Introduction
The nutritional imbalance that occurs in insects, due to a diet based solely on sap, has resulted in a mutualistic association with prokaryotic or endosymbiont organisms found within the insect in specialized cells called bacteriocytes (Baumann et al., 1995; Buchner, 1965; Dixon, 1998). Endosymbionts have been shown to be involved in metabolic processes such as food synthesis and digestion, as well as in the recycling of nitrogenous waste (Subandiyah, Nikoh, Tsuyumu, Somowiyarjo, & Fukatsu, 2000; Su, Zhou, & Zhang, 2013). However, some of these bacteria negatively interfere with the metabolic functions of their host or activate the plant’s defense mechanism during the feeding process (Chaudhary, Atamian, Shen, Briggs, & Kaloshian, 2014; Feldhaar & Gross, 2009).
Endosymbionts are classified, according to their function, into two groups: primary and secondary. The primary ones have an obligatory-mutualistic relationship with the host insect and are transmitted vertically from mother to offspring, while the secondary ones may or may not be necessary for the insect's survival; their transmission is horizontal, but once inside the insect it becomes vertical (Eleftherianos, Atri, Accetta, & Castillo, 2013; Su et al., 2013).
The presence of a great diversity of endosymbionts associated with Diaphorina citri, mainly Candidatus Carsonella ruddii, C. Profftella armatura, C. Wolbachia spp. and C. Liberibacter spp., has been found (Gill, Chu, & Pelz-Stelinski, 2016; Hussain et al., 2017; Subandiyah et al., 2000). The Asian citrus psyllid, D. citri Kuwayama (Liviidae), is considered the most dangerous pest of this crop because it transmits C. Liberibacter spp., the causal pathogen of Huanglongbing (HLB) (Hall, Richardson, Ammar, & Halbert, 2013). D. citri was first described in 1907 with the name of Euphalarus citri (Halbert & Manjunath, 2004). In India, the transmission efficiency of C. Liberibacter asiaticus (CLas) by D. citri was determined by observing the first symptoms in plants between 25 and 40 days after exposure to psyllids that previously fed on infected plants. These insects also carried the citrus tristeza virus (CTV); however, they were unable to transmit it, which demonstrated their close relationship with the bacterium (Capoor, Rao, & Viswanath, 1967).
In Mexico, HLB is associated with CLas and mainly affects sour citrus fruits such as Mexican lime (Citrus aurantifolia [Christm] Swingle) (Esquivel-Chávez et al., 2012), contrary to reports from Brazil and the United States, where this disease has a greater impact on sweet citrus fruits (Bové, 2006; Gottwald, da Graça, & Bassaneziu, 2007). Mexico’s Pacific region is considered to be at high risk of suffering an HLB epidemic and its consequent commercial impact due to the occurrence and intensity of the disease (Mora-Aguilera et al., 2014; Salcedo et al., 2010).
Endosymbionts, as well as their role in the insect vector and in the transmission of CLas, belong to one of the least studied lines of research today. Therefore, the aim of this review was to explore the importance and function of endosymbionts associated with D. citri, and their role in the transmission of Candidatus Liberibacter asiaticus.
Methodology
The information in this paper was obtained from the following databases, search engines, journals and full-text books: ACSESS, Agris, Agricola, American Association for the Advancement of Science, Annual Reviews Sciences Collection, Association for Computing Machinery Base, BioOne, Cold Spring Harbor Laboratory, CORE, CRC net BASE, CRC Press, Crossref, Dimensions, EBSCO, ELSEVIER, Europe PubMed Central, Global NDLTD, Google Scholar, Harvard Dataverse, JSTOR, Microsoft Academic, Nature, Oxford Academic, PNAS, PROQUEST, PubMed, Redalyc, Scholexplorer, SciELO, Science, ScienceOpen, Scopus, SpringerLink, SpringerNature, Taylor and Francis, Web of Science Group, Wiley-Blackwell, Wisdom and Zenodo.
Transmission of Candidatus Liberibacter asiaticus by D. citri
The damage caused by D. citri adults and nymphs feeding on citrus trees consists of a distortion in shoots and an alteration in the growth of young trees. However, its main importance lies in its function as a transmitter of CLas (Ortega-Arenas, Villegas-Monter, Ramírez-Reyes, & Mendoza-García, 2013). The insect acquires the bacterium between 5 and 7 hours after feeding on diseased plants, with a transmission efficiency of 40 % (Ammar, Ramos, Hall, Dawson, & Shatters, 2016). D. citri adults have an 8-h probing period in 40 % of CLas-infected plants, while in healthy plants it is shorter (Luo et al., 2015). This behavior can be attributed to the histological change that the leaves undergo due to infections caused by CLas, since it causes a thickening of the leaf cuticle and the accumulation of starch, which could explain the long period spent probing for ideal feeding sites (Cen et al., 2012a; Luo et al., 2015).
CLas establishes a persistent circulative relationship in its transmitter (Inoue et al., 2009). When adults free of the bacterium feed on CLas-positive trees, they require from 1 to 25 days to be able to transmit it to a healthy tree, while individuals from nymphs fed on positive trees are infected as soon as they emerge (Ammar et al., 2016), this because the bacterial load in nymphal stages increases considerably in a shorter period than in adults (Ammar et al., 2016; Inoue et al., 2009). Likewise, the transmission efficiency of CLas is higher in D. citri adults from infected nymphs than in insects that were exposed to Las in the adult stage. These facts suggest that the midgut and salivary glands act as a barrier to the transmission of the bacterium (Ammar et al., 2016; Cen et al., 2012a; Luo et al., 2015).
The percentage of CLas acquisition by D. citri adults fed on plants positive for this bacterium varies between geographic regions. In experiments carried out with populations of Japanese origin, 88 % of the psyllids acquired CLas after 24 h of feeding (Inoue et al., 2009), while, in Florida populations, only 35 % of adult psyllids acquired the bacteria after five weeks of exposure to infected plants (Pelz-Stelinski, Brlansky, Ebert, & Rogers, 2010).
Candidatus Carsonella ruddii
The proteobacterium Candidatus Carsonella ruddii (CCr) is a primary endosymbiont of psyllids, associated with food synthesis (Dan, Ikeda, Fujikami, & Nakabachi, 2017). Inside the carrier insect, the bacteria are lodged in tubular uninucleate bacteriocytes on the ovaries (Figure 1). Bacteriocytes increase in size during nymphal development, and since they do not possess genes for cell division, it is suggested that D. citri controls this process (Dan et al., 2017). CCr has one of the smallest genomes in nature, with 159,662 bp and 6 % GC (Riley, Kim, & Hansen, 2017). Some studies propose its transformation to a new sub-cellular entity, between living cells and organelle, since most genes for the biosynthesis of amino acids have been lost (Tamames et al., 2007).
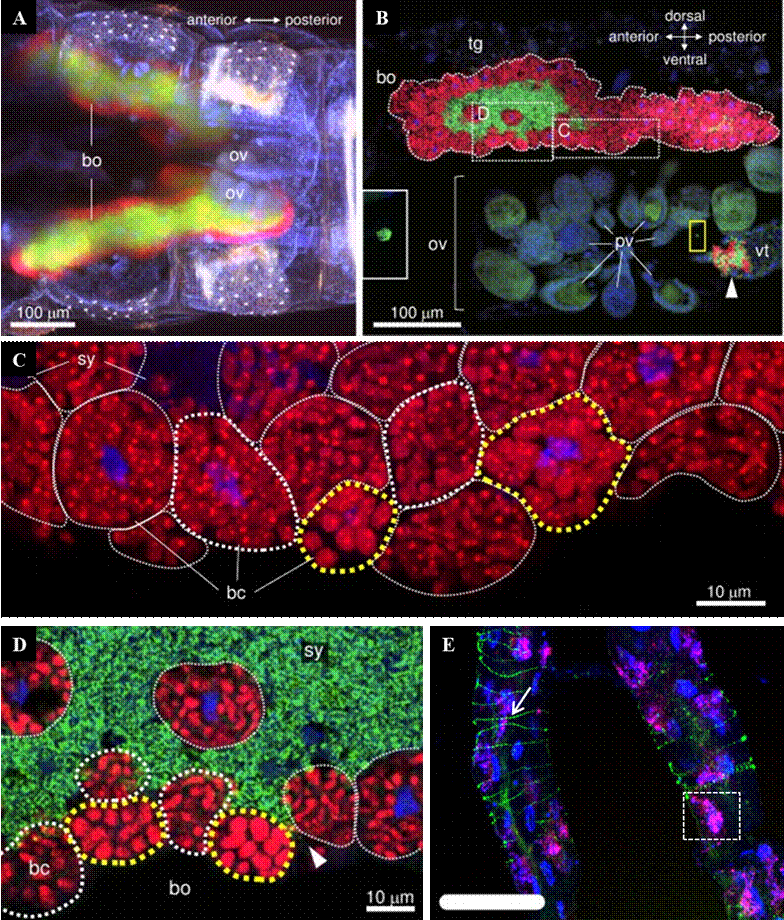
Figure 1 Localization of endosymbionts in D. citri by fluorescence in situ hybridization (FISH) A) Confocal micrograph of the abdomen of an adult female 5 days post-eclosion (ov = immature ovaries; bo = bacteriome), B) confocal micrograph of a sagittal cross-section of an adult female (C = uninucleate Carsonella bacteriocytes with red signals; D = syncytial cytoplasm harboring Profftella with green signals; bo = bacteriome; tg = D. citri cells with blue signals from the nuclei; ov = oogonium in ovary; vt = vitellogenic oocytes; pv = previtellogenic oocytes), C) enlarged section of the dotted C box of image B (bc = bacteriocytes with large cells [yellow dotted line] and thin tubular cells [white dotted line]), D) enlarged section of the dotted D box of image B (sy = syncytial cytoplasm harboring Profftella; the arrow between Carsonella bacteriocytes [red signals] indicates the syncytium on the periphery of the bacteriome), E) adult psyllid intestine of D. citri, CLas is observed in green and the arrow points to the specific oligonucleotide probe and the white dotted box shows Wolbaquia in red and pink tones. Source: A, B, C and D taken from Dan et al. (2017) and E modified from Mann et al. (2018).
Each species of psyllid is considered to correspond to a bacterial strain; for D. citri it is the DC strain (Gill et al., 2016; Katsir et al., 2018). In healthy psyllid hemolymph, the DC strain is composed of 362 unique proteins, while in CLas-infected psyllids the number decreases to 91 proteins. Of the total, 162 are unique proteins and 109 coincide with those of CCr. Most of the CCr proteins present in the D. citri hemolymph have as their main functions energy production and conversion, amino acid transport and metabolism, translation ribosomal structure and biogenesis, protein turnover, post-translational modifications and formation of chaperone proteins (Gill et al., 2016). In relation to the synthesis of chaperone proteins, it has been proven that the endosymbiont Buchenera sp. allows phytopathogenic viruses, mainly luteovirus, to move freely within the aphid Myzus persicae without being degraded and to reach the salivary glands. The interaction between virus and endosymbiont is determined by these proteins, which was demonstrated with antibiotic treatments in leutovirus-carrying aphids. The antibiotic reduced the concentration of symbionin (specific chaperone protein) and the formation of the protein capsid of the virus, which inhibited the transmission of the pathogen (Van den Heuvel et al., 1997).
Candidatus Profftella armatura
Candidatus Profftella armatura (CPa) is a widely studied primary endosymbiont in D. citri. This beta-proteobacterium is found in a syncytial cytoplasm within the insect bacteriome (Ramsey et al., 2015). In addition, it has genes related to the synthesis of pederin (polyketide toxin), which confers cytotoxic activity on D. citri, allowing it to protect itself from natural enemies (Nakabachi et al., 2013). In CLas-positive psyllids, the concentration of the polyketide toxin is higher than in non-carrier psyllids (Nakabachi et al., 2013; Ramsey et al., 2015). This compound is related to the suppression of mitosis and DNA synthesis in predators (Nakabachi et al., 2013; Wu et al., 2015).
The YCPA strain of CPa is found in D. citri hemolymph and has 263 and 116 total proteins in non-CLas-carrier and CLas-carrier psyllids, respectively, of which 156 are unique proteins in non-infected insects and 19 in infected ones, and 107 are unique proteins in prokaryotes (Gill et al., 2016; Wu et al., 2015). Among the functions that unique proteins perform in the insect are amino acid biosynthesis and degradation, carbohydrate degradation, aminoacyl-tRNA biosynthesis, carbohydrate metabolism, carotenoid biosynthesis, cofactor biosynthesis, genetic information processing, lipid metabolism, one-carbon metabolism, protein modification, pyrimidine metabolism, sulfur metabolism, and tRNA modifications (Gill et al., 2016).
Candidatus Wolbachia sp.
Candidatus Wolbachia sp. (CW) is an alpha-proteobacterium that is classified into eight supergroups with distinct evolutionary lineage. It is estimated that CW infects up to 65 % of D. citri individuals; however, it is not an arthropod-specific endosymbiont, since its infection has been reported in nematodes and mammals. The WGS strain of CW reported in D. citri corresponds to supergroup B (Ramírez-Puebla et al., 2015).
Hemolymph analyses of D. citri show an imperfect infection of CLas and CW, suggesting that they are facultative parasites (Subandiyah et al., 2000). The highest concentration of CW in infected D. citri occurs in adults; however, they exhibit high levels of cell necrosis or karyorrhexis compared to nymphs, thus inferring a close interaction between CLas and CW during the development of psyllids exposed to infected plants (Mann et al., 2018). The SC1 and SC2 genes have been described in all CLas strains reported in the world. SC1 is involved in holin synthesis, which causes the lytic cycle of the bacterium, although the proteins of CW strains obtained from D. citri are able to suppress the holin promoter, which is activated on contact with the plant, but not in the host psyllid. Such interaction is vital for the survival of these prokaryotes, which also explains why adults from positive nymphs are more efficient in transmitting CLas (Jain, Fleites, & Gabriel, 2017).
CW has been widely studied for its ability to interfere with the reproduction of its host (Guidolin & Cosoli, 2013). It has also been reported that Wolbachia species have a close relationship with whitefly and psyllids (Spaulding & Dohlen, 1998).
Candidatus Liberibacter asiaticus
HLB is an endemic disease that has been reported in Asia since 1870, and in 1956 it was associated with Candidatus Liberibacter spp. So far, three genera of this bacterium are known: C. Liberibacter americanus (CLam; reported in Brazil and Asia), C. Liberibacter africanus (CLaf; reported and distributed in Africa) and CLas (distributed in Asia, Brazil, Florida in the United States, the Caribbean and Mexico) (Bové, 2006; Santivañez, Mora-Aguilera, Díaz-Padilla, López-Arroyo, & Vernal-Hurtado, 2013).
The main form of dispersion of CLas is through its vector: D. citri (Liviidae); however, it has been observed that Trioza erytreae (triozidae) is able to transmit this bacterium. It is worth mentioning that the Asian citrus psyllid can act as a transmitter of Ca. Liberibacter africanus experimentally (Capoor et al., 1967; Lallemand, Fos, & Bové, 1986). Until a few years ago, D. citri was the only reported psyllid species capable of acquiring and transmitting CLas; however, other insects associated with this bacterium have been reported, such as Diaphorina communis (Liviidae) and Cacopsylla citrisuga (Psyllidae) in Asia, and Ferrisia virgata (Pseudococcidae) in the United States (Cen, Zhang, Xia, Guo, & Deng, 2012b; Donovan et al., 2012; Pitino et al., 2014). However, so far, there are no studies demonstrating their efficiency as vectors of the bacterium, and therefore, they are only considered as insect carriers (Cen et al., 2012b; Donovan et al., 2012; Pitino et al., 2014).
The efficacy and spread of a pathogen necessarily depends on the fitness, characteristics and interactions with its transmitter. Endosymbionts play a key role in the insect’s survival, and vice versa. In the co-evolution of CLas and D. citri, the bacterium benefits from the insect in its transmission to new host plants, in addition to ensuring its survival within the arthropod (Cen et al., 2012a; Luo et al., 2015). This alpha-proteobacteria lodges, as pleomorphic bodies, in the hemolymph, midgut, Malpighian tubules, ovaries and, with a higher concentration, in the muscle and fat tissues, the alimentary canal and the salivary glands (Ammar, Shatters, & Hall, 2011).
On the other hand, the bacterium potentiates the flight of the insect at short distances due to an increase in the lipophorin receptor and fatty acid binding proteins. In addition, females with a higher bacterial load are more attractive to males (Martini, Hoffmann, Coy, Stelinski, & Pelz-Stelinski, 2015). In Mexico, the longevity of psyllids developed in CLas-positive plants decreased, while fertility and the population growth rate benefited (Ramírez-Sánchez, Ortega-Arenas, Velázquez-Monreal, & Valdez-Carrasco, 2016). CLas-positive D. citri adults have been reported to be more susceptible to the entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae and Isaria fumosorosea. Tiwari, Pelz-Stelinski, and Stelinski (2011) observed that D. citri adults infected with CLas are more susceptible to insecticides when compared to healthy psyllids (Orduño-Cruz, Guzmán-Franco, & Rodríguez-Leyva, 2015). CLas not only modifies the biological fitness of D. citri, it also affects the interaction between its endosymbionts, which causes a reduction in protein synthesis. In the case of CW, it can decrease the number of unique proteins from 737 to 148 (Gill et al., 2016).
One of the remaining issues concerning the microbial interaction of the endosymbionts found in D. citri is the question of what controls the pre-existing dynamics between these microorganisms. Studies conducted in other insect-associated prokaryotes suggest that this activity may be regulated by chemical signaling processes, known as quorum sensing, through gene expression (Bassler, 1999). During this activity, bacteria induce changes in behavior that regulate population density through the production and release of chemical molecules called autoinducers, which increase their concentration when they detect an increase in cell density (Miller & Bassler, 2001). An example of this is the quorum sensing system of Sodalis gossinidus, an endosymbiont of the tsetse fly that has two regulatory proteins that synthesize an acylated homoserine lactone signaling molecule, which allows the bacterium to modulate gene expression in accordance with cell density (Pontes et al., 2008).
Perspectives
Endosymbionts have been extensively studied in both mammals and insects. The study of cooperative interactions between prokaryotes and vector insects allows us to understand the bases that regulate their mutual survival and reproductive success. C. Carsonella ruddi and C. Profftella armatura are considered the main endosymbionts of D. citri; however, the interaction with secondary endosymbionts, such as C. Wolbachia and C. Liberibacter asiaticus, allows the psyllid to develop greater efficacy as a vector of the bacterium. Current knowledge of the endosymbiont-psyllid relationship is largely due to molecular advances based on the study of genomes and interaction between organisms, this coupled with proteomics. From our perspective, future research efforts should include investigating the relationship of genomes between host-insect and endosymbiont-plant-host, as well as their function at the physiological level. The study of these interactions would allow us to deepen our understanding of the function of the genes involved in these mutualistic relationships and, therefore, to genetically manipulate the host or silence genes in the pursuit of an integrated management of both D. citri and CLas.
Conclusions
The key to the evolutionary success of insects is closely linked to the symbiotic association with bacteria. Endosymbionts have undergone major changes, ranging from size reduction to decreased protein synthesis, as is the case of Candidatus Carsonella ruddi and C. Profftella, primary endosymbionts of Diaphorina citri. In infected psyllids, C. Wolbachia and C. Liberibacter asiaticus seem to be complementing the functions of the primary endosymbionts, to such an extent that the presence of C. Wolbachia is essential for D. citri to be able to transmit C. Liberibacter asiaticus.











 texto em
texto em 



