Introduction
According to the U.S. Census Bureau, 19 % of the total U.S. population in 2020 is Hispanic and by the year 2060 this will reach 11.2 M people (Vespa, Medina, & Armstrong, 2020). A recent trend among this community is to reside in different areas in addition to the typical “gateway” states of Texas and California. In 2008, Hispanics were the largest ethnic minority in Massachusetts and represented 8 % of the population of the state (Mangan et al., 2008). This rapid expansion of new communities in New England has brought opportunities for commercial farmers to grow and market fresh products popular among these new and growing ethnicities.
In Massachusetts, research trials are actively conducted every year to identify crops that can be produced by local growers and sold in densely populated areas by the Latino community. This transition to the production of new specialty crops is a market opportunity for farmers to increase their profitability. One of the crops currently produced in Massachusetts is chipilín (Crotalaria longirostrata Hook. & Arn.), also called chepil, a leafy vegetable used in the traditional cuisine of Central America and Southern Mexico (Chávez-Quiñones, Roldan-Toriz, Sotelo-Ortiz, Ballinas-Díaz, & López-Zúñiga, 2009). This vegetable grows during the frost-free period with a weekly production of 800 kg·ha-1 and a wholesale value that can reach $8.83 USD per kilogram.
This article reports on one of the first attempts to commercially produce chipilín in the United States. In Massachusetts, this crop is grown under similar agronomic conditions to those described for collards (Brassica oleracea) in the New England Vegetable Management Guide (Howell, 2011). Although the initial economic investment for the establishment of the crop is moderately high, the cost is compensated by the multiple harvests and its excellent wholesale market price. In Massachusetts, it is recommended to grow chipilín under a synthetic row cover to manage the infestation of potato leafhopper (Empoasca fabae), and supply macro and micronutrients with inorganic fertilizers through drip irrigation at similar rates to those recommended for collards. As a legume crop, chipilín can naturally fix N from the atmosphere; however, 200 kg·ha-1 of inorganic N are applied to achieve the quality standards required by the market.
Food crops require N fertilizers to improve their yield and quality of crops. Currently, a large percentage of these N fertilizers are chemically synthetized through the Haber-Bosch process, a nitrogen fixation process that requires large amounts of fossil fuels (Olivares, Bedmar, & Sanjuán, 2013). The high environmental cost of synthetic N fertilization requires the identification of alternative biological sources of N. Biological nitrogen fixation (BNF) is a sustainable process that can potentially enhance the crop adaptability to environments with low N content. Overall, BNF supplies one fourth of the total amount of N fixed every year worldwide (Masson-Boivin, Giraud, Perret, & Batut, 2009) and decreases CO2 emissions emitted in the production of inorganic nitrogen fertilizers. Approximately, 80 % of total natural N fixation occurs via symbiosis between leguminous plants and alphaproteobacteria, especially Rhizobia (Olivares et al., 2013).
Nitrogen in the gas form (N2) is an unlimited resource in the atmosphere, but plants need to create a symbiotic relationship with prokaryotes to synthetize these molecules into N-rich compounds such as NH3, NO3 and NO2 (Nasholm, Kielland, & Ganeteg, 2009). The colonization by the symbiont is triggered when the flavonoids of the plant and the Nod proteins are present and develop CLOS Nod factors. CLOS Nod factors induce morphological changes in the plant that allow the bacteria to invade the stem cortex (Cooper, 2004). After intracellular colonization of the root system occurs, the specialized bacteria absorb N2 from the environment and the enzyme nitrogenase breaks the triple bond in the N2 to convert it into NH4 (Havlin, 2014). The efficiency of the N-fixing symbiosis is determined by the specificity between the legume crop and the symbiont. The most efficient nitrogen-fixing systems are symbioses involving Gram-negative bacteria from the genera Rhizobium and Bradyrhizobium (Olivares et al., 2013).
Preliminary trials identified strains from the genus Bradyrhizobium as symbionts of chipilín (Barnish & Spinelli, 2011; Isidoro & Messier, 2009). In these results, Bradyrhizobium USDA 3456 and 3384, as well as Bradyrhizobium PNL0i-Brady, efficiently colonized chipilín root systems and induced the fixation of N with potential for reducing applications of inorganic N (Isidoro & Messier, 2009). Specifically, an increase in N fixation and improvement in the overall health of the plants were associated with inoculations with Bradyrhizobium USDA 3456. In Central America, chipilín grows with minimum cultivation requirements and is infected by indigenous soil borne N-fixing bacteria. In a recent study, bacterial strains were isolated from nodules of plants collected in Central America and their efficiency to fix N was evaluated. Some of the bacterial strains were as efficient as inorganic N inputs in supplying this nutrient to the crop (Guamán-Díaz, Torres-Gutiérrez, Granda-Mora, & Nápoles-García, 2016).
In the present study, the colonization capacity of a group of Rhizobia strains in chipilín was evaluated and how this colonization affects the economic performance and the specific characteristics of the plant related to marketing was examined. Additionally, the use of bacterial inoculants in large-scale crop production systems was validated as an alternative source of N. To accomplish this, we have established the following specific objectives: 1) determine the N use efficiency of the crop, 2) evaluate the capacity of specific Rhizobia strains for colonization, and 3) indirectly estimate the N supplied by the bacterial colonization and assess the impact of the N fixation on quality traits and economic yield.
Materials and methods
Seedling production
Plant seedlings were produced from a single cultivar with seed provided by the Centro Nacional de Tecnologia Agropecuaria y Forestal (CENTA) in El Salvador. The seeds were sown in plastic trays, 54 by 28 cm, filled with peat moss and covered with 1 cm of vermiculite. Twenty days after sowing, the seedlings were transferred to plastic trays with 72 square cells and placed under controlled conditions until transplant to the field or greenhouse. The settings of the controlled environment were as follows: average temperature of 21 °C during the day, 16 °C at night, and 12 h of light and dark.
Multiplication of the Rhizobia strains
The Rhizobia strains evaluated were Bradyrhizobium sp. (Vigna) (R1), Bradyrhizobium USDA 3384 (R2), Rhizobium leguminosarum biovar (R3) and Bradyrhizobium USDA 2370 (R4). The strains R2 and R4 were multiplied in modified arabinose gluconate (MAG) liquid medium, with the pH adjusted to 6.6. Bacterial growth was induced by thawing a small portion of the frozen bacteria in 100 mL of liquid MAG medium and placing the solution on a rotary shaker at 200 rpm. After sufficient growth of the bacteria was observed, the solution was diluted in four concentrations (1:1, 1:10, 1:100 and 1:1,000) and transferred to individual petri dishes (100 x 15 mm) with solid MAG medium. The whole procedure was performed under a laminar flow hood. The inoculant for strains R1 and R3 was obtained from two commercial products developed by INTX microbials-llc, Indiana, USA. The strain R1 was inoculated with the commercial product N-DURE® inoculant for cowpea (Vigna unguiculata L.) and the strain R3 with the N-DURE® inoculant for beans (Phaseolus spp. L.).
A solution of each of the nitrogen-fixing bacterial strains was prepared with a minimum concentration of 2 x 108 colony-forming units (CFUs). The bacterial colonization of the root system was induced by applying 10 mL of the solution to the substrate in which the seedlings were growing using a plastic syringe.
Greenhouse experiment
A greenhouse trial (Greenhouse experiment 1) was conducted in 2012 to assess the inoculation efficiency of specific Rhizobia strains and determine the interaction of the bacterial colonization with applications of inorganic N. The experiment was carried out at the facilities of the College of Natural Sciences of the University of Massachusetts, Amherst. The general settings of the greenhouse were: 14 h of light with average temperatures of 21 °C during the day and 16 °C at night. The seedlings were transplanted into nursery pots, 18.5 cm in height and 16.2 cm in diameter, filled with a mix of river sand and perlite in 1:1 ratio.
The design of the experiment was a randomized complete block with four replications. In the trial, four Rhizobia strains (R1, R2, R3 and R4) and a control treatment (R0) were evaluated in combination with seven concentrations of N (0, 26.25, 52.5, 105, 157.5, 210 and 262.5 mg·L-1).
The N rates were supplied through a modified 0.5 M Hoagland nutrient solution (Hoagland & Arnon, 1950), using sodium nitrate (NaNO3) as the main N source. Macro and micronutrients were also included in the Hoagland solution as potassium phosphate (KH2PO4), magnesium sulfate (MgSO4), calcium chloride (CaCl2) and potassium chloride (KCl) in the following concentrations (mg·L-1): 234 potassium (K), 31 phosphorus (P), 48 magnesium (Mg), 64 sulfur (S), 200 calcium (Ca) and 525 chloride (Cl). The concentrations of the macro and micronutrients remained constant in all seven nutrient solutions, except for sodium (Na): 0, 44, 87, 173, 259, 345 and 432 mg·L-1.
The root systems of the plants were carefully washed, and the number of nodules per plant (NN) and the average weight per nodule (NW, in mg) were recorded once the crop cycle was concluded.
Field experiments
A field evaluation (Field experiment 1) was conducted in 2011 to determine the response of the crop to eight doses of inorganic N. In 2012, an additional experimental trial (Field experiment 2) was carried out to assess the inoculation efficiency of three Rhizobia nitrogen fixing bacteria strains under different rates of inorganic N. The two field trials were conducted at the Research Farm of the University of Massachusetts in South Deerfield, Massachusetts. The characteristics of the soil where the experiments were established are as follows: Occum fine sandy loam (coarse-loamy, mixed, mesic Fluventic Dystrudept) with a pH of 6.5, 2.4 % organic matter, 10 ppm P2O5, 53 ppm K, 556 ppm Ca, 65 ppm Mg and 91.23 ppm NO3.
The plots were established on raised beds, with a separation of 1.8 m, covered with degradable black mulch (BioTelo®). The seedlings were transplanted 30 cm apart in two rows per bed to obtain a plant density of 29,000 plants·ha-1. Irrigation was provided with a drip system and scheduled according to the information collected from the soil tensiometers (Irrometer®, USA) placed at 38 and 76 cm deep ground. Phosphorus (33.7 kg·ha-1 as P2O5) and potassium (123.3 kg·ha-1 as K2O) were supplied with inorganic fertilizer through drip irrigation according to the general recommendations for kale and collards in the New England Vegetable Management Guide, 2011-2012 (Howell, 2011).
Six rates of inorganic N were evaluated in Field experiment 1 (40, 80, 120, 160, 200 and 240 kg·ha-1), and two additional N rates were included in Field experiment 2 (0 and 280 kg·ha-1). Each rate of N was supplied in five applications containing 12.5 % of the total N each. The N source for both experiments was ammonium nitrate (NH4NO3). In Field experiment 2, the Rhizobia strains R1, R2, R3 and R4 were also evaluated in combination with seven concentrations of inorganic N. Both experimental trials were evaluated in a randomized complete block design with five replications.
The fresh shoots (FW, in kg·ha-1) were harvested five times when the marketable size for chipilín of 15 to 20 cm was reached and samples were dried in an oven for 48 h at 75 °C to obtain the dry weight (DW, in kg·ha-1). The color, vigor and uniformity of the crop were recorded on a visual scale from 1 to 5, where 1 was the worst and 5 was the best. Plant height (PH, in cm) was also measured. The following traits were examined only in Field experiment 2: leaf chlorophyll content, determined using a Minolta meter (SPAD-502, Mexico) (SPAD reading); total nitrogen content (TN, in mg·L) of dry matter, estimated using the Dumas combustion method in an element analyzer system; and NN and NW, obtained by cleaning the root system within a 35 cm radius around the plant.
Statistical analysis
The FW, DW, SPAD, PH, TN, NN and NW readings were subject to analysis of variance for a randomized completed block experiment.
In the model, Y ijk is the response variable for nitrogen rate i, with the Rhizobia strain j in the k block; μ is the overall mean; N i is the effect of the i th N rate; R j is the effect of the j th Rhizobia strain; R j x N i represents the interaction effect of the j th Rhizobia strain with the i th N rate; β k is the k th block effect and ε ijk is the random error effect for the i th N treatment and j th Rhizobia treatment within the k th block. For the statistical analysis of Field experiment 1 data, the terms R j and R j x N i were excluded from the linear model.
Tukey’s honestly significant difference test was applied for the main effects of Rhizobia and N. Additionally, the statistical association of the N rates and the response variables was evaluated by means of orthogonal polynomial contrasts (linear, quadratic and cubic). This analysis was conducted for the greenhouse trial and for both field trials (Field experiment 1 and 2). A regression model was fitted for the FW with the N rates. The whole analysis was conducted in the SAS software version 9.3 (SAS Institute Inc., 2011).
Results and discussion
Response of the crop to applications of inorganic nitrogen
Statistical differences were detected among the N treatments for FW and PH, with coefficients of variation of 16.9 and 7.7, respectively (Table 1). The total FW harvested during the crop cycle varied from 5.4 to 6.2 t·ha-1, with N rates of 240 and 40 kg·ha-1, respectively. A positive linear association between the BF and the N rate was observed, but only for N applications between 40 and 120 kg·ha-1. When N applications were high (240 kg·ha-1), the commercial yield was reduced by 25 %, compared to that at the N application of 40 kg·ha-1. The average PH of the crop ranged from 12.5 to 13.8 cm, but statistically only the PH of the NR6 (240 kg·ha-1) treatment was different from the PH of the other N treatments (Table 1). This suggests that the increase in FW associated with N applications from 40 to 120 kg·ha-1 (Figure 1) was due to the accumulation of biomass, rather than an increase in PH.
Table 1 Comparisons of means for N rates evaluated in the field for shoot fresh weight (FW) and plant height (PH). Field experiment 1, 2011.
| Treatment | N rate (kg·ha-1) | FW (kg·ha-1) | PH (cm) |
|---|---|---|---|
| NR1 | 40 | 6,293 abz | 13.5 ab |
| NR2 | 80 | 6,265 a | 13.6 ab |
| NR3 | 120 | 6,808 ab | 13.8 ab |
| NR4 | 160 | 6,610 ab | 13.8 ab |
| NR5 | 200 | 6,158 ab | 13.3 ab |
| NR6 | 240 | 5,393 b | 12.5 b |
| ANOVA significance | ** | * | |
| Coefficient of variation | 16.9 | 7.7 | |
| Nitrogen rate contrast1 | ** | * |
1Orthogonal contrast that corroborates the quadratic relationship between the rates of N and the response variable; ANOVA = analysis of variance; *, ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
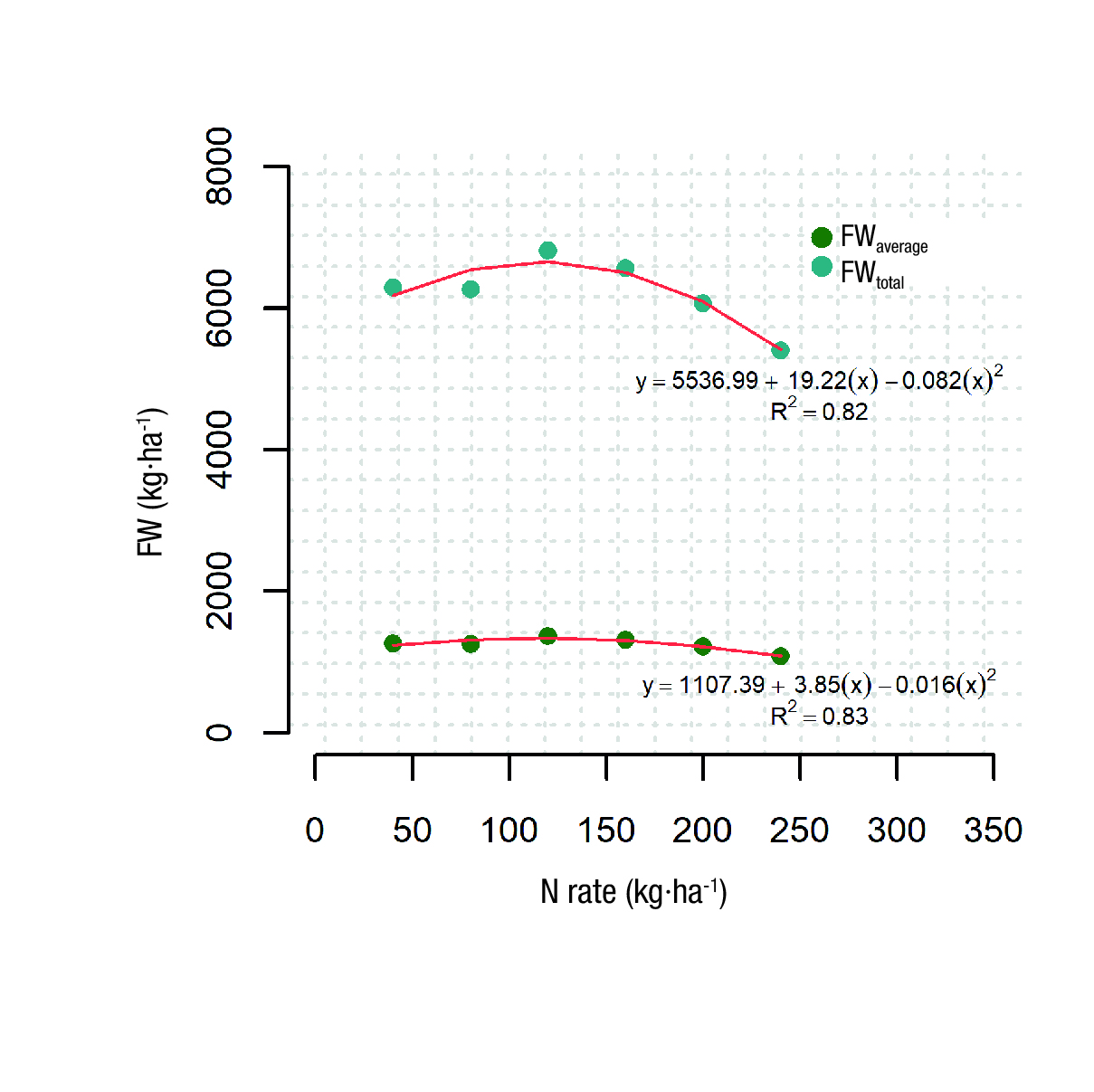
Figure 1 Average shoot fresh weight (FWaverage in kg·ha-1) and total shoot fresh weight (FWtotal in kg·ha-1) for six rates of inorganic nitrogen (N) evaluated. Field experiment 1, 2011.
A quadratic polynomial trend between the FW of the crop and the rates of inorganic N was observed (Figure 1). If two independent linear functions are fitted for N inputs and FW, a positive slope for the N rates of 40, 80 and 120 kg·ha-1 is observed, as well as a negative slope for the N rates of 160, 200 and 240 kg·ha-1. The slopes of these two models were 6.45 and -15.2, respectively. Then, per every kg·ha-1 of inorganic N applied there was an increase of 6.45 kg·ha-1 in FW; however, this trend was observed for N applications below 120 kg·ha-1. In contrast, a decrease of 15.21 kg·ha-1 in FW was observed per every kg·ha-1 of inorganic N applied above 120 kg·ha-1.
Inoculation efficiency of Rhizobia strains
As expected, inoculations with Rhizobia strains had a significant effect on NN and NW (P ≤ 0.01), and supplementary applications of inorganic N only affected NN. In contrast, the effect of inorganic N on the inoculation efficiency of Rhizobia was only observed in NN. Table 2 presents the average NN per plant and NW per nodule in response to inoculation with the Rhizobia strains. According to these results, the average NN detected with R1, R2, R3, R4 and R0 was 51, 38, 128, 39 and 23, respectively. All four strains of Rhizobia induced a larger NN than the native strains from Central America, for which the average NN was 9.4 (Guamán-Díaz et al., 2016). Thus, Rhizobium leguminosarum biovar induced the development of six times more nodules than the control treatment, with an average NW of 0.8 mg.
Table 2 Comparisons of means for Rhizobium strains (R1 to R4) and control treatment (R0) for number of nodules per plant (NN) and average weight per nodule (NW, in mg). Greenhouse experiment 1, 2012.
| Rhizobium strain | NN | NW |
|---|---|---|
| R0 = Control | 23.0 cz | 3.3 a |
| R1 = Bradyrhizobium sp. (Vigna) | 51.0 b | 2.5 a |
| R2 = Bradyrhizobium USDA 3384 | 38.0 bc | 2.8 a |
| R3 = Rhizobium leguminosarum biovar | 128.0 a | 0.8 b |
| R4 = Bradyrhizobium USDA 2370 | 39.0 bc | 1.6 ab |
| ANOVA1 significance | ** | * |
| Coefficient of variation | 7.2 | 11.7 |
1ANOVA = analysis of variance; *, ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
As reported by Isidoro and Messier (2009), Rhizobium leguminosarum biovar is a superior symbiont for chipilín and induces the production of a larger number of nodules in the root system. The strains R1, R2 and R4 also trigger the development of nodules with significantly larger sizes; however, the presence of nodules in roots does not always ensure an efficient N fixation process. In chipilín, only 20 % of the nodules collected from colonized plants by native bacterial strains in Central America contained leghemoglobin (Barnish & Spinelli, 2011), which buffers the concentration of free oxygen and ensures the proper functioning of root nodules.
Recently, an isolated bacterial strain (S.F3.1) with characteristics of the Rhizobiaceae family was identified and linked to a significant increase in fresh and dry weight, plant height, and root biomass (Guamán-Díaz et al., 2016). Furthermore, a detailed analysis to compare the N fixation efficiency of the isolated S.F3.1 and Rhizobium leguminosarum biovar in an intensive production system is needed.
The presence of nodules in the root system of the control treatment plants was also reported in a recent study (Guamán-Díaz et al., 2016). In our case, we attributed the development of nodules in plants of the control treatment to unknown Rhizobia strains present in the substrate used for producing the seedlings.
Table 3 presents the NN and PN for the N rates, and a negative trend is observed for the NN and the N concentration of the nutrient solution. When we compared the responses of HS0 and HS1, a minimum N supply of 26.25 mg·L-1 seemed to support the development of nodules. Similar to these results, an increase in the NN when 2 mmol·L-1 of N is supplied was reported in alfalfa (Medicago sativa) (Salles-de-Oliviera, Anchão-Oliveira, Corsi, Sanches-Duarte, & Mui-Tsai, 2004) and Crotalaria juncea (Miranda-Mendonça & Aparecida-Schiavinato, 2005). This minimum N concentration could be enough to support plant growth without negatively affecting the symbiotic and biological N-fixation process. However, when the N rate reaches 260 ppm (HS6), there is a clear reduction in the NN of approximately 82 % compared to HS1.
Table 3 Orthogonal polynomial contrasts of different N concentrations in the 0.5 M Hoagland nutrient solution for average number of nodules per plant (NN) and average weight per nodule (NW, in mg).
| Treatment | Nitrogen concentration (mg·L-1) | NN | NW |
|---|---|---|---|
| HS0 | 0 | 91.0 | 1.5 |
| HS1 | 26.25 | 105.2 | 3.4 |
| HS2 | 52.50 | 62.2 | 2.5 |
| HS3 | 105.00 | 51.3 | 1.9 |
| HS4 | 157.50 | 30.5 | 1.9 |
| HS5 | 210.00 | 40.3 | 1.9 |
| HS6 | 262.50 | 19.6 | 1.8 |
| Significance1 | ** | ns | |
| Trend (nitrogen) z | **L | ns |
L = significant linear relationship between nitrogen rate and the measured parameter; ns = not significant; *, ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively.
An analysis of the effect of inorganic N on the NN of plants inoculated with the Rhizobia strains is presented in Figure 2. In the graph, the standard concentration of the Hoagland nutrient solution (210 ppm) is represented as 1.0 in the abscissa axis; half of the standard concentration (105 ppm) is represented as 0.5, and 1.25 denotes 262.5 ppm. A clear trend towards a decrease in nodulation and N fixation with high doses of N fertilizer is observed.
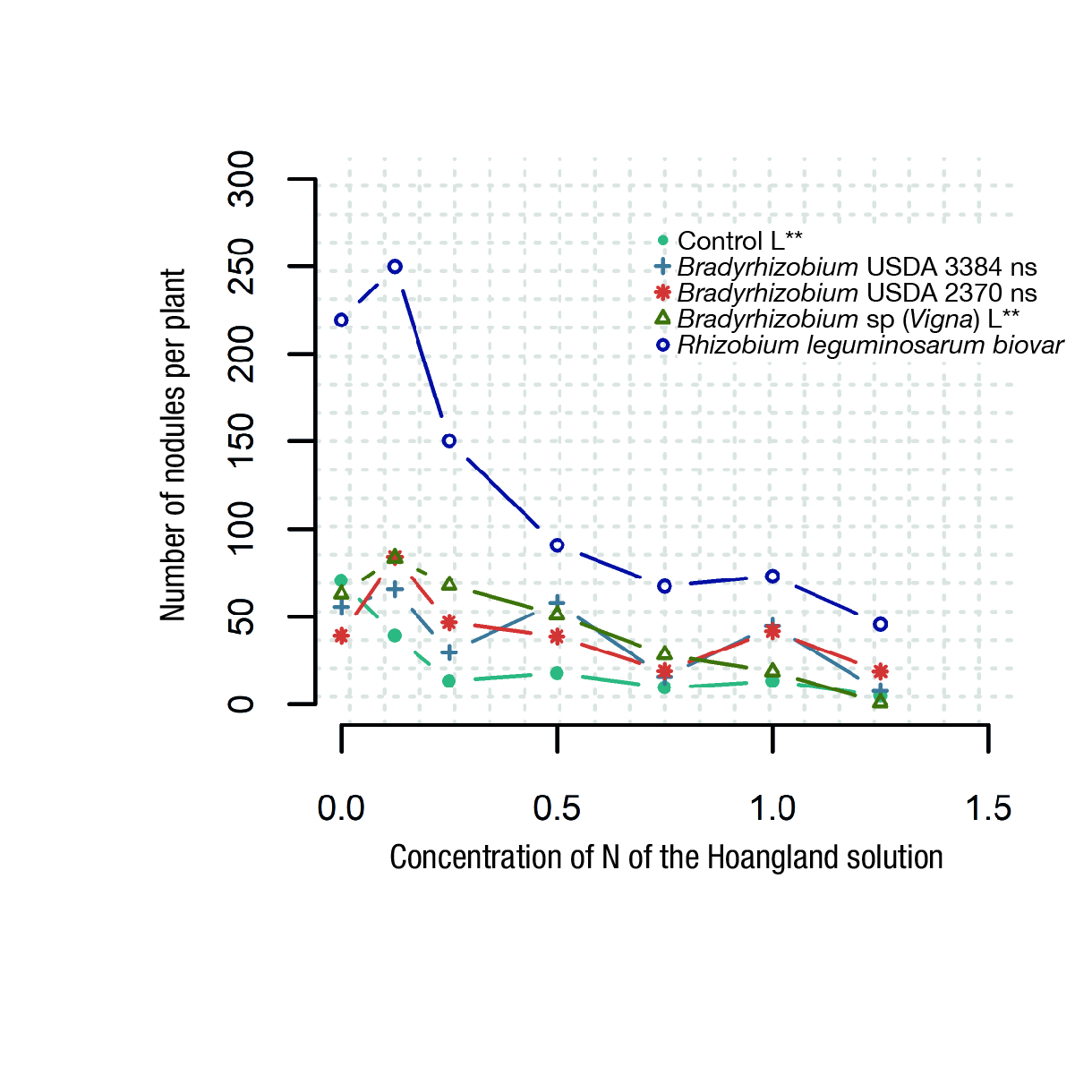
Figure 2 Number of nodules per plant in response to inoculation with four Rhizobia strains across the six nitrogen (N) rates of the 0.5 M modified Hoagland nutrient solution.
Although Rhizobium leguminosarum biovar was affected by a large supply of N, the high NN induced by the inoculation with this strain compensated for this inhibition (Figure 2). With N concentrations as high as 260 ppm, there was a reduction in the NN per plant of approximately 200 nodules. The capacity of Rhizobium leguminosarum biovar to cope with the inhibition of N fixation under high concentrations of inorganic N and to maximize fixation under low concentrations of N highlights the importance of identifying host-specific Rhizobia strains.
Constant increases in the prices of N fertilizers and fuels have a major effect on production decisions and profitability. Thus, emphasis should be placed on developing new production methods that are suitable both agronomically and economically. The capacity of Rhizobium leguminosarum biovar to convert stable N gas from the atmosphere into a biologically useful form is a major advantage in chipilín production by lowering production costs and mitigating the effects on climate change by replacing all or most of the N needed by the crop.
Nitrogen fixation efficiency of Rhizobia strains in commercial production systems
The colonization efficiency of the four Rhizobia strains was validated under field conditions. Table 4 presents the sources of variation N, Rhizobia strains (R) and their interaction (N x R) for all evaluated traits. Supply inorganic N to the crop showed a positive effect (P ≤ 0.01) on primary economic traits: color, vigor and uniformity, total FW and on the green color of leaves. This response coincided with preliminary observations, where a constant and large supply of inorganic N dramatically improved the marketable quality of the crop.
Table 4 Statistical significance of the analysis of variance of traits evaluated in chipilín (Crotalaria longirostrata Hook. & Arn.) under different doses of nitrogen (N) and inoculations of Rhizobium (R) strains. Field experiment 2, 2012.
| Dependent variable | N | R | (N x R) |
|---|---|---|---|
| Quantitative traits | |||
| Fresh weight (kg·ha-1) | ** | * | ns |
| Dry weight (kg·ha-1) | ** | * | ns |
| SPAD readings | ** | * | ns |
| Plant height (cm) | ** | ** | ns |
| Total nitrogen (mg·L-1) | ** | ns | ns |
| Number of nodules per plant | ns | * | ns |
| Weight per nodule (mg) | ns | ns | ns |
| Qualitative traits | |||
| Color | ** | ns | ns |
| Vigor | ** | ns | ns |
| Uniformity | ** | ns | ns |
ns = not significant; *, ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively.
Inoculations with the Rhizobia strains also had a positive effect (P ≤ 0.01) on the FW of the plants. Specific strains induced the increase in the total biomass of the plant but have no effect on the intensity of the green color of the leaves. Although the colonization with the bacterial strains increased the NN, there was no effect on the total N content of FW.
High doses of inorganic N decreased N fixation (Table 2). This negative effect on symbiosis was also reported for Sinorhizobium meliloti in alfalfa (Medicago sativa) when the annual N supply reached 450 kg-ha-1 (Salles-de Oliviera et al., 2004) and for Rhizobia in hemp (Miranda-Mendonça & Aparecida-Schiavinato, 2005). However, the NN was not affected by the continuous increase in N dose in Field Experiment 2 (Table 5). This discrepancy might be caused by differences in the number of times the crop was harvested, and the method employed for the application of the N treatments. Under field conditions, inorganic N was applied every time the crop was harvested, while in the greenhouse, the N was applied every week, and the crop was harvested only once. The constant regrowth of the plants in the field trial might have mined the soil N to the point where it did not affect the formation of nodules or N fixation.
The response of the economic yield of FW to N rates is presented in Table 5. If we analyze the tradeoff between the N application and the FW in Field experiment 2, we determine that per every kg of N supplied in the range from 0 to 80 kg·ha-1, there is an increase in FW of 30 kg·ha-1 (P ≤ 0.01). Additional applications above 80 kg·ha-1 are less cost-effective, with a maximum increase in FW of 1.12 kg·ha-1 per kg of N applied. Similar to the FW, a constant supply of N in excess of 80 kg·ha-1 provides an increase of only 0.175 kg·ha-1 (P ≤ 0.01) in DW per unit of N. The null supply of inorganic N produced only 0.61 t·ha-1 of DW per season, while in tropical areas, the total DW can reach 3.4 t·ha-1 per year (Sosa-Rubio, Cabrera-Torres, Rodriguez, & Ortega-Reyes, 2008). A simultaneous increase in the chlorophyll content and the TN content of leaves was observed with N rates as high as 280 kg·ha-1.
Table 5 Orthogonal polynomial contrasts of the eight rates of inorganic nitrogen evaluated (NR0 to NR7). Field experiment 2, 2012.
| Nitrogen rate (kg·ha-1) | FW (kg·ha-1) | DW (kg·ha-1) | SPAD | TN (mg·L-1) | PH (cm) | NN | NW (mg) |
|---|---|---|---|---|---|---|---|
| NR0 0 | 4,564 | 661 | 44.1 | 4.4 | 33.8 | 16.4 | 1.9 |
| NR1 40 | 5,892 | 838 | 45.5 | 4.9 | 36.9 | 14.1 | 1.4 |
| NR2 80 | 6,974 | 982 | 45.9 | 5.0 | 39.1 | 16.7 | 0.6 |
| NR3 120 | 6,950 | 982 | 46.3 | 5.1 | 38.6 | 13.2 | 1.9 |
| NR4 160 | 7,067 | 982 | 46.8 | 5.2 | 38.0 | 12.5 | 0.5 |
| NR5 200 | 6,870 | 941 | 46.8 | 5.2 | 37.5 | 12.0 | 0.4 |
| NR6 240 | 6,913 | 960 | 47.2 | 5.3 | 38.6 | 11.0 | 0.5 |
| NR7 280 | 7,229 | 1,031 | 48.1 | 5.3 | 38.7 | 12.8 | 0.4 |
| Significance | ** | ** | ** | ** | ** | ns | ns |
| Polinomial contrast | **L, **Q, **C | **L, **Q, **C | **L, **C | **L, **Q, **C | **L, **Q, **C | ns | ns |
1FW = shoot fresh weight; DW = shoot dry weight, TN = concentration of N in leaves; PH = plant height; NN = number of nodules in the roots per plant; NW = weight per nodule; ns = not significant; * , ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively; L, Q, C = linear, quadratic or cubic contrasts, respectively, between nitrogen treatment and the parameter evaluated.
The strong association between the chlorophyll content and the absolute N accumulation in leaves (r = 0.95, P ≤ 0.01) validates the use of the SPAD meter for in situ estimations of the plant N content. In rice, corn, barley and wheat, a similar association was determined between the SPAD readings and the concentration of N in leafs (Dwyer et al., 1995; Lin et al., 2010; Peltonen, Virtanen, & Haggrèn, 2008; Reeves, Mask, Wood, & Delaney, 2008; Shukla et al., 2004).
The importance of inorganic N in the expression of marketing quality traits for vegetable production was examined. Differences in the color, vigor and uniformity of the crop were detected. When N was not applied, the average color, vigor and uniformity were 2.3, 2.5 and 2.3, respectively, and with 280 kg·ha-1 of N, the corresponding scores were 3.5, 3.5 and 3.3. Further addition N more than 80 kg·ha-1, and up to 280 kg·ha-1, result in a minimum enhancement in the color of 0.2, in vigor of 0.3 and in uniformity of 0.4. The analysis to determine the effect of Rhizobia inoculations on these three quality characteristics did not show statistical significance.
Table 6 shows the statistical association between the Rhizobia strains and the FW, DW, SPAD reading, TN, PH, NN and NW. A non-significant increase of 158 kg·ha-1 in FW was linked to the inoculation of plants with Rhizobium leguminosarum biovar. On the other hand, plants colonized with Bradyrhizobium USDA 3384 showed a minimal increase in economic yield but significant improvement in the green color of leaves; however, the same strain significantly decreased NN. On average, Bradyrhizobium sp. (Vigna) and Rhizobium leguminosarum biovar induced the development of six more nodules per plant compared to Bradyrhizobium USDA 3384. Although colonization with the Rhizobia strains modified the NN, this factor did not affect the weight per nodule.
Table 6 Comparison of means of three Rhizobia strains (R1 to R3) and control treatment (R0). Field experiment 2, 2012.
| Rhizobium strain | FW (kg·ha-1) | DW (kg·ha-1) | SPAD | TN (mg·L-1) | PH (cm) | NN | NW (mg) |
|---|---|---|---|---|---|---|---|
| R0 = Control | 6,674.4 az | 934.8 a | 46.6 ab | 5.0 | 38.1 a | 14.3 a | 0.8 |
| R1 = Bradyrhizobium sp. (Vigna) | 6,646.4 a | 941.2 a | 46.0 b | 5.0 | 37.8 a | 15.8 a | 0.8 |
| R2 = Bradyrhizobium USDA 3384 | 6,077.2 b | 858.0 b | 48.0 a | 5.1 | 36.4 b | 9.0 b | 0.4 |
| R3 = Rhizobium leguminosarum biovar | 6,832.4 a | 956.4 a | 46.0 b | 5.1 | 38.3 a | 15.0 a | 1.7 |
| Significance | * | * | * | ns | ** | ** | ns |
1FW = shoot fresh weight; DW = shoot dry weight, TN = concentration of N in leaves; PH = plant height; NN = number of nodules in the roots per plant; NW = weight per nodule; ns = not significant; * , ** = significant with P ≤ 0.05 and P ≤ 0.01, respectively. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
The plant-microbe association of chipilín and Rhizobium leguminosarum biovar and Bradyrhizobium USDA 3384 was an efficient N-fixing system, but further efforts are needed to develop cultivars adapted to specific inoculants and to evaluate how biotic and abiotic factors can affect their fixation efficiency. Additionally, the presence of nodules in the root system of plants in the control treatment suggests potential competition between the native Rhizobia in the soil and the introduced strains. When competitors in the soil are poor N fixers, N fixation rates and plant productivity can decrease (Olivares et al., 2013). Therefore, it is important to consider multiple inoculations to ensure adequate colonization by the inoculant.
Conclusions
According to the results presented in this study, applications of inorganic N in excess of 80 kg·ha-1 provide a minimum economic return in terms of economic yield (FW); however, additional N can increase the marketable quality of chipilín. Bradyrhizobium and Rhizobium strains were able to colonize the crop, and specifically the strain Rhizobium leguminosarum biovar enhanced FW accumulation. However, the relationship between this colonization and the chlorophyll content of the crop is unclear. Any supplementary applications of inorganic N under field conditions have little impact on the effectiveness of the bacterial colonization, yet to maximize NN and enhance N fixation, it is recommended that N be supplied at a rate not greater than 80 kg·ha-1. Furthermore, there is a need to evaluate the effectiveness of periodic re-inoculations with the best nitrogen-fixing bacteria strains, and to identify genotype-specific nodulation to select an effective and competitive inoculant.











 text in
text in 


