Introduction
Arbuscular mycorrhizal fungi (AMF) are cosmopolitan rhizospheric microorganisms found in up to 80 % of terrestrial plant species. The main characteristic of this fungus is the ability to colonize the root system of plants and establish a mutualistic or symbiotic association (Brundrett & Tedersoo, 2018). The term arbuscular refers to fungal structures called arbuscules that form within plant root cells; therefore, an arbuscular mycorrhiza is defined as a mutualistic symbiosis with the ability to produce arbuscules within the cells of compatible plants (Köhl, Lukasiewicz, & van der Heijden, 2016).
Arbuscular mycorrhiza are endomycorrhiza whose hyphae initially enter the root cells and later penetrate inside them, forming feeding vesicles and arbuscules. An arbuscular mycorrhiza has three main components: the root, structures within the root cells, and extraradical mycelium in the soil (Smith & Smith, 2011). The symbiotic relationship occurs when the plant receives nutrients and water from the mycorrhiza (Camarena-Gutiérrez, 2012), and the fungus obtains carbohydrates and vitamins from the plant that it is unable to synthesize on its own (Thioub, Ewusi-Mensah, Sarkodie-Addo, & Adjei-Gyapong, 2019). Other important benefits received by the plant from said symbiosis are growth promotion, increased mineral uptake (Díaz-Hernández, Aguirre-Medina, & Díaz-Fuentes, 2018) and tolerance to soil pathogens (Tahat, Kamaruzaman, & Othman, 2010).
Some types of AMF have been identified such as Funneliformis mosseae, whose mycelium modifies root anatomy (such as their length, volume, and exploration area), which makes inoculated plants more efficient in absorbing soil nutrients such as nitrates (Huang et al., 2019). Likewise, fungi increase the uptake of poorly mobile or immobile mineral ions from the soil and their hyphae, by growing beyond the rhizosphere and thus increasing the absorbent surface area of roots. In general, this behavior is attributed to AMF (Vallejos-Torres, Saboya-Pisco, & Arévalo-López, 2021).
Plant growth-promoting bacteria (PGPR) are a group of microorganisms that can increase crop growth and productivity. Azospirillum is one of the best-known genera used in agriculture to benefit plant development through the production of phytohormones (Berendsen, Pieterse, & Bakker, 2012) and changes in plant growth by increasing germination speed, biomass accumulation (aerial and root), root respiration rate, and root system development of root hairs and root diameter) (Canto-Martín, Medina-Peralta, & Morales-Avelino, 2004).
Positive effects have been reported with the use of AMF or beneficial rhizobacteria on the growth and yield of fruiting vegetables (Chiquito-Contreras, Reyes-Pérez, Chiquito-Contreras, Vidal-Hernández, & Hernández-Montiel, 2020; Zulueta-Rodríguez, Hernández-Montiel, Reyes-Pérez, González-Morales, & Lara-Capistrán, 2020). However, research evaluating the interaction between AMF and A. brasilense in albahaca (Ocimum basilicum) is scarce. It is known that the interaction between different microorganisms can improve the uptake of nutrients such as nitrogen and phosphorus, elements of great importance for plant growth and development (García-Gutiérrez et al., 2012).
Basil is a commercially important aromatic plant used as food, as well as in the cosmetic and pharmaceutical industry (Juárez-Rosete et al., 2013). In Mexico, fresh basil is generally destined for the export market. In 2019, fresh basil was generally destined for the export market. In 2019, 3,103.4 t were produced, which generated a value of almost 50 million Mexican pesos.
Based on the above, the aim of this study was to evaluate the growth and development of basil inoculated with arbuscular mycorrhizal fungi and A. brasilense under limited nutrition.
Materials and methods
Experiment location
The experiment was carried out in a tunnel-type greenhouse with a white plastic cover, which provided 30 % shading, and with an anti-aphid mesh on the side walls, belonging to the Faculty of Agricultural Sciences of the Autonomous University of the State of Morelos, Chamilpa, Cuernavaca, Morelos, Mexico (18° 58’ 51’’ NL and 99° 13’ 55’’ WL, at 1,866 m a. s. l.). Environmental conditions in the greenhouse were monitored with a model U12 datalogger (Hobo®, Massachusetts, USA): average temperature of 25 °C, average relative humidity of 55 % and average solar radiation of 514 µmol·m-2·s-1.
Treatments evaluated
The treatments evaluated were: 1) without microorganism inoculation (control), 2) Rhizophagus intraradices, 3) Funneliformis mosseae, 4) Cerro del Metate AMF consortium, 5) Azospirillum brasilense, 6) R. intraradices + A. brasilense and 7) without microorganism inoculation and irrigated with Steiner's nutrient solution at 100 % concentration.
Treatments 1 to 6 were irrigated daily by hand with 0.5 L of Steiner's nutrient solution (Steiner, 1984) at 50 % during the first 21 days after transplanting (dat), and irrigated with 1.5 L of nutrient solution during the period from 22 to 50 dat, at which time the plants were harvested. Treatment 7 was used to compare the effect of nutrient concentration and inoculations with the microorganisms on plant growth.
Experimental unit and design
The experimental unit was a pot containing one basil plant. A completely randomized design with six replications was used.
Microbiological plant material and sowing of basil plants
The 'Sweet Nufar' basil cultivar (SunLine AG Inc, USA) was used. Seeds were sown on March 29, 2019 in 200-cavity polystyrene trays with Sunshine® Mix 3 germination substrate, previously sterilized in an autoclave (cv 300, AESA) for 15 min at 120 °C (0.98 to 1.05 kg·cm-2 steam pressure), according to the methodology described by Zulueta-Rodríguez et al. (2016). Azospirullum brasilense strain Cd was provided by the Biotechnology Center of the Autonomous University of the State of Morelos, and the AMF by the Phytopathology Laboratory of the Center for Research and Assistance in Technology and Design of the State of Jalisco (CIATEJ). The Funneliformis mosseae inoculum was isolated from the Cerro del Metate consortium, the Rizhophagus intraradices strain was isolated from the Micorriza INIFAP product, and the Cerro del Metate consortium was isolated from the Cerro del Metate mountain, located in Michoacán, from the rhizosphere of Agave cupreata (Trinidad-Cruz, Quiñones-Aguilar, Hernández-Cuevas, López-Pérez, & Rincón-Enríquez, 2017).
Inoculation of basil plants with arbuscular mycorrhizal fungi
Transplanting was performed on May 4, 2019 (38 days after sowing [das]). The basil seedlings were placed in 5-L plastic pots containing tezontle with granulometry ranging from 1 to 5 mm in diameter, previously autoclaved for 15 min at 120 °C (0.98 to 1.05 kg·cm-2 steam pressure). Treatments were established at transplanting; the AMF were applied at a dose of 150 spores per plant directly to the bare root. The fungi were found in river sand as an inoculation medium.
Inoculation of basil plants with A. brasilense
Basil plants were inoculated with A. brasilense strain Cd at a concentration of 1.04 x 109 colony-forming units (CFU) per gram of dry substrate (Bashan, 1986). CFU counting was performed according to the methodology described by Peña-Cortés, Peña-Cortés, and Gonzalo-Moreno (2011). The pots had 3 kg of tezontle, and the inoculations were carried out every two weeks from May 14, 2019 until harvest at 50 dat.
Plant growth variables
Harvesting was carried out at 50 dat and the following variables were determined: plant height (PH, cm), basal stem diameter (SD, mm), fresh aerial biomass weight (FBW, g), dry aerial biomass weight (DBW, g) and leaf area (LA, cm2).
Decreased basil growth due to a reduction in the nutrient concentration in the nutrient solution
The percentage reduction of the characters plant height, fresh biomass weight and leaf area was determined by decreasing the concentration of the nutrient solution from 100 % (treatment 7) to 50 % (treatments 1 to 6) using the following formula:
where Ȳ 7 is the mean of treatment seven (100 % nutrient solution concentration) and Y ij is the observation of the i-th treatment in its j-th replication (50 % nutrient solution concentration).
Subsequently, Tukey's multiple comparison test (P ≤ 0.05) was performed.
Macronutrient concentration
The aerial dry biomass (leaves and stems) of the six replicates was ground with an electric mill (004108-013-000, Osterizer®, USA). Subsequently, the concentration of N (by the microkjeldahl method), P (by colorimetry), K (by flamometry), and Ca and Mg (both by atomic absorption spectrophotometry) were determined (Cruz-Álvarez et al., 2020).
Mycorrhizal colonization and number of spores
As an indicator of the efficiency and functionality of the inocula in basil plants, the percentage of mycorrhizal colonization (PMC) by fungal structure was determined at 50 dat. For this, roots from each replicate were collected at the end of the experiment and fixed in FAA (formaldehyde: acetic acid: ethanol, 2:1:10:7); subsequently, the roots were cleared and stained (Phillips & Hayman, 1970). The percentage of mycorrhizal colonization was evaluated in 30 1-cm root segments, according to the method described by McGonigle, Miller, Evans, Fairchild, and Swan (1990). PMC was determined using a microscope (model k7, Zeiss, Germany).
The number of spores in 100 g of dry soil was determined from extraction by the wet sieving and decanting method (Gerdemann & Nicolson, 1963). Spore counting was performed visually with a stereo microscope (VE-S5C, Velab™). Wet sieving was observed in a gridded Petri dish (90 mm) and the number of spores was recorded.
Statistical analysis
Data expressed as percentages were transformed with the arcsine square root function. Normality and homogeneity of variance were checked using the Levene and Kolmogorov-Smirnov test. Subsequently, analysis of variance and Tukey's multiple comparison test (P ≤ 0.05) were performed using Statistical Analysis System version 9.1 software (SAS Institute Inc., 2004).
Results and discussion
Growth of basil inoculated with AMF and A. brasilense
When considering the treatments with 50 % nutrient solution (treatments 1 to 6) and comparing them with the control (without microorganisms) (Table 1), only A. brasilense significantly improved the development of 'Sweet Nufar' basil plants, by increasing PH, SD, FBW, DBW and LA (P ≤ 0.05), by 15, 18, 28, 27 and 24 %, respectively. The application of Rizhophagus intraradices and R. intraradices + A. brasilense increased FBW and LA (P ≤ 0.05), while Funneliformis mosseae only improved SD and LA (P ≤ 0.05). The Cerro del Mate consortium did not show advantages over the control without microorganisms.
Table 1 Growth of 'Sweet Nufar' basil plants inoculated with arbuscular mycorrhizal fungi and A. brasilense under greenhouse conditions.
| Treatment | PH (cm) | SD (mm) | FBW (g) | DBW (g) | LA (cm2) |
|---|---|---|---|---|---|
| Control (without microorganisms) | 35.2 bz | 6.6 b | 99.4 c | 10.6 b | 2026.6 b |
| Rizhophagus intraradices | 37.8 ab | 7.2 ab | 118.7 ab | 12.1 ab | 2492.5 a |
| Funneliformis mosseae | 37.7 ab | 7.4 a | 112.9 abc | 12.2 ab | 2373.8 a |
| Cerro del Metate consortium | 39.0 ab | 7.3 ab | 111.4 bc | 11.6 ab | 2302.4 ab |
| Azospirillum brasilense | 40.6 a | 7.8 a | 127.5 a | 13.5 a | 2518.2 a |
| R. intraradices + A. brasilense | 36.5 ab | 7.3 ab | 123.0 ab | 12.1 ab | 2516.3 a |
| CV (%) | 7.54 | 6.61 | 7.33 | 10.34 | 7.12 |
| LSD | 5.00 | 0.78 | 14.87 | 2.18 | 296.57 |
PH = plant height; SD = stem diameter; FBW = fresh biomass weight; DBW = dry biomass weight; LA = leaf area; CV = coefficient of variation; LSD = least significant difference. zMeans with the same letter within each column did not differ statistically (Tukey, P ≤ 0.05).
Cruz-Romero, Barrios-Díaz, Rodríguez-Mendoza, Espinoza-Victoria, and Tirado-Torres (2016) reported similar effects on growth when inoculating broccoli plants with A. brasilense; the increases were 11 % in SD, 38 % in LA, and 29 % in DBW. Likewise, it has been documented that A. brasilense increases nitrogen assimilation in species such as maize and wheat (Mariani-Zeffa et al., 2019; Shintate-Galindo et al., 2020), which favors plant development, even when fertilization levels are limited. The above coincides with the results of the present study, since a 15 % increase in nitrogen concentration was observed in the tissues of the plants inoculated with A. brasilense with respect to the control (Table 2). In this regard, Pereira-Coelho et al. (2021) note that A. brasilense forms symbiosis with some plants and increases their water and nutrient assimilation capacity, which improves the growth and yield of agricultural crops.
Inoculation of R. intraradices improved FBW by 7 % and LA by 22 %, while F. mosseae increased SD by 12 % and LA by 17 % compared to the control (Table 1). Favorable effects from the use of AMF have also been reported in tomato (Bona et al., 2017) and broad bean (Abd-Alla, El-Enany, Nafady, Khalaf, & Morsy, 2014). This implies that the species R. intraradices and F. mosseae are also an option to improve basil crop development, although their effect is less than that achieved with A. brasilense.
The combination of R. intraradices + A. brasilense increased FBW by 24 % and LA by 24 % compared to the control. This coincides with the findings reported by Mujica-Pérez, Medina-Carmona, and Rodríguez-Guerra (2017), who point out that the inoculation of growth-promoting bacteria in combination with AMF generates a synergistic effect that is reflected in better plant development.
Basil growth due to reducing the nutrient solution concentration
When comparing the effect of nutrient concentration (100 and 50 %) on crop development, inoculation with A. brasilense had the smallest decrease in basil growth (P ≤ 0.05) due to the effect of nutrient reduction on the variables PH, FBW and LA; however, its response was similar to the decrease in growth with AMF inoculation and the combination of R. intraradices + A. brasilense (Figure 1). The greatest decreases in growth occurred when no beneficial microorganism was inoculated. Specifically, plants without microorganisms had a decrease of 15.6 % in PH, 49.9 % in FBW and 46.6 % in LA. This indicates that inoculation with growth-promoting rhizobacteria has the ability to improve basil plant development and increase PH and FBW, determining variables in the growth of this crop (Chiquito-Contreras et al., 2018).
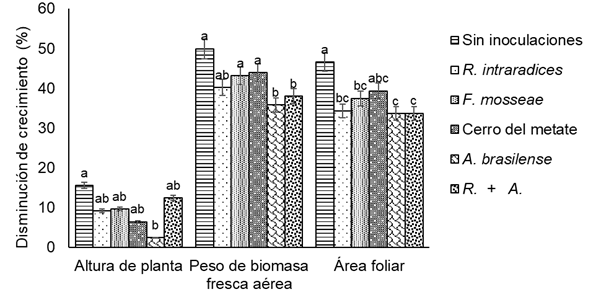
Figure 1 Decreased growth in 'Sweet Nufar' basil grown with 50 % nutrient solution compared to 100 % nutrient solution treatment, 50 days after transplanting. Each bar represents the average of six replicates ± standard error. R.+ A.: R. intraradices + A. brasilense. Bars with the same letter between each response variable do not differ statistically (Tukey, P ≤ 0.05).
Macronutrient concentration
The use of beneficial microorganisms improved the concentration of nutrients in the basil plant tissue, mainly N and K (P ≤ 0.05), compared to the control without microorganisms (Table 2). The N concentration increased (P ≤ 0.05) in plants inoculated with R. intraradices (17 %), A. brasilense (16 %) and with the combination of both microorganisms (15 %). Regarding P, only A. brasilense increased (P ≤ 0.05) its concentration in plant tissues, by 11 %, while K concentration had no significant changes due to the effect of microorganism inoculation. The highest Mg concentrations occurred with the inoculation of R. intraradices and F. mosseae, with significant increases (P ≤ 0.05) of 18 and 20 %, respectively, compared to the control. It has been reported that the use of beneficial microorganisms such as rhizobacteria and AMF allow plants to absorb greater amounts of essential nutrients (Díaz-Hernández et al., 2018). In the case of Ca concentration, there were no significant effects from the inoculation of microorganisms.
Table 2 Effect of the inoculation of beneficial microorganisms on macronutrient concentration in 'Sweet Nufar' basil grown in tezontle and under greenhouse conditions.
| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (%) | |||||
| Control (without microorganisms) | 2.99 bz | 0.44b | 2.53 c | 2.02 a | 1.06 bc |
| Rizhophagus intraradices | 3.49 a | 0.47 ab | 2.71 bc | 1.93 a | 1.28 a |
| Funneliformis mosseae | 3.22 ab | 0.47 ab | 2.95 ab | 2.02 a | 1.25 a |
| Cerro del Metate consortium | 3.12 ab | 0.44 b | 3.04 a | 2.10 a | 1.07 bc |
| Azospirillum brasilense | 3.46 a | 0.49 a | 2.86 ab | 1.26 b | 1.20 ab |
| R. intraradices + A. brasilense | 3.44 a | 0.46 b | 2.93 ab | 1.79 a | 1.01 bc |
| CV (%) | 4.67 | 2.69 | 3.19 | 8.66 | 4.41 |
| LSD | 0.42 | 0.03 | 0.24 | 0.47 | 0.14 |
CV = coefficient of variation; LSD = least significant difference. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
Mycorrhizal colonization and number of AMF spores
Figure 2A shows a longitudinal root section of the control, where no AMF structure was observed. In the colonization of mycorrhizal fungi R. intraradices (Figure 2B), F. mosseae (Figure 2C) and the Cerro del Metate consortium (Figure 2D) on basil roots, the presence of some characteristic AMF structures, such as intraradical hyphae, vesicles and arbuscules, was observed (Figures 2E, 2F and 2G). The functionality of mycorrhiza on plant nutrition is associated with the degree of maturity of the mycorrhiza and with the conditions in which it develops (Lara-Pérez et al., 2014).

Figure 2 Longitudinal sections of 'Sweet Nufar' basil roots colonized with arbuscular mycorrhizal fungi (AMF): A) control (cv = vascular cylinder), B) Rhizopagus intraradices, C) Funneliformis mosseae, D) Cerro del Metate AMF consortium, E) intraradical hyphae (hi), F) vesicles (v), and G) arbuscules (a) and base of the arbuscule (ba).
AMF inocula colonized plant roots, as the percentage of mycorrhizal colonization by hyphae was the highest (35.9 - 37.7 %), followed by arbuscules (13.0 - 14.8 %); however, there were no significant differences (P ≤ 0.05) between them (Figure 3). Regarding vesicles, the range was 4 - 8 %, and differences (P ≤ 0.05) were found between R. intraradices and the rest of the AMF. These results differ with those reported by Elhindi, El-Din, and Elgorban (2017), who found a PMC between 60.6 and 71.1 % in 'Nano Compatt' basil, while Zulueta et al. (2016) reported a PMC between 75 and 87 % in 'Nufar' basil. These differences can be attributed to the growing conditions, the use of different substrates, the inoculated AMF and the exposure time of the plants to the inoculum, since in those studies the duration of the experiments was 70 and 120 days, respectively.
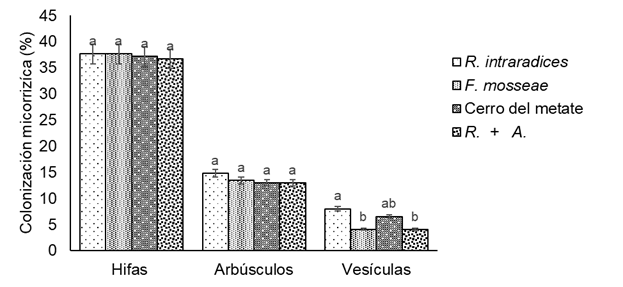
Figure 3 Mycorrhizal colonization on 'Sweet Nufar' basil roots inoculated with arbuscular mycorrhizal fungi (AMF) 50 days after transplanting. Each bar represents the average of six replicates ± standard error. R. + A.: Rizhophagus intraradices + Azospirillum brasilense. Bars with the same letter between each response variable do not differ statistically (Tukey, P ≤ 0.05).
With the co-inoculation of R. intraradices + A. brasilense there was no positive effect on the PMC of AMF, which contrasts with the findings reported by Meng et al. (2015) in maize and soybean, since the interaction of AMF and growth-promoting bacteria tends to increase the population of both individuals in the rhizosphere and, consequently, increases in PMC can occur. In addition, it was confirmed that the control and the treatment with A. brasilense presented 0 % colonization, since these treatments did not contain AMF.
Another indicator to determine mycorrhizal efficiency is the number of spores present in the substrate where the plants developed. At 50 dat, no differences (P ≤ 0.05) were found in the number of spores, where values ranged from 240 to 272 spores per 100 g of substrate. These values are considered low compared to those reported by Quiñones-Aguilar, López-Pérez, Hernández-Acosta, Ferrera-Cerrato, and Rincón-Enríquez (2014), who in papaya cultivation found 1,000 to 4,500 spores after 120 days of inoculation on organic substrates.
It is important to note that AMF sporulation is a survival mechanism (Johnson, 1984), which could be influenced by the type of substrate. Corbera, Paneque, Calaña, and Morales (2008) point out that the physical and biological properties of the substrate are determining factors in the proper development of AMF, as well as the age of the plant (Agüero-Fernández et al., 2016). In the present study, tezontle was used as growth medium and, since it is an inert substrate, it could have interfered in the sporulation process; however, that same inert characteristic guaranteed that the results obtained are due to the effect of the microorganisms and their interaction with the basil plants.
Conclusions
The arbuscular mycorrhizal fungi Rizhophagus intraradices and Funneliformis mosseae, the mycorrhizal consortium Cerro del Metate and the bacterium Azospirillum brasilense improved the growth of 'Sweet Nufar' basil, which was expressed in the increase in fresh biomass weight and leaf area. This increase was associated with a higher concentration of nitrogen and potassium in the plant tissue. The microorganisms evaluated in the growth of basil grown with a reduced level of mineral nutrition can serve as a sustainable alternative in the agronomic management of this crop under greenhouse conditions.











 texto en
texto en 



