Introduction
Grape vines form symbiosis with microorganisms known as arbuscular mycorrhizal fungi (AMF) (Eftekhari, Alizadeh, Mashayekhi, Asghari, & Kamkar, 2010; Trouvelot et al., 2015), which are beneficial for the development and health of plants (Bhat et al., 2017). These microorganisms favor the absorption of water and mineral nutrients from the soil by the plant and this, in turn, provides carbon and other essential substances to them (Ortas, Rowell, & Harris, 2004; Rouphael et al., 2015; Begum et al., 2019).
In Brazilian viticulture, the ‘IAC 766’ (Ripária do Traviú x Vitis caribaea) rootstock, also known as 'Campinas', is the most widely used because it adapts to different environmental conditions, is pest-resistant and diseases, has a good rate of fruit set and high vegetative vigor (Souza, Mota, Cardozo, Pimentel, & Regina, 2015; Silva et al., 2019; Cardoso-Campos et al., 2020), in addition to having excellent yield and grape quality (Nuzzo & Mathews, 2006).
Recently, the propagation method of vine rootstocks using woody cuttings in containers has increased significantly, to obtain grafted seedlings in less time for subsequent transplantation in the definitive field (Ollat et al., 2016; Grohs, Almança, Fajardo, Halleen, & Miele, 2017).
The rootstock greatly influences the nutritional status of the vine in relation to factors such as soil structure and nutritional requirements (Trouvelot et al., 2015; Zhang, Marguerit-Jacob, Rossdeutsch, Ollat, & Gambetta, 2016), in addition to affecting the high absorption capacity and the possibility of reduced nutrient application (Keller, Kummer, & Vasconcelos, 2001). In this sense, the colonization of roots of plants in full growth by AMF, mainly in soils with low fertility levels, significantly favors the absorption of nutrients such as phosphorus (P) (Smith & Read, 2008; Chatterjee & Franzen, 2020). Likewise, it is important to inoculate with AMF at the beginning of seedling formation, to ensure the benefits of symbiosis at this stage and during the acclimatization period (Silva, Melo, & Yano-Melo, 2017; Begum et al., 2019; Diagne et al., 2020). Also, soils with a significant presence of P can favor the quantity and diversity of AMF species (Mahecha-Vásqueza, Sierrab, & Posada, 2007; Vilcatoma-Medina et al., 2021).
The formation of AMF-inoculated rootstocks, in addition to balanced P fertilization in the greenhouse, can be a viable and profitable alternative for the production of good quality grapevine seedlings. Therefore, this study aimed to evaluate the effect of inoculation of AMF species and phosphorous fertilization on rooted cuttings of ‘IAC 766’ grapevine rootstocks in a greenhouse.
Material and methods
This study was conducted in a greenhouse located in the Agricultural Sciences Sector of the Federal University of Paraná - UFPR, Curitiba-PR (25° 25’ 47” S and 49° 16’ 19” W, at 950 m a. s. l.).
On November 15, 2018, woody cuttings 15 to 20 cm long and 5.8 mm in diameter were collected from four-year-old Campinas grapevine ‘IAC 766’ rootstocks at the Canguiri Station Experimental Center of the UFPR (Pinhais -PR) (25° 23’ 42” S and 49° 08’ 12” W, at 950 m a. s. l.), which were subsequently placed in 133 cm3 plastic tubes containing a medium-textured vermiculite substrate to promote rooting. The rootstocks were kept for 45 days in a greenhouse with manual irrigation every 24 h. After rooting, the rootstocks were transplanted into 3 L plastic bags containing a mixture of a commercial substrate based on biostabilized pine bark (Mecplant Brasil) + washed sand (1:1) for the application of the treatments.
A completely randomized design was used, with eight repetitions per treatment, totaling 80 plastic bags. The treatments consisted of incorporating and mixing 30 g of material with different AMF species (Gigaspora margarita; Gigaspora margarita + Dentiscutata heterogama; Dentiscutata heterogama; Rhizophagus intraradices; Acaulospora colombiana; Acaulospora colombiana + Acaulospora scrobiculata; and Acaulospora scrobiculata), two doses of fertilization phosphated (12.5 and 25 mg·kg-1 of P2O5) using commercial simple superphosphate, and the control.
Inoculum of AMF species were multiplied on Brachiaria (Brachiaria decumbens) in 5 L pots at the DFF greenhouse condition, with manual irrigation every 48 h. The substrate used was a mixture of washed sand and medium texture vermiculite (1:1), previously sterilized in an autoclave at 120 °C for two periods of 1 h at an interval of 24 h.
After 90 days of inoculation and fertilization of the rootstock cuttings, the following characteristics were evaluated: seedling height (H), basal stem diameter 5 cm from the substrate surface (D), length of the largest root (RL), shoot (SFW) and roots fresh weight (RFW), and shoot (SDW) and roots dry weight (RDW).
The analysis of macro and micronutrients was carried out in the Plant Nutrition Laboratory, in the Department of Soils and Agricultural Engineering of UFPR, according to the methodology adapted from Martins and Reissmann (2007). Approximately 0.5 g of material was used and incinerated in porcelain crucibles in a muffle furnace at 500 °C for 4 h. Then, 10 mL of 3 mol·L-1 HCl was added and the crucibles were left for 10 min on a heating plate at 70 °C. After this period, the digestion solutions were filtered through filter paper (pore diameter 8 µm) and the extracts collected in 50 mL volumetric flasks. To measure the volumetric flasks, deionized water was used. In the obtained extracts, the following were determined: P, K, Ca, Mg, Fe, Cu, Mn, and Zn using an inductively coupled plasma-optical emission spectrometer (ICP-OES), (720-ES, Varian, USA).
To evaluate mycorrhizal colonization, the thinnest roots were selected and kept in 10 % KOH for 24 h, after being treated in a water bath at 80 °C for 1 h. Next, they were bleached by adding H2O2, then washed and stained with blue ballpoint ink and kept again in a water bath for 5 min more, when lactoglycerol was applied. For the counting of mycorrhizal colonization, the roots were placed in a 1x1 cm square plate using a stereoscopic microscope, according to the methodology described by Giovanetti and Mosse (1980).
The variables evaluated (growth parameters, macro and micronutrients and the percentage of root colonization) were subjected to analysis of variance and, when there was a significant effect, the means were compared using the Tukey (P ≤ 0.05). These explanatory variables were also subjected to correlation and principal component analyzes (PCA), using the statistical software R (R Core Team, 2018).
Results and discussion
According to the results in Table 1, most of the growth parameters of the ‘IAC 766’ rootstock showed significant differences when inoculated with AMF species and fertilized with P2O5. Only the stem diameter was not influenced by the treatments.
Table 1 Growth parameters of rooted cuttings of ‘IAC 766’ vine rootstocks inoculated with arbuscular mycorrhizal fungi (AMF) species or phosphate fertilization (Curitiba, Brazil).
| Treatments | Height | Root length | Diameter (mm) | Fresh weight | Dry weight | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||||||
| cm | g·seedling-1 | |||||||||
| G. margarita | 59.8 efz | 43.0 cd | 3.0 ns | 27.8 gh | 26.3 e | 7.3f g | 7.0 ef | |||
| G. margarita + D. heterogama | 67.0 de | 44.6 cd | 3.5 | 29.9 efg | 27.9 de | 7.7 ef | 7.6 d | |||
| D. heterogama | 61.2 ef | 44.0 cd | 3.5 | 28.4 fgh | 27.6 de | 7.4 f | 7.4 de | |||
| R. intraradices | 72.6 cd | 45.0 bcd | 3.1 | 30.9 def | 28.5 cde | 8.0 def | 7.6 d | |||
| A. colombiana | 87.4 ab | 49.8 b | 3.4 | 35.3 bc | 30.7 bc | 8.9 c | 8.2 c | |||
| A. colombiana + A. scrobiculata | 89.4 a | 56.6 a | 3.5 | 36.8 b | 32.2 ab | 11.5 b | 8.6 b | |||
| A. scrobiculata | 96.0 a | 58.8 a | 3.6 | 41.5 a | 34.0 a | 13.7 a | 9.1 a | |||
| 12.5 P2O5 | 79.4 bc | 46.2 bc | 3.6 | 33.0 cd | 28.7 cd | 8.7 cd | 7.7 d | |||
| 25 P2O5 | 80.6 bc | 46.6 bc | 3.3 | 32.7 cde | 28.2 de | 8.2 cde | 7.6 d | |||
| Control | 57.8 f | 40.6 d | 3.2 | 26.1 h | 26.2 e | 6.6 g | 6.8 f | |||
zMeans followed by the same letter in the column do not differ from each other (Tukey, P ≤ 0.05). ns = Not significant.
Height showed significant differences between the treatments, with the species A. scrobiculata (96.0 cm) and A. colombiana + A. scrobiculata (89.4 cm) standing out, but without differing in relation to A. colombiana. Also, the RL showed significant differences, highlighting the same species, A. scrobiculata (58.8 cm) and A. colombiana + A. scrobiculata (56.6 cm). Regarding FW and DW, of shoot (S) and root (R), A. scrobiculata presented the highest values, except for RFW where the same species (34.0 g) did not differ from A. colombiana + A. scrobiculata (32.2 g) (Table 1).
The inoculation of AMF species and phosphate fertilization favored the growth of rooted cuttings of the rootstock ‘IAC 766’, differing from the control in terms of growth parameters, presence of macro and micronutrients and root colonization when inoculated into the cuttings. As well as the positive results obtained in the present study, the beneficial effects of AMF inoculation in the formation of vigorous and healthy vine seedlings were previously emphasized by several researchers (Aguín, Mansilla, Vilariño, & Sainz, 2004; Caglar & Bayram, 2006; Camprubí et al., 2008; Ozdemir et al., 2010; Ambrosini et al., 2015).
In the present study, in general, the species of the genus Acaulospora stood out both individually and mixed in the evaluations carried out in comparison with the rest of the treatments. The positive effect of the inoculation of species of this genus was confirmed by the work carried out by Meyer, Botha, Valentine, Archer, and Louw (2005) and Ambrosini et al. (2015), in which root colonization was also favored in commercial plantations with different rootstocks in the cultivar Merlot and young vines subjected to high levels of Cu, respectively.
Another species of mycorrhiza that deserves to be highlighted according to the results obtained in the present work was Rizophagus intraradices, which promoted considerable benefit in the growth and percentage of colonization in the rootstock. Similar responses were found in studies carried out by Ambrosini et al. (2015) and Rosa et al. (2016) with R. clarus species subjected to high levels of Cu on Paulsen 1103 rootstocks, and Rosa et al. (2020) with R. irregularis on North American rootstocks in commercial substrate (expanded clay). The results obtained show that the species of the genus interact favorably in the different rootstocks and obtain a wide adaptability to different environmental conditions, thus being commercially produced for more than two decades (Hijri, 2016; Fracasso, Telò, Lanfranco, Bonfante, & Amaducci, 2020).
In general, the inoculation of AMF species on the ‘IAC 766’ rootstock provided greater gain in H, RL, FW and DW, both in shoots and roots. The results presented in the present study are confirmation of those obtained in research by Agostini and Souza (2003) and Anzanello, Souza, and Casamali (2011) with inoculations of species of the genera Glomus and Scutellopora on rootstocks of Paulsen vines 1103, 101-14, SO4 and 043-43. It is also important to mention similar responses in studies with rootstocks from other crops such as peach and citrus with inoculations of species Glomus clarum, Glomus etunicatum and Acaulospora sp. (Nunes, Souza, Marodin, & Fachinello, 2008; Back, Reith, Giuliani, & Souza, 2017).
The significant differences show that the AMF contributed to the increase in the architecture of the root system such as root length, accelerating the growth in height and leaf area of the rootstocks, allowing greater photosynthetic areas and, consequently, a higher level of photoassimilates production and accumulation of biomass (Wu, Zou, Liu, & Lu, 2012; Wu, Zou, & Huang, 2013; Wright, Scholes, & Read, 1998). The behavior between the actors is interpreted as functional specificity, since there is compatibility between the symbionts (Lindermann & Davis, 2001; Locatelli & Lovato, 2002; Silveira, Souza, & Koller, 2002).
Table 2, in general, shows significant differences when calculating the presence of macro and micronutrients in the rootstocks after 90 days of AMF inoculation and phosphate fertilization. When inoculated with the genus Acaulospora, the highest average value of P was obtained in the shoot (16.6 mg·kg-1), while in the root the presence of this element was higher (20.9 mg·kg-1) when fertilized with the highest dose of P2O5, followed by the lowest dose of phosphorus applied. Among the inoculated species, the highest concentration of this nutrient was obtained for D. heterogama, not differing from A. scrobiculata. For K, the highest averages in both shoots and roots were obtained with the inoculation of A. scrobiculata and R. intraradices, respectively. Finally, Ca and Mg presented higher averages, both in the shoot and in the root, when inoculated with species of the genus Gigaspora and fertilized with the lowest dose of P.
Table 2 Macronutrients and micronutrients present in shoot and root of rooted cuttings of ‘IAC 766’ vine rootstocks inoculated with arbuscular mycorrhizal fungi (AMF) or phosphate fertilization (Curitiba, Brazil).
| Treatments | P | K | Ca | Mg | Fe | Cu | Mn | Zn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·kg-1 | ||||||||||||||||
| Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | |
| G. margarita | 6.2 fz | 14.7 f | 4.6 g | 4.1 f | 2.7 c | 1.3 b | 1.3 a | 2.0 a | 30.0 a | 56.5 f | 1.4 de | 3.6 b | 6.5 e | 10.5 d | 4.1 e | 7.6 b |
| G. margarita + D. heterogama | 5.0 g | 16.3 de | 5.1 ef | 6.1 cd | 3.4 a | 0.9 cd | 1.3 a | 2.0 a | 19.9 e | 63.1 d | 1.3 ef | 2.6 cd | 8.1 b | 9.2 e | 5.1 cd | 5.3 e |
| D. heterogama | 7.7 e | 16.8 c | 5.0 ef | 6.3 c | 2.5 c | 0.9 cd | 1.0 bc | 1.8 ab | 22.4 c | 73.2 a | 1.6 cd | 1.8 f | 5.1 g | 9.4 e | 4.3 e | 5.0 ef |
| R. intraradices | 7.9 e | 14.6 f | 4.9 fg | 8.0 a | 3.2 ab | 0.8 d | 1.2 ab | 1.6 bc | 17.2 f | 45.7 i | 1.6 cd | 1.9 ef | 6.3 e | 6.5 g | 4.3 e | 9.1 a |
| A. colombiana | 11.0 d | 13.5 g | 7.0 c | 6.7 b | 2.6 c | 0.8 cd | 0.8 c | 1.2 d | 21.9 d | 27.1 j | 1.8 c | 3.7 b | 3.9 h | 4.4 h | 5.1 d | 7.3 b |
| A. colombiana + A. scrobiculata | 16.6 a | 15.9 e | 5.3 e | 5.9 d | 2.7 c | 1.0 bcd | 1.2 ab | 1.4 cd | 23.2 b | 51.9 h | 4.1 a | 2.1 ef | 8.6 a | 8.1 f | 6.6 a | 4.9 f |
| A. scrobiculata | 16.3 ab | 16.6 cd | 10.0 a | 4.2 f | 2.6 c | 1.2 bc | 1.2 ab | 1.9 ab | 23.1 b | 64.5 c | 2.9 b | 4.4 a | 6.3 e | 8.3 f | 5.4 c | 6.4 d |
| 12.5 P2O5 | 15.8 b | 18.6 b | 7.6 b | 2.5 g | 1.5 e | 1.9 a | 1.4 a | 1.9 ab | 13.9 i | 58.8 e | 1.0 f | 2.9 c | 7.3 c | 21.1 b | 6.0 b | 6.8 c |
| 25 P2O5 | 13.6 c | 20.9 a | 6.3 d | 4.2 f | 1.9 d | 1.4 b | 0.9 c | 2.1 a | 15.8 g | 53.6 g | 2.6 b | 2.2 de | 5.7 f | 25.9 a | 4.0 e | 4.5 g |
| Control | 4.8 g | 12.1 h | 7.7 b | 5.0 e | 3.0 b | 1.9 a | 1.2 ab | 2.0 a | 14.3 h | 71.9 b | 1.0 f | 3.5 b | 6.9 d | 16.1 c | 3.6 f | 6.2 d |
zMeans followed by the same letter in the column do not differ from each other (Tukey, P ≤ 0.05).
Regarding the micronutrients, the presence of Fe in the shoot was presented by the species G. margarita and in the root by D. heterogama. While for Cu the highest averages both in the shoot and in the root were of the species of the genus Acaulospora. In the case of Mn, the highest average was obtained in the shoot when inoculated with a mixture of species of the genus Acaulospora and in the root it was when fertilized with the highest dose of P. Finally, higher concentrations of Zn were also verified in the shoot, when it was inoculated with a mixture of species of the Acaulospora genus and in the root, when it was inoculated with R. intraradices.
The time that the experiment lasted was sufficient to demonstrate that there was a higher content of macro and micronutrients in the shoots and roots of the rootstock ‘IAC 766’, in the presence of AMF species, similar to what occurred in the vine cuttings of the cultivar Cabernet Sauvignon inoculated with the species Claroideoglomus etunicatum and Entrophospora infrequens (Coutinho, Silva, & Yano-Melo, 2017). In the studies carried out by Alarcón, González-Chávez, Ferrera-Cerrato, and Villegas-Monter (2001) and Anzanello et al. (2011), with twice the duration of the present experiment, verified a significant increase in the absorption of macro and micronutrients in different rootstocks inoculated with the species Glomus fasciculatum, G. etunicatum and Scutellospora heterogama, respectively. According to Agostini and Souza (2003), the effect of AMF on grapevine rootstocks in a short period of time does not allow them to express their full potential.
In Figure 1, it can be verified that the percentage of root colonization of the ‘IAC 766’ rootstock was favored by the inoculation of the five AMF species, and the highest value of this variable (53 %) was obtained when the species of the genus Acaulospora were associated (AC+ASC).
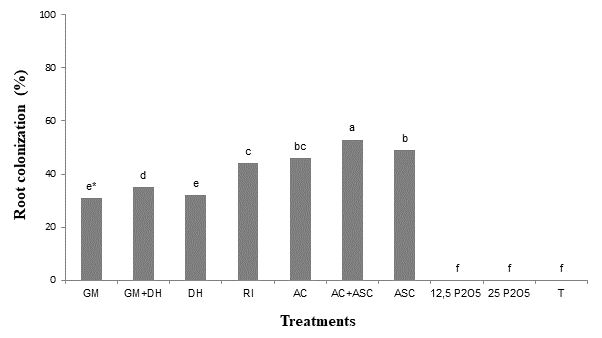
Figure 1 Root colonization of rooted cuttings of ‘IAC 766’ vine rootstock inoculated with arbuscular mycorrhizal fungi (AMF) or phosphate fertilization (Curitiba, Brazil). GM = Gigaspora margarita; DH = Dentiscutata heterogama; RI = Rizophagus intraradices; AC = Acaulospora colombiana; ASC = Acaulospora scrobiculata; 12.5 P2O5 = 12.5 mg·kg-1 P2O5; 25 P2O5 = 25 mg·kg-1 P2O5; T = control. *Means followed by the same letter do not differ (Tukey, P ≤ 0.05).
The fungi actively colonized the roots of the ‘IAC 766’ rootstocks; however, the species of the same genus had different behaviors when they were inoculated individually or mixed. Similar responses were also confirmed by Alarcón et al. (2001), Agostini and Souza (2003), and Rosa et al. (2016), who evaluating different rootstocks obtained a high root colonization. On the other hand, Nunes et al. (2008) and Back et al. (2017) evaluating rootstocks of other crops, verified that root colonization was almost 100 %. These differences in the intensity of the root colonization present in the rootstocks were also mentioned by Silveira et al. (2002), as a consequence of the degree of affinity between the seedlings and the AMF species.
Figure 2 shows the correlation matrices that exist between the averages of the treatments on the percentage of colonization, growth parameters and the content of macro and micronutrients in the rooted cuttings up to 90 days. It is verified that there is a positive correlation between RL, SFW, SDW, RFW, RDW and H. Likewise, the micronutrients Zn and Cu of the shoot had a positive correlation with most of the growth parameters, while the percentage of root colonization (% Col) had a high positive correlation with the Fe in the shoot (FeS).
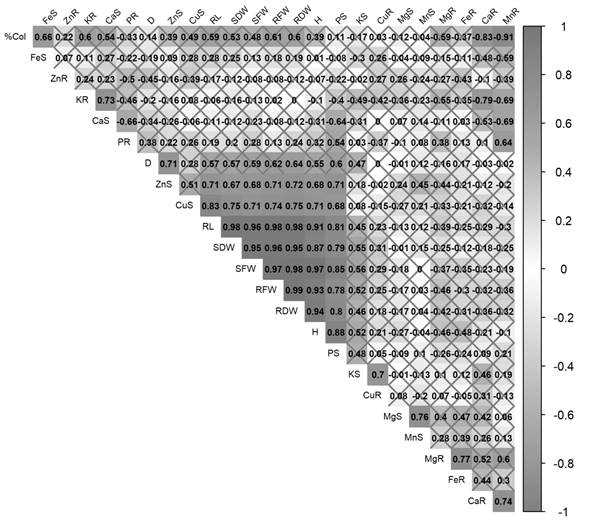
Figure 2 Correlation matrix of treatment means in the percentage of root colonization, growth parameters, presence of macro and micronutrients in rooted cuttings of rootstock ‘IAC 766’ inoculated with arbuscular mycorrhizal fungi (AMF) species or fertilized with phosphorus (Curitiba, Brazil).
As observed in the results, the efficiency of AMF was to confer greater vigor to the ‘IAC 766’ rootstocks, reflecting positively in the correlation of growth parameters. Therefore, it could be said that the rootstocks showed moderate mycorrhizal dependence, since the microorganisms facilitated sufficient absorption of nutrients which were contained in the substrate used in the experiment. A clear example of this fact was demonstrated by Schreiner (2003), who evaluating different rootstocks grafted with a clone of Pinot Noir in the final field, verified high yields in crops with moderate doses of nutrients, especially P. The growth of the vine depends on strongly from AMF in certain types of soils and the increase in growth parameters is mainly due to a greater absorption of P and other nutrients (Schreiner, 2007). These results were also verified by Nogueira and Nogueira-Cardoso (2006), and Miranda, Mello, and Kupper (2018), when inoculating species of the Glomus genus on rootstocks of Rangpur lime (Citrus limonia) with low concentrations of P in the substrates.
The results of the PCA based on the order between growth parameters, presence of macro and micronutrients and colonization percentage of the rooted cuttings with the species inoculated with AMF and phosphate fertilization up to 90 days, are shown in Figure 3. The perpendicular axes 1 and 2 indicate the separation of most of the treatments in relation to the parameters evaluated.
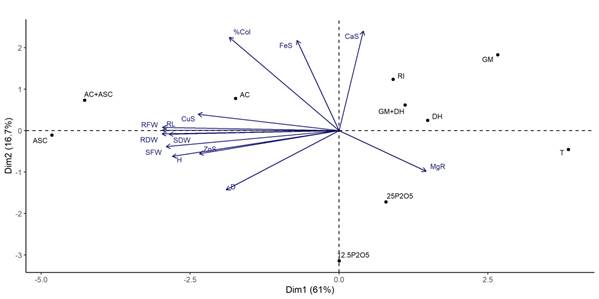
Figure 3 Principal component analysis (PCA) diagram in rootstock ‘IAC 766’ rootstocks inoculated with arbuscular mycorrhizal fungi (AMF) species or fertilized with phosphorus. Curitiba, Brazil. With the presence of growth parameters, macro and micronutrients and percentage of root colonization (% Col). GM = Gigaspora margarita; DH = Dentiscutata heterogama; RI = Rizophagus intraradices; AC = Acaulospora Colombiana; ASC = Acaulospora scrobiculata; 12.5 P2O5 = 12.5 mg·kg-1 P2O5; 25 P2O5 = 25 mg·kg-1 P2O5; T = control; H = height; D = diameter; RL = length of root; SFW = shoot fresh weight; SDW = shoot dry weight; RFW = root fresh weight; RDW = root dry weight; CaS = calcium shoots; MgR = magnesium root; FeS = iron shoots; CuS = copper shoots; ZnS = zinc shoots.
The axes represented 61.0 and 16.7 % of the data variability, which explains 77.7 % of the total accumulated variability. The growth parameters (RL, SDW, SFW, RFW, RDW, H and D), root colonization percentage (% Col) and micronutrients Cu and Zn of the shoots are favored by the presence of the species of the genus Acaulospora individually or as a mixture. On the other hand, although the P fertilizer and control results were different, they were far apart.
The efficiency demonstrated by the AMF in the rootstocks happened mainly with the presence of the species of the genus Acaulospora, as observed in the PCA, where the mentioned species had a favorable influence on root colonization, growth parameters and the absorption of micronutrients from the shoot. Similar results were observed by Schreiner and Mihara (2009), and Chatzistathis, Orfanoudakis, Alifragis, and Therios (2013) in plantations of the Pinot Noir cultivar and olive tree (Olea europaea) in soils with different levels of fertility.
In the present study it is important to highlight the presence of low levels of P in the rootstock ‘IAC 766’, which interacts favorably in the growth and formation of roots, as it was verified in different rootstocks when they were grafted with vinifera cultivars in soil with P availability of 8 mg·kg-1 (Grant & Matthews, 1996). These results were confirmed by Simões-Neto et al. (2009), Veneklaas et al. (2012), and Tecchio, Teixeira, Terra, Moura, and Paioli-Pires (2011), who recommend the application of small concentrations of phosphorus to improve the efficiency of its use, in addition to optimizing the absorption capacity of the rootstock, and which will serve as the basis for future fertilization of vineyards in the final field.
Favorable results also confirm the adaptability of the ‘IAC 766’ rootstock in Brazilian soils with a low presence of P, offering vines with greater vigor and growth (Cardoso-Campos et al., 2020). Likewise, Tofanelli et al. (2011), Bruna and Back (2015), Souza et al. (2015) and Dias et al. (2017) verified vines with high productivity when grafted with the cultivars ‘Niagara Rosada’, ‘Cabernet Sauvignon’ and ‘Syrah’. While in Chile, under hyperarid conditions, the works of Ibacache and Sierra (2009) and Verdugo-Vásquez et al. (2021) demonstrated the great influence that different rootstocks can have on the nutritional composition of vine cultivars for the production of table grapes and “pisco” in the presence of P with an average of 60 mg·kg-1 in addition to other macronutrients.
Conclusion
The inoculation of AMF species provides favorable responses on growth parameters, root colonization and the extraction of macro and micronutrients in the cuttings of the 'IAC 766' rootstock, highlighting the inoculation with species of the genus Acaulospora either individually or in association.
Phosphorus fertilization also influences growth parameters and the extraction of macro and micronutrients, but to a lesser degree than that found by inoculation with AMF species.











 texto en
texto en 



