forest Ecology and Management 258:2353-2364. [ Links ]
Reichstein, M., J.D. Tenhunen, O. Roupsard, J.M. Ourcival, S. Rambal, F. Miglietta, A. Peressotti, M. Pecchiari, G. Tirone and R. Valentini. 2002. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites. Revision of current hypotheses? Global Change Biology 8(10):999-1017. [ Links ]
Robles-Díaz, E., E. Jurado, M. Ruíz-López, L. Yáñez-Espinosa and J. Flores. 2014. Heat shock effect in breaking physiscal dormancy in seeds of Lupinus elegans and L. rotundifolius from Jalisco, México. Botanical Sciences 92(1):123-129. [ Links ]
Rundel, P., A. Smith and F. Meinzer. 1994. Tropical Alpine environments. Cambridge University Press. UK. [ Links ]
Sáenz-Romero, C., G.E. Rehfeldt, N.L. Crookston, P. Duval P, R. St-Amant, J. Beaulieu and B.A. Richardson. 2010. Spline models of contemporary, 2030, 2060 and 2090 climates for Mexico and their use in understanding climate-change impacts on the vegetation. Climatic Change 102:595-623. [ Links ]
SAS Institute Inc. 2004. SAS/STAT® 9.1 User’sGuide. Cary, NC: SAS Institute Inc. 5136 p. [ Links ]
Soto-Correa, J.C., C. Sáenz-Romero, R. Lindig-Cisneros and E. de la Barrera. 2013. The neotropical shrub Lupinus elegans, from temperate forests, may not adapt to climate change. Plant Biology 15(3):607-610. [ Links ]
Tenopala, J., F.J. Gonzalez and E. de la Barrera. 2012. Physiological responses of the green manure, Vicia sativa, to drought. Botanical Sciences 90(3):305-311. [ Links ]
Van der Maaten-Theunissen, M., H.P. Kahle and E. van der Maaten. 2013. Drought sensitivity of Norway spruce is higher than that of silver fir along an altitudinal gradient in southwestern Germany. Annals of Forest Science 70(2):185-193. [ Links ]
Vitasse, Y., S. Delzon, E. Dufrêne, J.Y. Pontailler, J.M. Louvet, A. Kremer and R. Michalet. 2009. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar response? Agricultural and Forest Meteorology 149(5):735-744. [ Links ]
Vitt, P., K. Havens, A.T. Kramer, D. Sollenberger and E. Yates. 2010. Assisted migration of plants: Changes in latitudes, changes in attitudes. Biological Conservation 143(1):18-27. [ Links ]
Yu, D.P., Q.L. Wang, G.G Wang and L.M. Dai. 2006. Dendroclimatic response of Picea jezoensis along an altitudinal gradient in Changbai mountains. Science in China Series E: Technological Sciences 49 (Suppl.1):150-159. [ Links ]
Zhang, W.T., Y. Jiang, M.Y. Dong, M.Y. Kang and H.C. Yang. 2012. Relationship between the radial growth of Picea meyeri and climate along elevations of the Luyashan mountain in north-central China. Forest Ecology and Management 265:142-149. [ Links ]
Note
This paper most be cited as: Soto-Correa, J.C., C. Sáenz-Romero, H. Paz y R. Lindig-Cisneros. 2015. Drought stress in provenances of Lupinus elegans from different altitudes. Madera y Bosques 21(1):35-43.














