Introduction
Broccoli (Brassica oleracea var. italica) is one of the most economically important vegetables in Mexico, after green chili pepper (Capsicum annuum L.), potato (Solanum tuberosum L.), onion (Allium cepa L.), tomato (Lycopersicum esculentum Mill.) and watermelon (Citrullus lanatus). In 2020, 33,930 ha of broccoli were grown in Mexico, with a total production of 583,645 t (Secretaría de Agricultura y Desarrollo Rural - Servicio de Información Agroalimentaria y pesquera [SADER-SIAP], 2020). Currently, the leading producing state is Guanajuato, with 24,233 ha of broccoli.
As in many plant species, diseases caused by phytopathogens are a significant problem for cruciferous vegetable production around the world (Akram et al., 2020; Dillard, Cobb & Lamboy, 1998; Fraire-Cordero et al., 2010; Pattanamahakul & Strange, 1999). In Mexico and several parts of the world, one of the diseases that reduces broccoli yield and quality is head rot, which occurs when crop development coincides with rainfall greater than 10 mm (at an approximate temperature of 30 °C and relative humidity of 70 %), and the problem is aggravated if this occurs at harvest time. Losses caused by this disease are higher than 40 % (Editorial Agro Cultivos S. C de R. L. de C. V. [EAC], 2017).
This disease has been associated with different phytopathogens in different regions. In 2009, the fungus Alternaria brassicicola was reported as the causal agent of broccoli head rot in Shanghai province, China (Liu et al., 2009), while Li et al. (2009) identified the bacterium Pseudomonas fluorescens in broccoli fields with rot symptoms in Zhejiang province. In another study, Alternaria alternata was isolated and identified on broccoli florets with the same symptom in Tanggu, Tianjin district, China (Guo, Cui, Zhu, Song, & Wang, 2015). Finally, in 2018, the first report of Fusarium tricinctum as a cause of the rot in the same crop in Hubei province was published (Zhao, Li, Yan, Huang, & Zheng, 2018).
In Mexico, the work of Fraire-Cordero et al. (2010) is an important precedent for research and knowledge of broccoli head rot. This study reported the presence of Alternaria tenuissima, Alternaria alternata and Fusarium oxysporum as fungi causing this disease in the commercial broccoli varieties Marathon, Patron (Sakata Seed Corporation) and ‘Monaco’ (Syngenta-Rogers Company) in Guanajuato, specifically in the municipality of Apaseo el Grande. Despite the importance of this report, the research was restricted to a single geographic area, hence the current need to increase the study area to generate information at the regional level of the phytopathogens involved in the development of this disease in the state of Guanajuato.
Ochoa, Hernández-Montiel, Latisnere-Barragán, León-de la Luz, and Larralde-Corona (2007) point out that the study of the causal agents of diseases worldwide suggests that the problems of each region are different, due to management issues as well as soil and weather conditions. Therefore, and given that head rot can be caused by different pathogens, it is essential to identify and characterize the microorganisms associated with head rot in each region. This would allow generating information on the frequency and importance of these pathogens, in order to make a correct diagnosis of the disease, since the choice of an appropriate management method that can avoid damage and severe crop losses depends largely on this.
Based on the above, the objective of this work was to identify, morphologically and molecularly, the causal agents of broccoli head rot in the municipalities of León, San Francisco del Rincón, Valle de Santiago, Abasolo, Juventino Rosas and Dolores Hidalgo in the state of Guanajuato, Mexico.
Materials and methods
Sample collection
During July and August 2017, 64 broccoli florets of the Avenger variety (Sakata Seed Corporation), at harvest stage with rot symptoms, were collected in the municipalities of León (16 florets), San Francisco del Rincón (10 florets), Valle de Santiago (12 florets), Abasolo (9 florets), Juventino Rosas (9 florets) and Dolores Hidalgo (8 florets), Guanajuato, Mexico. The samples were transferred to the microbiology laboratory of the Universidad Politécnica de Pénjamo. Upon arrival, the samples were stored at 4 °C and processed within a maximum period of 24 h.
Isolation and morphological identification of the causal agent
The collected florets were washed with running water and tissue sections were cut for the bacterial flow test, which consisted of placing the pieces in a test tube containing 3 mL of sterile distilled water and shaking for 5 min (Jiménez, Contreras, & Nass, 2004).
Once the presence of bacterial flow was ruled out, isolations were performed. For this, small sections (1 cm2) were taken from each broccoli floret, disinfested with 1.5% sodium hypochlorite for 2 min, washed three times with sterile distilled water and placed on sterile blotting paper. Subsequently, the pieces were placed in Petri dishes with potato dextrose agar (PDA) medium and incubated at 28 °C for 7 days. Each fungal colony developed was isolated and purified by monosporic culture.
Three groups of fungal strains with different characteristics were isolated from broccoli samples with head rot symptoms. One group presented morphological characteristics similar to Alternaria alternata (AA) (Chen & Zhong, 2017), while the other two were identified as Fusarium oxysporum (FO) (Leslie & Summerell, 2006) and Fusarium verticillioides (FV) (Morales-Rodríguez, Yáñez-Morales, Silva-Rojas, García-de los Santos, & Guzmán-de Peña, 2007). Two representative isolates were selected from each group to perform the pathogenicity tests.
Pathogenicity tests
Healthy Avenger variety broccoli plants (Sakata Seed Corporation) produced under greenhouse conditions (28 ± 2 °C and 70 % relative humidity) were used. For this, broccoli seeds were sown in plastic pots containing a substrate mixture of peat moss, perlite and vermiculite, at a ratio of 70, 20 and 10 % (v/v), respectively. The plants were constantly irrigated with distilled water and fertilized twice a week (100 mg·L-1 N, 44 mg·L-1 P and 83 mg·L-1 K), until floret development, at which time the isolates were inoculated.
Inoculation was carried out with selected isolates of AA (León and San Francisco del Rincón), FO (Juventino Rosas and Dolores Hidalgo) and FV (Juventino Rosas and Valle de Santiago), grown in calcium carbonate culture medium for AA, and PDA medium for FO and FV. After 15 days of incubation at 28 °C, conidial suspensions were prepared in sterile distilled water at a concentration of 1 x 106 conidia·mL-1. For inoculation, a wound was made on the surface of each floret (dome) with a sterile hypodermic needle, and 100 µL of each conidial suspension was placed on each wound.
Ten plants per inoculated isolate and 10 control plants (which were wounded similarly to those described above, but inoculated with sterile distilled water) were used in a completely randomized arrangement. All plants were covered with transparent polyethylene bags to ensure high relative humidity (close to 100 %) and facilitate the infection process. At 72 h, the bags were removed and the plants were kept in the greenhouse under the conditions described above (28 ± 2 °C and 70 % relative humidity) until symptom development. The trial was conducted in triplicate. At the end of each experiment (25 days post-inoculation), fungi were re-isolated from infected florets to complete Koch's postulates.
DNA extraction
From purified isolates, DNA extraction was performed using the 3 % CTAB protocol (Zhang, Uyemoto, & Kirkpatrick, 1998), with some modifications. A piece of mycelium was transferred to a 1.5 mL tube and macerated with 800 µL of CTAB extraction buffer (3 % hexadecyltrimethylammonium bromide, 1.4 M NaCl, 20 mM EDTA, 100 mM Tris-HCl, pH of 8 and 0.2 % mercapto-ethanol) preheated to 60 °C; the mixture was then incubated at 60 °C for 30 min. Samples were extracted with chloroform-isoamyl alcohol (24:1), 15 µL of RNAsa A (1 µg·µL-1) was added and incubated at 37 °C for 10 min. The aqueous DNA layer was precipitated with 600 µL of isopropanol at -20 °C. The DNA pellet was washed with 70% ethanol and dried at room temperature.
The amount and purity of the DNA samples were evaluated using a NanoDrop spectrophotometer (ND-1000-UV-Vis, NanoDrop Technologies, USA). Finally, the DNA obtained was stored at -20 °C for later analysis.
Molecular identification of the causal agent
For the amplification of the internal transcribed spacer (ITS) regions of the DNA of the isolates obtained, the polymerase chain reaction (PCR) technique was used with the universal primers ITS1 (5’TCCGTAGGTGAACCTGCGG3’) and ITS4 (5’ TCCTCCGCTTATTGATATGC3’), which amplify a region of 500 to 750 bp (White, Bruns, Lee, & Taylor, 1990).
PCR was performed in 0.2 mL eppendorf tubes, with a total reaction volume of 25 µL containing 1 µL of DNA, 1X of buffer, 2 mM of MgCl2, 0.2 µM of each primer, 0.2 mM of each triphosphate nucleotide (dNTP) and 1 unit of Taq polymerase (Invitrogen Life Technologie, Brazil). The reaction was carried out in an automatic thermal cycler (C1000 Thermal Cycler, BioRad, USA), with a denaturation cycle at 95 °C for 4 min, 30 successive three-step PCR cycles (denaturation at 95 °C for 1 min, annealing at 60 °C for 1 min and elongation at 72 °C for 2 min) and a final extension cycle at 72 °C for 5 min.
PCR products were analyzed by electrophoresis in 1 % agarose gels, stained with ethidium bromide (2 mg·µL-1), and visualized in a UV transilluminator (Gel Doc XR+ Gel Documentation System, Bio-Rad, USA). Finally, PCR products were purified with the E.Z.N.A® Cycle Pure Kit (P692-02, Omega, USA), following the manufacturer's specifications, and sequenced (ABI PRISM 377, Applied Biosystems, USA). The sequences obtained were compared against all those reported in the NCI gene bank database (National Center for Biotechnology Information, www.ncbi.nih.gov) using the BLAST program.
Phylogenetic analysis
The Clustal W method (Thompson, Higgins, & Gibson, 1994) was used to align the ITS sequences of the Fusarium isolates, together with 27 representative sequences of different species of that genus reported in the NCBI gene bank. Alternaria alternata ITS sequences were also aligned with other Alternaria species in the gene bank. Phylogenetic trees were built by the maximum likelihood method using MEGA version 11 software (Tamura, Stecher, & Kumar, 2021). Node stability was estimated by bootstrap analysis of 1,000 replicates.
Results and discussion
Isolation and morphological identification of the causal agent
Of the 64 broccoli florets with rot symptoms collected in the field (Figure 1), 185 fungal isolates were obtained: 102 were identified as Alternaria alternata and 83 showed characteristics of the genus Fusarium (35 were identified as Fusarium oxysporum and 48 as Fusarium verticillioides).
The fungus A. alternata was isolated with a frequency of 55 % and presented the following morphological characteristics on PDA medium: brown or dark olive green mycelium (Figure 2A), and obclavate, muriform, elliptical or ovoid conidia in chains (from 1 to 5 transverse and 0 to 3 longitudinal septa, 13.22 to 50.30 µm long and 8.7 to 13.3 µm wide) (Figure 2B and 2C) (Chen & Zhong, 2017).
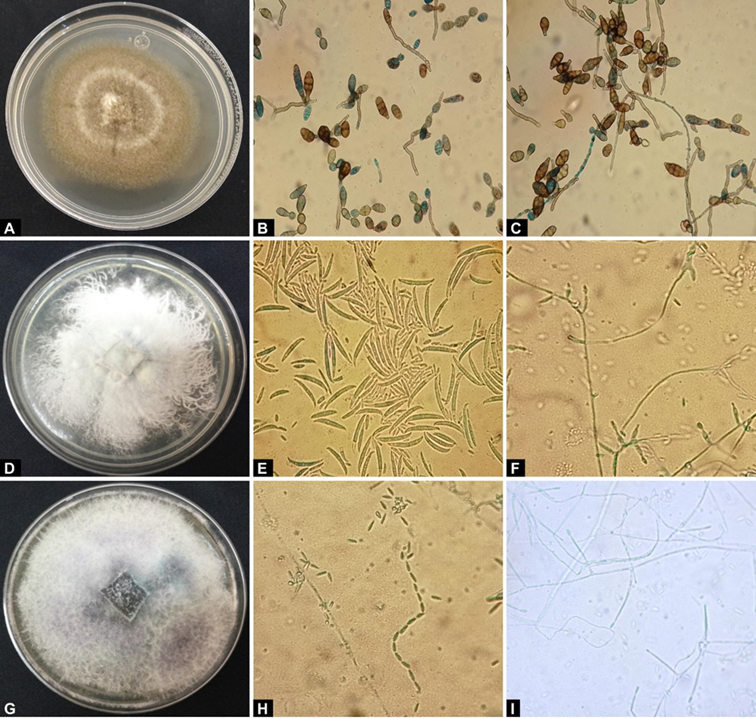
Figure 2 Macroscopic and microscopic analysis of fungal isolates causing broccoli head rot: A) mycelial growth of A. alternata (isolate L1-3F-A4) on PDA medium, B) and C) typical morphological characteristics of A. alternata, D) mycelial growth of F. oxysporum (isolate L1-8F-A1) on PDA medium, E) and F) typical morphological characteristics of F. oxysporum, G) mycelial growth of F. verticillioides (isolate L7-5F-A3) on PDA medium, and H) and I) typical morphological characteristics of F. verticillioides.
For fungi of the genus Fusarium, F. oxysporum was isolated with a frequency of 19 % and presented the following morphological characteristics on PDA medium: pink mycelium (Figure 2D), elongated, curved macroconidia with pointed ends (with three to five cells of 28.76 to 40.5 µm long and 3.9 to 4.2 µm wide) (Figure 2E), oval-shaped microconidia (5.2 to 7.5 µm long and 1.81 to 2.2 µm wide) and short phialides (Figure 2F). According to these cultural and morphological characteristics, the isolates were identified as Fusarium oxysporum (Leslie & Summerell, 2006).
In the case of F. verticillioides, it was isolated with a frequency of 26 % and presented the following characteristics on PDA medium: white mycelium with a purple hue in the center of the dish (Figure 2G), few macroconidia (when present they were long, slightly straight, and with a curved apical cell and another foot-shaped basal cell with three to five septa), abundant microconidia (in long, oval chains with a flattened base) (Figure 2H and 2I) and absent chlamydospores (Morales-Rodríguez et al., 2007).
Based on these results, it can be inferred that the main phytopathogenic agent causing head rot in broccoli crops in the state of Guanajuato is A. alternata, with an isolation frequency of more than half (55 %) of the samples analyzed in this study. In this sense, it is important to note that the presence of bacteria was not detected in any of the samples analyzed by means of the bacterial flow test.
Pathogenicity tests
Broccoli florets inoculated with conidial suspensions of isolates AA, FO and FV showed rotting symptoms (Figure 3A, 3B and 3C). The development of small dark lesions started 10 days after inoculation, and these increased in size until they covered the entire floret 20 to 25 days post-inoculation. Control florets did not develop disease symptoms (Figure D3).
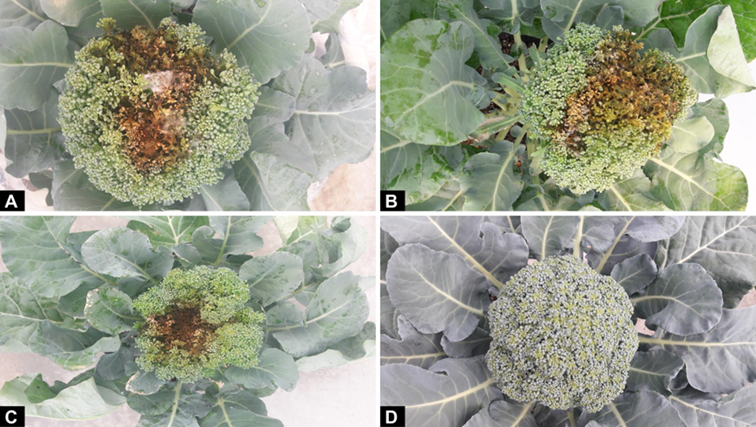
Figure 3 Symptoms induced by inoculation of phytopathogenic fungi in broccoli florets: A) broccoli head rot induced by A. alternata (isolate L1-3F-A4), B) broccoli head rot induced by F. oxysporum (isolate L1-8F-A1), C) broccoli head rot induced by F. verticillioides (isolate L7-5F-A3) and D) control plant.
To comply with Koch's postulates, isolates were made from symptomatic tissue; AA, FO and FV were re-isolated from inoculated tissue, thus confirming their pathogenicity and their importance as causal agents of broccoli head rot in the municipalities of Guanajuato evaluated in the present study.
Alternaria alternata is mainly a saprophytic fungus common in soil and decaying plant tissue (Thoma, 2003). However, it has been reported as a pathogen of different agronomically important crops around the world, such as potato in Israel (Droby, Dinoor, Prusky, & Barkai-Golan, 1984), tomato in California (Morris, Connolly, & Clair, 2000), peach in Japan (Inoue & Nasu, 2000) and citruses in China (Huang et al., 2015), in which it induces symptoms such as leaf spots, fruit lesions, dark sunken spots on fruit, blight on branches, and fruit and young leaf drop, among other symptoms. In China, A. alternata has been reported as one of the main pathogens of broccoli, leading to chlorosis and rotting, which causes increased senescence and a shorter shelf life of the product (Guo et al., 2015). In addition to the floret, this fungus can severely affect broccoli foliage, causing circular leaf spots with concentric rings surrounded by a yellow halo, which causes significant economic losses of this vegetable (Nira et al., 2022).
In Mexico, A. alternata and F. oxysporum have been reported as causal agents of rotting or spotting of broccoli (Fraire-Cordero et al., 2010), specifically in the municipality of Apaseo el Grande, Guanajuato. This report also highlights the presence of Alternaria tenuissima as the causal agent of the disease, which contrasts with the results of the present study, since Fusarium verticillioides was detected for the first time as part of the complex of causal agents of the disease and not the fungus Alternaria tenuissima. Fraire-Cordero et al. (2010) reported that the isolation frequency of the genus Alternaria ranged between 70 and 80 %, and of the genus Fusarium between 20 and 30 %. This differs from our research, where a lower isolation frequency (55 %) was found for AA and a higher frequency (45 %) for the genus Fusarium (FO and FV). This may be due to the fact that in the present study the sampling covered a wider region of broccoli-producing areas in the state of Guanajuato (León, San Francisco del Rincón, Valle de Santiago, Abasolo, Juventino Rosas and Dolores Hidalgo), compared to only one municipality (Apaseo el Grande).
The identification of Fusarium oxysporum and F. verticillioides, as causal agents of broccoli head rot, represents an important step in the search for strategies that can minimize the impact on production. In Brassica vegetables, Fusarium wilt (Fusarium oxysporum f. sp. conglutinans) is one of the most important and devastating diseases in cabbage, cauliflower and broccoli around the world (Mehraj et al., 2020). In these crops, Fusarium oxysporum f. sp. conglutinans causes leaf yellowing, wilting, defoliation, and eventually death of infected plants (Liu, Fang, Yang, Zhang, & Lv, 2018; Mehraj et al., 2020). In contrast, Fraire-Cordero et al. (2010) reported Fusarium oxysporum as a causal agent of floret spotting and rot symptoms in broccoli in Guanajuato, Mexico, which coincides with the results of our study, since F. oxysporum is one more member of the fungal complex associated with head rot in our country, so this would be the second report of the presence of this pathogen as a causal agent of the disease.
Fusarium verticillioides has been identified mainly as a pathogen in corn, where it produces toxins in the tissue and grain that harm the health of animals and humans (Duncan & Howard, 2010; Oren, Ezrati, Cohen, & Sharon, 2003). However, this fungus has also been reported in other crops. Akram et al. (2020) made the first report of this pathogen on Chinese cabbage (Brassica rapa L. parachinensis), where it produces rotting symptoms on leaves and flowers. Additionally, in the present study, pathogenicity tests with Fusarium verticillioides, isolated from florets with rot symptoms, confirmed that this fungus is part of the complex of causal agents of this disease, this being the first report in Mexico and the world.
Molecular identification of the causal agent
PCR amplification of DNA extracted from the isolates yielded amplicons of approximately 546 and 573 bp from the ITS region of Fusarium spp. and Alternaria alternata, respectively (data not shown). A portion of the purified PCR products were directly sequenced in both directions with the primers ITS1 and ITS4. DNA sequences obtained from representative isolates were deposited in the NCBI gene bank and the following accession numbers were obtained: OL721612, OL721613 and OL721614, for isolates L7-5F-A3, L1-8F-A1 and L1-3F-A4, respectively.
BLAST analysis revealed that the sequence of isolate L7-5F-A3 shares the highest similarity percentages (98.9 to 98.72 %) with Fusarium verticillioides strains isolated in India (accession numbers MN652651.1, MN652650.1, MN652649.1, MN652645.1, MN652644.1 and MN652643.1), Canada (accession number MG515226.1), China (accession numbers MW928602.1 and MW386816.1), Jordan (accession numbers MN335235.1, MN335234.1, MN335233.1, MN335232.1 and MN335231.1) and Nigeria (accession number MT742824.1).
Isolate L1-8F-A1 showed 99.63 % identity with Fusarium oxysporum isolates identified in Kenya, China, India and Nigeria (accession numbers MT420633.1, MH707084.1, KU671046.1 and MW260086.1, respectively). Finally, the sequence of isolate L1-3F-A4 showed 98.26 % similarity with Alternaria alternata strains identified in Pakistan (accession number MN548780.1) and China (accession numbers MH892844.1 and KY397985.1). The above confirms the results found with morphological identification.
Other phytopathogens have been reported in different regions of the world as causing rot symptoms in broccoli, such as the bacteria Peudomonas fluorescens (Li et al., 2009), Alternaria brassicicola (Liu et al., 2009), Alternaria alternata (Fraire-Cordero et al., 2010; Guo et al., 2015), Alternaria tenuissima, Fusarium oxysporum (Fraire-Cordero et al., 2010) and Fusarium tricinctum (Zhao et al., 2018). The present investigation confirms the presence of Alternaria alternata and Fusarium oxysporum as causal agents of broccoli head rot, and reports for the first time Fusarium verticillioides as a causal agent of the disease in crop fields in Guanajuato. This result reinforces the need for accurate identification and characterization of phytopathogens associated with diseases in each region for the development of more efficient management strategies.
Phylogenetic analysis
The sequences of the ITS regions of Fusarium isolates associated with rotting symptoms were compared with 27 representative sequences of different species of that genus reported in the NCBI gene bank (Figure 4A). Alternaria alternata ITS sequences were also aligned with other Alternaria species in the gene bank (Figure 4B), and in both cases Penicillium spp. was used as an outgroup. The analysis indicated that isolates L1-8F-A1, L7-5F-A3 and L1-3F-A4 were grouped in the same phylogenetic branch as strains belonging to Fusarium oxysporum, Fusarium verticillioides and Alternaria alternata, respectively, which is consistent with the results found in the morphological and molecular identification.
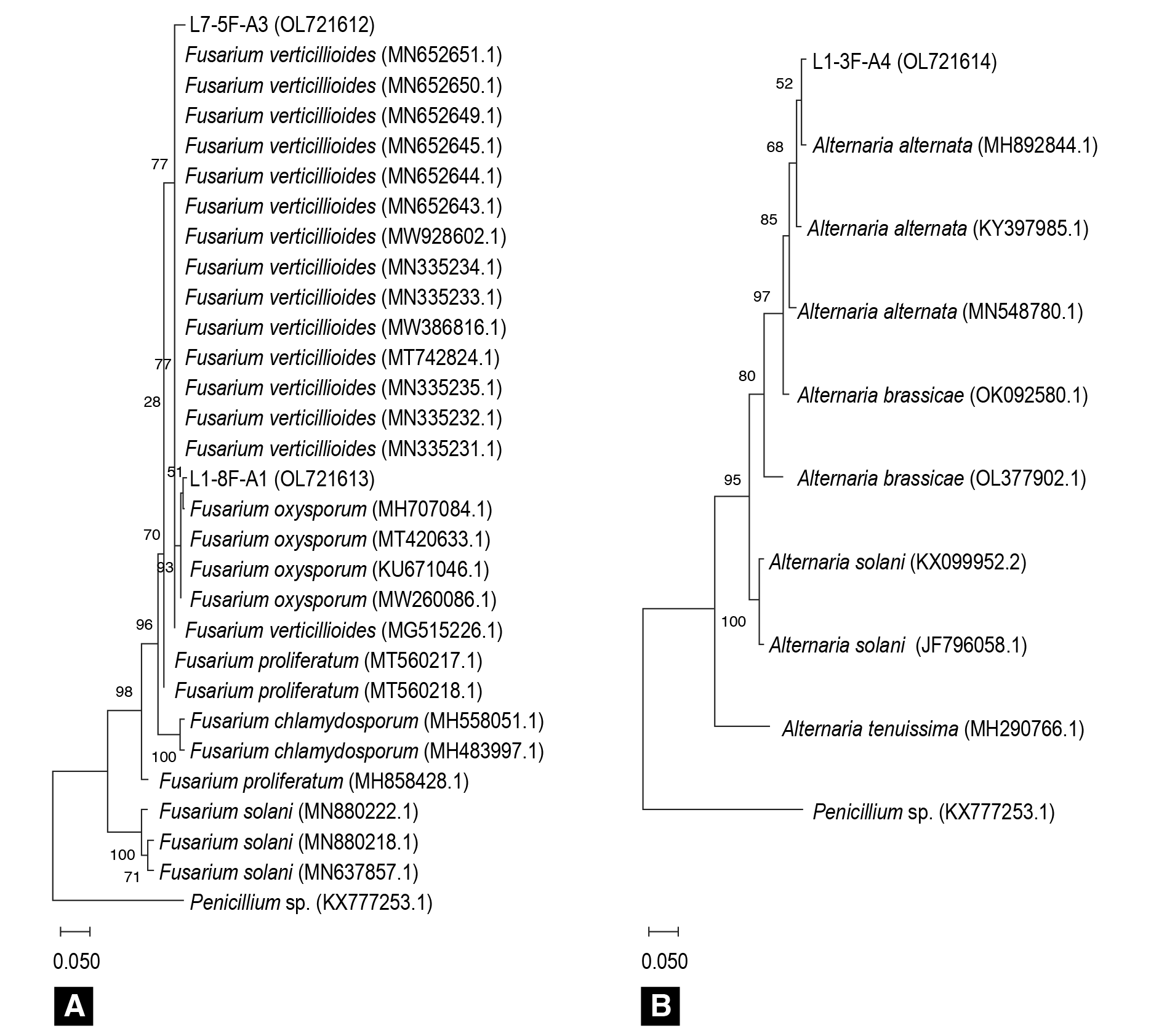
Figure 4 Maximum likelihood phylogenetic trees based on ITS sequences showing the phylogenetic relationships of isolates L1-8F-A1, L7-5F-A3 and L1-3F-A4 with other Fusarium (A) and Alternaria (B) species. Penicillium spp. (KY777253.1) was included as an outgroup. Bootstrap values are indicated at the nodes. Sequence accession numbers are shown in parentheses.
Conclusion
Based on cultural, morphological and molecular characteristics, Alternaria alternata, Fusarium oxysporum and Fusarium verticillioides were identified as causal agents of broccoli head rot in the state of Guanajuato, Mexico. This is the second report of Alternaria alternata and Fusarium oxysporum as causal agents of head rot in broccoli grown in Mexico, and the first report to identify Fusarium verticillioides as part of the complex of causal agents of this disease.











 texto en
texto en 




