Introduction
Worldwide, Mexico is one of the countries with the greatest diversity of flora with 22,000 plant species, of which more than 4,000 have characteristics for ornamental, food or both uses (Mejía-Muñoz et al., 2020). A particular case is the genus Dahlia (Asteraceae: Coreopsideae), considered the national flower of Mexico, consisting of 38 described species, with 90 % being endemic (Reyes-Santiago, Islas-Luna, Macías-Flores, & Castro-Castro, 2018). This genus is well known for its great diversity of petal colors, shapes and sizes, whose use is limited to that of a cut or pot flower, or as a structural element in garden design (Granados-Balbuena et al., 2022). Currently, it is one of the most appreciated ornamental plants, and it is estimated that there are around 50,000 varieties, product of the genetic improvement of D. coccinea, D. pinnata, D. merckii and D. imperialis, which dates back to the beginning of the 18th century in France, Italy, Germany and England (Jiménez, 2015; Deguchi, Tatsuzawa, Hosokawa, Doi, & Ohno, 2016).
In the last decade, there has been increased interest in characterizing, revaluing and incorporating flowers and inflorescences from rose (Rosa hybrida L.), sunflower (Helianthus annuus L.), cempasúchil (Tagetes erecta L.), flame coral tree (Erythrina coralloides DC.), nopal (Opuntia ficus-indica), sávila (Aloe vera L.), maguey pulquero (Agave salmiana Gentry), and dahlia (Dahlia sp.), among others (Lara-Cortés et al., 2014; Rivera-Espejel et al., 2019b; Pensamiento-Niño et al., 2021). These flowers and inflorescences have a number of bioactive compounds, and their intake seeks to promote the care and prevention of the occurrence of various cardiovascular and neurodegenerative diseases and some types of cancer (Deguchi et al., 2016; Avendaño-Arrazate et al., 2021; Martínez-Damián, Mejía-Muñoz, Colinas-León, Hernández-Epigmenio, & Cruz-Álvarez, 2021).
Paleobotanical and historical evidence suggests that the diet among populations settled in Mesoamerica, and other cultural regions of the Americas, was supplemented by the consumption of tuberous roots and inflorescences of various plants, including dahlias (Castro-Castro, Zuno-Delgadillo, Carrasco-Ortiz, Harker, & Rodríguez, 2015; Costa-Silva et al., 2020). Dahlia tuberous roots are characterized by having a high content of carbohydrates (inulin), fiber, protein, minerals (K+, Ca2+, Mg2+, P and Zn2+) and vitamins (B2, B6, B7 and E) (Arenas et al., 2011; Rivera-Espejel et al., 2019a). Also, ligulate flowers, because of their color and shape, were used to improve the appearance, texture and flavor of many traditional dishes (Rivera-Espejel et al., 2019b).
Previous studies by Lara-Cortés et al. (2014), Deguchi et al. (2016), Rivera-Espejel et al. (2019b), Martínez-Damián et al. (2021) and Granados-Balbuena et al. (2022) report the presence of carbohydrates, organic acids, phenolic compounds, anthocyanins, fiber, protein, minerals and vitamins in ligulate flowers of some wild species and varieties of dahlia. Some of the mentioned compounds are directly related to the presence of bright colors (yellow, orange, red, green, violet and variegated), and when consumed fresh or minimally processed they can be beneficial for health care, due to their biological and nutritional activity (Kaisoon, Siriamornpun, Weerapreeyakul, & Meeso, 2011; Kaisoon, Konczak, & Siriamornpun, 2012; Barriada-Bernal et al., 2014; Kumari et al., 2017; Estrada-Beltrán et al., 2021).
Phenolic compounds are the most abundant secondary metabolites in fruit and vegetable products and are related to several aspects, including color, flavor, astringency, nutritional characteristics, and antioxidant properties (Lara-Cortés et al., 2014; Frías-Moreno et al., 2019; Pires et al., 2021). In this sense, the creation of new dahlia varieties should be preceded by their phenotypic, morphological and molecular evaluation. However, it is important to generate basic information that allows knowing the content of some metabolites of interest and nutritional value, in order to diversify their use (medicinal, food or both) and contribute to the efforts made in Mexico for the conservation and utilization of this endemic flower (Castro-Castro et al., 2015; Mejía-Muñoz et al., 2020). Considering the above, the aim of this research was to determine the phenolic profile and nutritional value of ligulate flowers from some different colored D. x hortorum clones.
Materials and methods
Experiment location, plant material and crop management
The study was conducted at the Department of Plant Science, Universidad Autónoma Chapingo, Mexico. The plant material consisted of inflorescences made up of ligulate and tubular flowers (capitula) of seven dahlia (D. × hortorum) clones planted in the “San Martín” Experimental Agricultural Field (19° 29’ 23” north latitude and 98° 53’ 37” west longitude, at 2,246 m a. s. l.), with mean annual temperature and precipitation of 15.6 °C and 608 mm, respectively.
The management of the plant material began in January 2020 with the conditioning of the tuberous roots obtained in the 2019 growing season. For this, the material was placed in containers with a mixture of peat and perlite (1:1) for two weeks. After this period, cuttings 10 ± 1 cm in length were selected and rooted; for this, 200-cavity plastic trays with peat and perlite (2:1) as substrate were used. Subsequently, they were transplanted into the open field with a distance between plants and rows of 0.5 and 0.9 m, respectively. Water and nutrients were supplied by a drip irrigation system with a frequency of three times per week (1 to 3.5 L per plant), considering the phenological stage and environmental conditions prevailing during the growth and development of the crop. The application of N-P-K (120-0-200) was made prior to transplanting and flower bud formation. Weed and disease control was carried out manually and by phytosanitary pruning, respectively.
For the selection and harvesting of flower heads, the opening of stamens, stems of 15 ± 1 cm in length and the absence of defects caused by biotic (pests and diseases) and abiotic (including wind, precipitation, ambient temperature and relative humidity) factors were considered (Figure 1). The samples were transported in insulated foam containers (20 x 15 x 11 cm). These activities were carried out in the first week of August 2020 during the first hours of the day (between 6:00 and 8:00 am), in order to minimize the impact of solar radiation.
Separation, sorting and freeze-drying of ligulate flowers
Prior to physical separation of the ligulate flowers from the floral disc, they were sorted according to color using a portable sphere colorimeter (X-Rite, SP62®, USA). The color coordinates (L = lightness, C = chroma and h = hue angle) were as follows: C1Va - variegated L* 80.88, C* 15.69 and h* 20.82; C2Gui -cherry L* 33.26, C* 46.6 and h* 15.27; C3Roj - red L* 32.06, C* 50.96 and h * 26.68; C4Na - orange L* 56.73, C* 34.46 and h* 35.49; C5Ama - yellow L* 71.58, C* 36.78 and h* 46.34; C6Fuc - fuchsia L* 44.82, C* 52.89 and h * 5.81, and C7Ros - pink L* 67.2, C* 31.82 and h* 11.34.
The ligules were freeze-dried in a FreeZoneTM 18L console freeze drier (LabconcoTM, USA); for this, 500 g were placed in 600-mL lyophilized vials and kept at -80 °C in an ultra-low temperature freezer (ULT185, Thermo Fisher, USA) for 4 h. Subsequently, they were lyophilized for 72 h (until constant weight) in the equipment previously stabilized at -50 °C and 0.180 mBar.
Experimental design
The trial was established under a completely randomized experimental design with three replications, where the experimental unit consisted of two inflorescences with stems of 15 ± 1 cm in length.
Parameters evaluated
Extract preparation
The extract was prepared according to the method described by Aguiñiga-Sánchez et al. (2017), with slight modifications. Prior to extraction, the ligulate flowers were ground with a double blade blender (BLST3A-R2T, Oster® Xpert Series™ vaso Tritan™, USA), 1 g of sample was taken and 8 mL of 80 % (v:v) methanol was added. The mixture was stirred for 1 min with a digital vortex (SI-0236 Vortex-Genie 2, Thermo Scientific®, USA), homogenized for 10 min with a sonicator (505, Fisherbrand™, USA) and allowed to stand for 5 min; this process was performed twice. After this time, it was centrifuged at 7 g for 5 min (5427 R, Eppendorf®, Germany). Finally, 1 mL of the supernatant was taken for analysis by high- performance liquid chromatography (HPLC).
Phenolic profile
The content of phenolic compounds was determined in a liquid chromatograph (series 1100®, Agilent Technologies Inc, USA). The stationary phase was a 125 x 4.0 mm column (Hypersil ODS, Hewlett Packard, USA). The analysis was by gradient, with the mobile phase being (A) HPLC-grade water adjusted dropwise to pH 2.5 with trifluoroacetic acid and (B) acetonitrile. The analysis started between 0-10 min, with a ratio of 85 % A and 15 % B for 20 min, and finally 65 % A and 35 % B for 5 min. The flow rate was 1 mL·min-1 at 30 °C, the diode array detector was set at 254, 280 and 330 nm, and the injection volume was 20 μL.
Identification and quantification of phenolic compounds
The identification of phenolic acids and flavonoids was performed by verifying the retention time with respect to that of the standards (gallic acid, caffeic acid, chlorogenic acid, hesperidin and quercetin). Likewise, the absorption spectra of the standards were compared with those of the compounds identified by retention time to ensure the identity of the compounds. Quantification was performed by constructing standard curves of the reference compounds, in a concentration range of 1 to 16 µg·µL-1 obtained under the same chromatographic conditions. The results were expressed on a dry weight basis (µg·g-1).
Proximal composition
For the determination of moisture, dry matter, crude protein, crude fat and fiber, the AOC procedures (934.01, 2001.11, 954.02 and 962.09) indicated by the Association of Official Analytical Chemists (AOAC, 2016) were used. Nitrogen-free extract (NFE), also known as total carbohydrates, represents sugars and starches, and were calculated by difference. The results were expressed as a percentage.
Statistical analysis
The data obtained were normalized and homogenized with the Kolmogorov-Smirnov and Bartlett tests, respectively (Sokal & Rohlf, 1995). Multiple analysis of variance and Tukey's range test (P ≤ 0.05) were performed. In all procedures, SAS version 9.0 (SAS Institute, 2002) software was used.
Results and discussion
Phenolic profile
The data obtained from the profile of phenolic compounds in ligulate flowers of D. x hortorum are shown in Table 1 and Figure 2. These compounds are present in a wide variety of fruit and vegetable products, and are valued for their antioxidant activity and ability to eliminate or reduce free radical activity (Frías-Moreno et al., 2019; Blanda et al., 2020). In this research, phenolic acids (gallic, caffeic and chlorogenic) and flavonoids (quercetin and hesperidin) were detected. Gallic acid content ranged from 1.0 to 3.19 µg·g-1, where C7Ros, C1Var, C3Roj, C5Ama and C4Nar were the most outstanding (P ≤ 0.05). These results do not agree with those reported by Lara-Cortés et al. (2014) in purple (12.9 µg·g-1), pink (9.7 µg·g-1), orange (9.4 7 µg·g-1), yellow (6.4 µg·g-1) and white (13.7 µg g-1) ligules of some wild species of D. australis, D. appiculata, D. brevis, D. coccinea, D. campanulata and D. pinnata, respectively. However, the gallic acid values of the analyzed materials exceed those reported for edible flowers of “cacaloxóchitl” (Plumeria obtusa) with 2.7 ± 0.1 µg·g-1 (Kaisoon et al., 2011). There are few reports on gallic acid content in dahlia; however, the observed variation can be linked to genetic aspects, agronomic management and the solvent used for its extraction (Karimi, Oskoueian, Hendra, Oskoueian, & Jaafar, 2012; Terán-Erazo et al., 2019).
Table 1 Phenolic compound content of ligulate flowers of Dahlia x hortorum.
| Clone | Phenolic acids (µg·g-1) | Flavonoids (µg·g-1) | ||||
|---|---|---|---|---|---|---|
| Gallic | Caffeic | Chlorogenic | Quercetin | Hesperidin | ||
| C1Var | 2.36 abz | nd | nd | 81.46 a | 10.06 de | |
| C7Ros | 3.19 a | 0.68 b | nd | 25.13 c | 24.30 c | |
| C3Roj | 2.15 ab | 0.48 b | 4.11 c | 3.49 d | 14.34 d | |
| C5Ama | 2.34 ab | nd | 14.72 ab | 57.62 b | 34.39 b | |
| C6Fuc | 1.00 b | nd | 17.53 a | 23.73 c | 111.55 a | |
| C4Nar | 1.91 ab | nd | 7.57 bc | 22.79 c | 14.84 d | |
| C2Gui | 1.59 b | 2.29 a | 1.36 c | 27.97 c | 9.31 e | |
zMeans with the same letter within each column do not differ statistically (P ≤ 0.05, Tukey). nd = not determined. Data are expressed on a dry weight basis (µg·g-1).
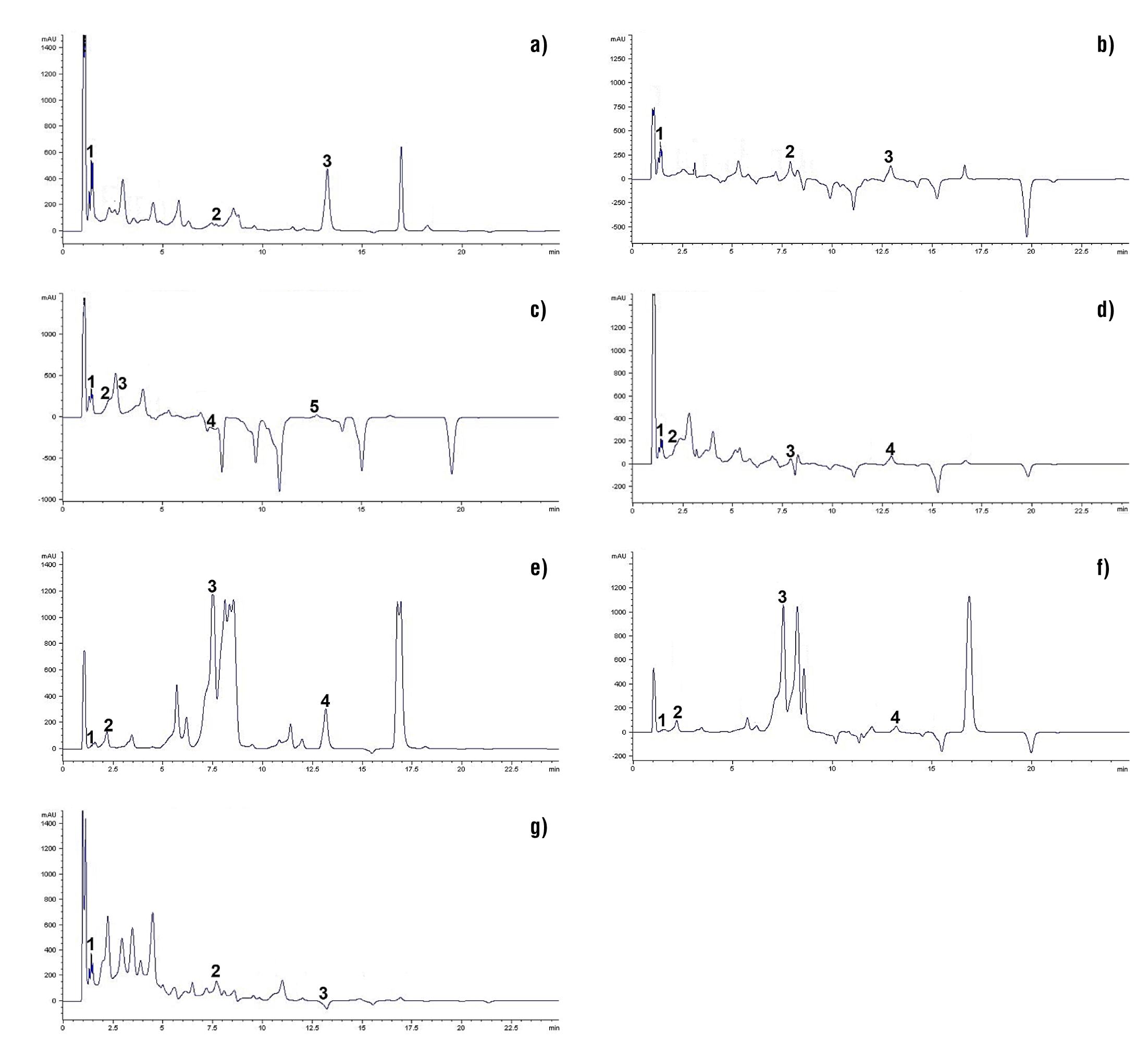
Figure 2 Phenolic profile by HPLC of D. x hortorum flowers (detection at 254 nm): a) C1Va, b) C2Gui, c) C3Roj, d) C4Na, e) C5Ama, f) C6Fuc and g) C7Ros. Retention time data are shown in Table 2.
Table 2 Identification of compounds found in D. hortorum ligulate flowers, according to their maximum retention time.
| Clone | Peak | PC | RT (min) | λmax (nm) |
|---|---|---|---|---|
| C1Va | 1 | Ga | 1.39 | 254, 280 |
| 2 | Hs | 7.67 | 254, 280 | |
| 3 | Qc | 13.23 | 254, 280 | |
| C2Gui | 1 | Ga | 1.38 | 254,280 |
| 2 | Hs | 7.89 | 254,280 | |
| 3 | Qc | 12.92 | 254 | |
| C3Roj | 1 | Ga | 1.40 | 254, 280 |
| 2 | Cha | 2.06 | 254, 330 | |
| 3 | Ca | 2.86 | 254, 330 | |
| 4 | Hs | 7.41 | 254, 280 | |
| 5 | Qc | 12.72 | 254 | |
| C4Nar | 1 | Ga | 1.39 | 254, 280 |
| 2 | Cha | 2.13 | 254, 330 | |
| 3 | Hs | 7.90 | 254, 280 | |
| 4 | Qc | 12.94 | 254 | |
| C5Ama | 1 | Ga | 1.40 | 254, 280 |
| 2 | Cha | 2.17 | 254, 330 | |
| 3 | Hs | 7.49 | 254, 280 | |
| 4 | Qc | 13.16 | 254,330 | |
| C6Fuc | 1 | Ga | 1.33 | 254, 280 |
| 2 | Cha | 2.17 | 254, 330 | |
| 3 | Hs | 7.52 | 254, 280 | |
| 4 | Qc | 13.19 | 254,330 | |
| C7Ros | 1 | Ga | 1.89 | 254, 280 |
| 2 | Hs | 7.68 | 254, 280 | |
| 3 | Qc | 13.21 | 254 |
Ga = gallic acid; Cha = chlorogenic acid; Hs = hesperidin; Ca = caffeic acid; Qc = quercetin; PC = phenolic compound; RT = retention time; λmax (nm) = maximum absorbance for compound identification by UV-Vis spectrum.
Caffeic acid content ranged from 0.48 to 2.29 µg·g-1, and was only detected in C7Ros, C3Roj and C2Gui, where the last one showed the highest content (P ≤ 0.05). Lara-Cortés et al. (2014) published contrasting data on wild dahlias with purple, cherry, pink, orange, yellow, red and white ligules (3.1, 0.9, 3.4, 1.0, 4.3, 1.4 and 4.7 µg·g-1, respectively). It is important to note that these authors report the content of this phenolic acid in yellow, pink and orange flowers. The variation between results may be due, in the first instance, to the extraction and quantification method (Blanda et al., 2020); however, genetic and adaptive variation between wild and cultivated species can be included (Castro-Castro et al., 2015; Jiménez, 2015). In this sense, wild species, being adapted to unfavorable edaphoclimatic conditions, develop multiple survival and defense mechanisms against damage caused by oxidative stress, which include the synthesis and accumulation of phenolic compounds (Kaisoon et al., 2011; Rivera-Espejel et al., 2019a; Granados-Balbuena et al., 2022). On the other hand, the presence of caffeic acid in tea plant (Camellia sinensis L.), grape (Vitis vinífera L.), Brussels sprout (Brassica oleracea var. gemmifera) and peanut (Arachis hypogaea L.) (Quiñones, Miguel, & Aleixandre, 2012).
In all dahlia materials evaluated, gallic acid content could be quantified; however, chlorogenic acid was only detected in C3Roj, C5Ama, C6Fuc, C4Nar and C2Gui (4.11, 14.72, 17.53, 7.57 and 1.36 µg·g-1, respectively). Lara-Cortés et al. (2014) report a similar content in orange flowers (16.4 µg·g-1), and without being able to detect its presence in yellow ones. This difference may be a consequence of the degree of floral opening and the solvent used for extraction (Karimi et al., 2012; Avendaño-Arrazate et al., 2021). Martínez-Damián et al. (2021) point out that any modification in the balance of the interaction between phenolic acids and anthocyanins can generate variation in their content and color expression in dahlia flowers. Additionally, the presence of chlorogenic acid is reported in Arabic coffee (Coffea arabica L.) beans and in species of the genus Prunus (plum, apricot, and peach, among others), where its consumption can improve lipid and glucose metabolism (Naveed et al., 2018).
Regarding the content of some flavonoids (quercetin and hesperidin), the maximum mean values corresponded to C1Var (81.46 µg·g-1) and C6Fuc (111.55 µg·g-1), respectively. These compounds are directly related to some characteristics of flavor, color, palatability and nutritional value in fruits, vegetables and flowers (Terán-Erazo et al., 2019; Avendaño-Arrazate et al., 2021). The results obtained exceed those reported by Lara-Cortés et al. (2014) in dahlia flowers with cherry-colored ligules (36.4 µg·g-1). However, a higher quercetin content (185.37 ± 0.11 µg·g-1) was reported by Karimi et al. (2012) in edible flowers of bitter orange (Citrus aurantium). The presence of this flavonoid has been reported in various flowers, including cempaxúchitl (Tagetes erecta L.) (13.57 mg 100·g-1), sulfur cosmos (Cosmos sulphureus Cav.) (9.45 mg 100·g-1), bougainvillea (Bougainvillea glabra Chosy) (Kaisoon et al., 2012) and Agave durangensis (0.4 g·mL-1) (Barriada-Bernal et al., 2014).
The flowers and fruits of sweet orange (Citrus sinensis (L.) Osbeck) and lemon (Citrus x limón) have the highest hesperidin content (Wilmsen, Spada, & Salvador, 2005). The values obtained for these flavonoids in this study are low compared to those reported by Lara-Cortés et al. (2014), with values of 160.8 and 398.9 µg·g-1 in dahlia with pink and purple ligulate flowers, respectively. However, clone C6Fuc exceeded the 70.9 µg·g-1 published by these researchers for white flowers.
Overall, the content of phenolic compounds detected in this study was variable; however, dahlia flowers can be considered as a complementary source of antioxidant agents.
Nutritional value
Proximal analysis data are shown in Table 3. No variation (P > 0.05) was found between the mean moisture (M) (89.53 to 91.10 %) and dry matter (DM) (8.80 to 10.47 %) values of the D. x hortorum materials. Lara-Cortés et al. (2014) obtained similar results in ligules of some wild dahlia species (D. australis, D. appiculata, D. brevis, D. coccinea, D. campanulata and D. pinnata). However, a previous study by Martínez-Damián et al. (2021), on different colored ligules in some D. x hortorum clones, shows variation in M and DM parameters. The contrast between the results can be associated with the morphological variation of the analyzed material; that is, between cultivated varieties and wild species (Mejía-Muñoz et al., 2020).
Table 3 Nutritional value of ligulate flowers of Dahlia x hortorum.
| Clone | M | DM | A | Cf | Cfa | Cp | TC |
|---|---|---|---|---|---|---|---|
| % | |||||||
| C1Var | 89.53 az | 10.47 a | 5.55 bc | 17.91 a | 7.28 a | 13.04 ab | 56.21 c |
| C7Ros | 90.30 a | 9.70 a | 5.96 a | 16. 84 b | 3.02 c | 13.68 a | 60.70 b |
| C3Roj | 90.08 a | 9.92 a | 5.56 bc | 16.65 bc | 2.68 c | 12.79 b | 62.37 ab |
| C5Ama | 91.20 a | 8.80 a | 5.76 ab | 17.15 ab | 3.03 c | 13.72 a | 59.73 b |
| C6Fuc | 89.82 a | 10.18 a | 5.56 bc | 15.75 cd | 6.95 ab | 11.76 c | 60.23 b |
| C4Nar | 90.16 a | 9.84 a | 5.16 d | 16.81 b | 4.47 bc | 11.98 c | 61.64 ab |
| C2Gui | 91.10 a | 8.90 a | 5.4 cd | 15.25 d | 2.84 c | 12.40 bc | 64.09 a |
M = moisture; DM = dry matter; A = ash; Cf = crude fiber; Cfa = crude fat; Cp = crude protein; TC = total carbohydrates. zMeans with the same letter within each column do not differ statistically (P ≤ 0.05; Tukey). Data are expressed on a dry weight basis.
In general, the ligulate flowers presented a high carbohydrate content, followed by fiber, protein, crude fat and ash. This represents a number of advantages for their fresh consumption (Granados-Balbuena et al., 2022).
An indirect way of evaluating mineral nutrient content is through the percentage of ash. In this study, only C7Ros (5.96 %) and C5Ama (5.76 %) showed statistical variation among the clones analyzed. These results are higher than those observed by Lara-Cortés et al. (2014) in some wild species (from 0.6 to 0.9 %), although these authors report no variation in this parameter. In contrast, Rivera-Espejel et al. (2019b) obtained values of 8.7 and 7.3 % in D. campanulata and D. brevis, respectively. However, ligulate flowers from D. coccinea (orange) and D. x hortorum (cherry and red) presented similar values of 5.5, 5.8 and 5.9 %, respectively (Rivera-Espejel et al., 2019b). This behavior may be the result of edaphoclimatic conditions, degree of domestication, fertilization practices and flowering stage. On the other hand, the values obtained in this work are relatively low when compared with the data obtained for this parameter by Pensamiento-Niño et al. (2021) in flowers of Hudson pear (Cylindropuntia rosea) (11.21 ± 0.04 %), xoconostle ulapa (Opuntia oligacantha var Ulapa) (17.73 ± 0.10 %) and pitaya (Echinocereus cinerascens) (16.83 ± 0.35 %).
Flowers from C1Var and C5Ama showed an outstanding performance (P ≤ 0.05) with respect to crude fiber content, and C1Var, together with C6Fuc, was also higher in crude fat. In this sense, a high fiber content was found when comparing the data obtained with those reported in some flowers from wild and cultivated dahlia species (Rivera-Espejel et al., 2019b) and from some cacti (Pensamiento-Niño et al., 2021). De Lima-Franzen, Rodrigues-de Oliveira, Lidório, Farias-Menegaes, and Martins-Fries (2019) report similar fiber values in sunflower (Helianthus annuus L.), pot marigold (Calendula officinalis L.) and rose (Rosa x grandiflora Hort.) flowers, although they report 12.5 % crude fat for pot marigold.
The obtained protein data were similar to those reported by Rivera-Espejel et al. (2019b) for some wild dahlia species (D. brevis, D. coccinea and D. campanulata). However, they did not exceed the range of 14.84 to 19.25 % published by Martínez-Damián et al. (2021) for D. x hortorum with cherry, yellow, variegated, pink and purple ligulate flowers. In other species with edible flowers such as cornflower (Centaurea cyanus L.) and pot marigold (Calendula officinalis), Pires, Dias, Barros, and Ferreira (2017) obtained lower protein values (5.79 and 6.43 %, respectively), and 5.93 % in dwarf dahlias (D. mignon).
Clones C2Gui, C4Nar and C3Roj were outstanding for their high total carbohydrate (TC) content. These results are similar to those reported for flowers of the wild dahlia species D. merckii (Rivera-Espejel et al., 2019b) and some cacti (Pensamiento-Niño et al., 2021). However, the results obtained are not higher than those indicated for dahlia species such as D. brevis, D. coccinea and D. campanulata, with values of 65.08, 68.64 and 69.88 %, respectively (Rivera-Espejel et al., 2019b).
According to the results obtained, dahlia could be considered as a complementary source of crude fiber and protein in the diet, similar to that reported for other edible flowers (Granados-Balbuena et al., 2022). In this sense, existing studies have shown that edible flowers, including dahlia, can complement the list of functional foods, since they are characterized by their low lipid content and variable carbohydrate and mineral content. In addition, it allows diversifying their use and favoring their conservation, due to the risk posed by the alteration of their native habitat by anthropogenic activities.
Conclusions
Five phenolic compounds were identified, including phenolic acids (gallic, caffeic and chlorogenic acids) and flavonoids (quercetin and hesperidin). Among the clones evaluated, C7Ros, C2Gu and C6Fuc were the most outstanding with respect to gallic acid, caffeic acid and chlorogenic acid content, respectively. Likewise, quercetin and hesperidin content was higher for C1Var and C6Fuc. Proximal analysis revealed that C1Var presents ligules with high fiber, fat and protein content; however, in this last parameter it was similar to C7Ros (13.68 %) and C5Ama (13.72 %). The highest total carbohydrate content was found in C2Gui, C4Nar and C3Roj. Dahlia flowers have a significant content of phenolic compounds, so they can be considered as a functional food for fresh consumption.











 texto en
texto en 




