Introduction
Salinity stress is a key environmental factor limiting plant growth and development that may entail several other stresses like ion toxicity, osmotic stress, and oxidative stress (Gezi, Peng, Wei, & Kang, 2013). Grasses form a major part of green spaces in urban areas and they cannot be replaced by other plant species in most cases because of their high tolerance for heavy traffic (Torgeon, 2008). Kentucky bluegrass (Poa pratensis L.) is one of the most widely used grasses in landscaping, due to its beauty, resistance and stability, especially in regions with cold and wet climates (Bushman, Amundsen, Warnke, Robins, & Johnson, 2016).
Salinity stress is a major limiting factor in the grass industry as it deteriorates grass quality, so it is a key concern in grass protection. Therefore, extensive research has focused on growth regulators that can alleviate stress damages in order to generate resistance. Among these compounds, salicylic acid (SA) is a plant growth regulator belonging to the family of phenols. SA plays an essential role in plant growth, respiration, and resistance to pathogens (Janda, Hidog, Szalai, Kovacs, & Janda, 2012). It has been documented that SA improves the growth of plants exposed to chilling, high temperature, salinity, osmotic stress, ozone or ultraviolet stresses, drought, or herbicides (Belkhadi et al., 2010). As well, SA may induce plant tolerance to different stresses through signaling molecules (Loutfy, El-Tayeb, Hassanen, Sakuma, & Inouhe, 2012).
Plants and animals contain many steroid compounds that act as signaling molecules during complicated growth and development processes (Erdal, Genisel, Turk, & Gorcek, 2012). Mammal sex hormones (MSHs), including progesterone (P4), estrogen, and testosterone, form another group of steroids. These hormones have key roles in controlling development and propagation process as well as mineral and protein metabolism in mammals (Erdal & Dumlupinar, 2010; 2011). Steroid sex hormones, such as P4, estrone, β-estradiol, and testosterone, stimulate growth, development, callus development, cell division, root and stem elongation, and pollination in plants and have desirable effects on coping with salinity stress (Genisel, Turk, & Erdal, 2013; Metwally, Awad, Abou-Leila, Tayeb, & Habba, 2015; Rojek, Pawełko, Kapusta, Naczk, & Bohdanowicz, 2015).
An interesting impact of MSH is its ability to improve plant resistance to various abiotic stresses (Erdal et al., 2012). Erdal and Dumlupinar (2011) reported that MSH treatment significantly alleviated the negative impacts of salinity stress by stimulating the activity of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase and catalase, and increasing proline content. In another study, it has been revealed that MSH treatment induced antioxidant systems and synthesis reactions and reduced reactive oxygen species (ROS) in wheat seedlings (Erdal & Dumlupinar, 2011).
Given the special role of Kentucky bluegrass in landscapes and the positive impact of SA and progesterone (P4) on salt stress damages in most experimental works, the present study aimed to evaluate the effect of SA and P4 on physiological and biochemical parameters in Kentucky bluegrass under salinity stress.
Materials and methods
The experiment was carried out in a greenhouse (average day/night temperature of 25 °C, photoperiod of 16 h, photosynthetically active radiation of 1,470 ± 11 μmol·m-2·s-1; relative humidity of 50 %) in Chaharbagh, Karaj, Iran. For this, a factorial experiment was established based on a randomized complete block design with four replications. The treatments included salinity at four levels (0, 2, 4, or 6 dS·m-1) and two plant growth regulators (P4 and SA) at six levels (control, 1 mg·L-1 P4, 10 mg·L-1 P4, 1 mM SA, 3 mM SA, and 1 mg·L-1 P4 + 1 mM SA). The SA was obtained from Merck Group, Germany, and the P4 treatments were applied on the basis of 4-pregnene-3, 20-dione (Sigma-Aldrich Corp., USA).
Kentucky bluegrass seeds were obtained from the Iran Bazr Company and were sown in 25 × 35 cm plastic pots. The substrate was a mixture of 49 % sand, 12 % clay, and 39 % silt. The electrical conductivity (EC) in control treatment (conventional water) was 1,200 µS·cm-1. Two months after sowing and irrigation with conventional water, salinity was applied in a 40-day period. SA and P4 treatments were applied in three phases (two weeks before salt stress initiation, two weeks after stress initiation, and four weeks after stress initiation).
Among the variables evaluated, the quality of the grass was determined considering its color, density and texture by means of the chlorophyll content and electrolyte leakage (EL). Total chlorophyll content and leaf carotenoids were measured using the methodology proposed by Arnof (1946) and the following equations:
where V is the volume of infiltrated solution (supernatant taken from centrifuge), A is the absorption at 663, 645 and 470 nm and W is the sample fresh weight (g). Absorption rate was recorded by microplate-reader (ELX808, BioTek, US) at 663, 645, 470, and 520 nm.
The proline content (μM·g-1 fresh weight) of plant samples was estimated by Bates, Waldren, and Teare (1973)’s method using a spectrophotometer (Z-2000, Hitachi Ltd., Japan) at 520 nm.
Malondialdehyde (MDA) was measured by the procedure described by Huang and Xu (2008) using a plate reader. Ascorbate peroxides (APX) enzyme activity was measured by formazan synthesis using Mittler and Zilinskas (1993)’s procedure with slight modifications, in which the capability of APX to suppress pertaining ascorbate was determined through reducing nitro blue tetrazolium (NBT) in the presence of hydrogen peroxide (H2O2). To measure superoxide dismutase enzyme, the method described by Beauchamp and Fridovich (1970) was used with some modifications.
All variables were analyzed every week for seven weeks and the results obtained were subjected to an analysis of variance and Fisher’s least significant difference test (P ≤ 0.05).
Results and discussion
Color and apparent quality of grass
Analysis of variance showed that the simple and interaction effects of salinity stress and plant growth regulators (PGRs) were significant on color and apparent quality of grass at all measurement times (Table 1). Under the influence of both salinity and PGRs, the apparent quality was lost over time (Table 2). Means comparison revealed that the apparent quality was the highest in weeks two and seven at interaction of control treatment of salinity (i.e. without salinity) with every level of PGRs treatment, while it was the lowest at 6 dS·m-1 salinity and no PGR application.
Table 1 Analysis of variance of the apparent quality of Kentucky bluegrass.
| Sources of variation | DF | Week | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Replication | 3 | 0.40* | 0.19 ns | 0.09 ns | 0.17 ns | 0.53 ns | 0.21 ns | 0.26 ns |
| Salinity (A) | 3 | 5.90** | 15.36** | 24.79** | 34.69** | 48.36** | 76.96** | 100.39** |
| PGRs (B) | 5 | 0.34* | 1.97** | 2.92** | 3.95** | 3.34** | 1.97** | 1.99** |
| AB | 15 | 0.30* | 0.49** | 0.59** | 0.94** | 0.74** | 1.45* | 0.39** |
| Error | 69 | 0.14 | 0.11 | 0.16 | 0.17 | 0.25 | 0.20 | 0.16 |
| Coefficient of variation (%) | 4.55 | 4.19 | 5.25 | 5.63 | 7.16 | 7.01 | 6.59 | |
1DF = degrees of freedom; *: significant at P < 0.05; **: significant at P < 0.01; ns: non-significant
Table 2 Means comparison for the interaction effect of salinity and plant growth regulators (PGRs) on apparent quality of grass.
| Salinity | PGRs | Week | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Control | Control (no PGRs) | 9 az | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a |
| 1 mg·L-1 P41 | 8.75 ab | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | |
| 10 mg·L-1 P4 | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | |
| 1 mM SA | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | |
| 3 mM SA | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | |
| P4 + SA | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | 9 a | |
| 2 dS·m-1 | Control (no PGRs) | 8 cd | 7 e | 6.75 e-g | 6 f | 6 b-e | 5 ef | 5 c |
| 1 mg·L-1 P4 | 8.50 a-c | 7.75 bc | 7.75 bc | 7 c-e | 6 d-f | 5 ef | 5 c | |
| 10 mg·L-1 P4 | 8 cd | 7.50 cd | 7.25 c-e | 7.25 cd | 7 b | 6 bc | 6 b | |
| 1 mM SA | 8.50 a-c | 8 b | 8 b | 8 b | 7 b | 6 bc | 6 b | |
| 3 mM SA | 8.25 bc | 7.75 bc | 7.75 bc | 6.75 de | 6.25 c-e | 5.75 cd | 5.75 b | |
| P4 + SA | 8.50 a-c | 8 b | 8 b | 7.25 cd | 7 b | 6 bc | 6 b | |
| 4 dS·m-1 | Control (no PGRs) | 8 cd | 7 e | 6 hi | 5.25 g | 5 gh | 4.75 fg | 4.50 cd |
| 1 mg·L-1 P4 | 8 cd | 7.25 de | 7 d-f | 6.50 ef | 6.25 c-e | 5.50 c-e | 5 c | |
| 10 mg·L-1 P4 | 8.25 bc | 7.50 cd | 7.50 b-d | 7 c-e | 7 b | 6.50 b | 5.75 b | |
| 1 mM SA | 7.50 de | 7 e | 7 d-f | 6.50 ef | 6 d-f | 5.50 c-e | 5 c | |
| 3 mM SA | 8 cd | 7 b | 7.50 b-d | 7 c-e | 6.75 bc | 6 bc | 5.75 b | |
| P4 + SA | 8.25 bc | 7 b | 8 b | 7.50 bc | 6.75 bc | 5.75 cd | 5.75 b | |
| 6 dS·m-1 | Control (no PGRs) | 7 e | 6 f | 5.50 i | 5 g | 4.50 h | 4.25 g | 3.50 e |
| 1 mg·L-1 P4 | 8 cd | 7 e | 6.25 gh | 6 f | 5.75 ef | 5.25 e-f | 4.25 d | |
| 10 mg·L-1 P4 | 8 cd | 7.50 cd | 7.25 c-e | 7 c-e | 6.50 b-d | 5.50 c-e | 5 c | |
| 1 mM SA | 8 cd | 7 e | 6.50 f-h | 6 f | 5.50 fg | 4.75 fg | 4 de | |
| 3 mM SA | 8 cd | 7.75 bc | 7 d-f | 7 c-e | 6.25 c-e | 5.25 d-f | 4.50 cd | |
| P4 + SA | 8 cd | 8 b | 7.25 c-e | 7 c-e | 6.50 b-d | 5.50 c-e | 4.50 cd | |
1P4 = progesterone; SA = salicylic acid. zMeans with the same letter within each column do not differ statistically (Fisher, P ≤ 0.05).
The negative effect of salinity on grass quality showed through the loss of leaf chlorophyll content as reported in other research (Borowski, 2018; Uddin & Juraimi, 2013). The positive effect of simultaneous use of P4 and SA was evident in different weeks. When these growth regulators were applied concurrently, the apparent quality of grass was reduced with a gentler slope (Table 2). The increase in leaf color and greenness can be attributed to the increased density of leaf mesophyll cells and higher chlorophyll content arising from the application of SA + P4. In another study, SA application could partially alleviate the negative effects of salinity on the apparent quality of grass (Kurepin et al., 2017).
It has been reported that exogenous SA application may influence a range of different processes in plants, such as photosynthesis and growth rate; in addition, it may improve nutrient uptake under salinity conditions, and this may increase growth (Arghavani, Savadkoohi, & Mortazavi, 2017). On the other hand, Beiraghdar, Yazdanpoor, Naderi, & Zakerin (2014) state that it is possible that SA increases the chlorophyll content in leaves that are at the beginning of senescence.
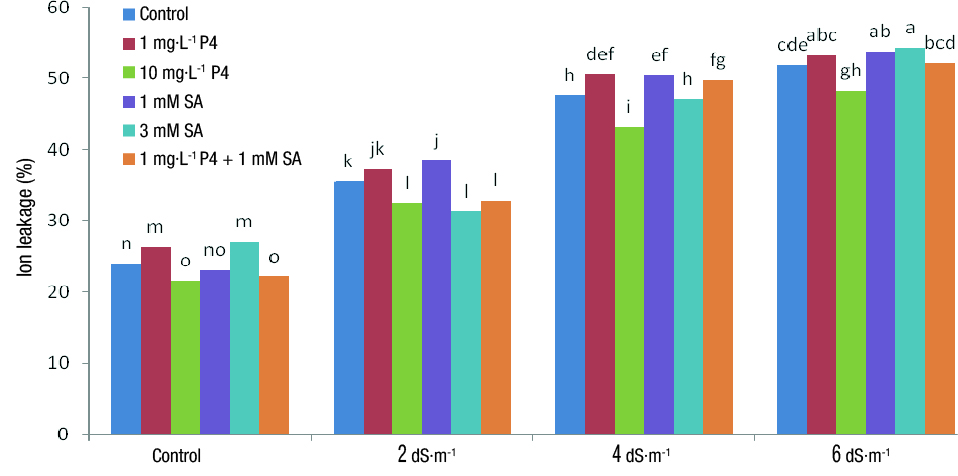
Electrolyte leakage
EL was significantly influenced by both simple and interaction effects of salinity and PGRs (Table 3). According to the results of means comparison, the highest EL was observed in the treatment with 6 dS·m-1 salinity and 3 mM SA, while the lowest EL was obtained with the control salinity treatment and 10 mg·L-1 P4, as well as with the control salinity treatment and the combination of both PGRs (Figure 1). These results coincide with those reported by Lu et al. (2017) and Zhang, Wu, Ervin, Shang, & Harich (2018). In salt stress conditions, the synthesis of free oxygen radicals, which causes the peroxidation of membrane fatty acids and the replacement of Na+ with Ca2+ in cell membrane, increases membrane permeability and reduces its stability, so EL is increased. It is likely that SA and P4, which act as signaling molecules, improve plants’ antioxidant defensive capacity against the damages of ROS, thereby increasing membrane stability and reducing EL (Ahemad & Kibert, 2014). The slight or no effect of PGRs on EL under 6 dS·m-1 salinity maybe attributed to the very high rate of EL under this concentration.
Table 3 Analysis of variance of the studied physiological traits of Kentucky bluegrass.
| Sources of variations | DF | Electrolyte leakage | Leaf carotenoid | Total chlorophyll | Proline | MDA | SOD | APX |
|---|---|---|---|---|---|---|---|---|
| Replication | 3 | 3.61 ns | 0.004 ns | 0.140 ns | 27.44 ns | 11.99 ns | 2.82 ns | 0.53 ns |
| Salinity (A) | 3 | 4011.84** | 0.241** | 2.736** | 13146.94** | 1895.06** | 98.06** | 145.9** |
| PGRs (B) | 5 | 60.91** | 0.007ns | 0.156* | 464.43** | 178.01** | 30.81** | 4.91** |
| AB | 15 | 14.43** | 0.010* | 0.123* | 860.01** | 51.91* | 36.49** | 9.29** |
| Error | 69 | 1.59 | 0.005 | 0.066 | 86.43 | 28.07 | 1.75 | 0.45 |
| Coefficient of variation % | 3.17 | 17.03 | 15.08 | 11.12 | 11.18 | 14.66 | 11.83 |
1DF = degrees of freedom; MDA = malondialdehyde; SOD = superoxide dismutase; APX = ascorbate peroxidase. * = significant at P < 0.05; ** = significant at P < 0.01; ns: non-significant.
Leaf carotenoids
Analysis of variance revealed that the simple effect of salinity and its interaction with PGRs induced significant differences in leaf carotenoid content (Table 3). The mean comparison data for the interaction of salinity × PGRs indicated that the treatment with 6 dS·m-1 salinity with no PGRs caused the lowest leaf carotenoid content and treatment without salinity × SA + P4 caused the highest mean leaf carotenoid content. SA and P4 application to grasses exposed to salinity significantly increased carotenoid content compared to plants not treated with these regulators (Figure 2). Taibi et al. (2016) reported that salinity reduced carotenoid content by 20 %. As well, Idrees, Khan, Aftab, Naeem, and Hashmi (2010) note that the application of growth regulators increases plant pigments both under stressful and normal conditions. According to Chaparzadeh and Hosseinzad-Behboud (2015), SA application under salinity stress increased leaf carotenoid.
Total chlorophyll
Total chlorophyll content was significantly influenced by the simple and interaction effects of salinity and PGRs (Table 3). Means comparison revealed that the highest and lowest total chlorophyll contents were observed in plants treated with 2 dS·m-1 salinity and 10 mg·L-1 P4 and those treated with 6 dS·m-1 salinity and no growth regulators, respectively (Figure 3), although they did not significantly differ from other treatments. In general, salinity stress reduced total chlorophyll content. The loss of photosynthetic pigments in plants under salinity stress might mainly be associated with the degradation of chloroplast structure and photosynthesis system, photooxidation of chlorophylls and their reaction with oxygen radicals, the destruction of chlorophyll synthesis precursors, the inhibition of biosynthesis of new chlorophylls, the activation of chlorophyll oxidizing enzymes (like chlorophyllase), and hormone disorders (Grzeszczuk, Salachna, & Meller, 2018; Stepien & Klobus, 2006; Taiz & Zeiger, 2009). However, the accumulation of Na+ and Cl- in leaves under salinity stress has some negative impact on chlorophyll content (Stepien & Klobus, 2006). Arghavani et al. (2017) reported that SA application improved total chlorophyll content under salinity and this effect was most evident at 80 mM salinity.
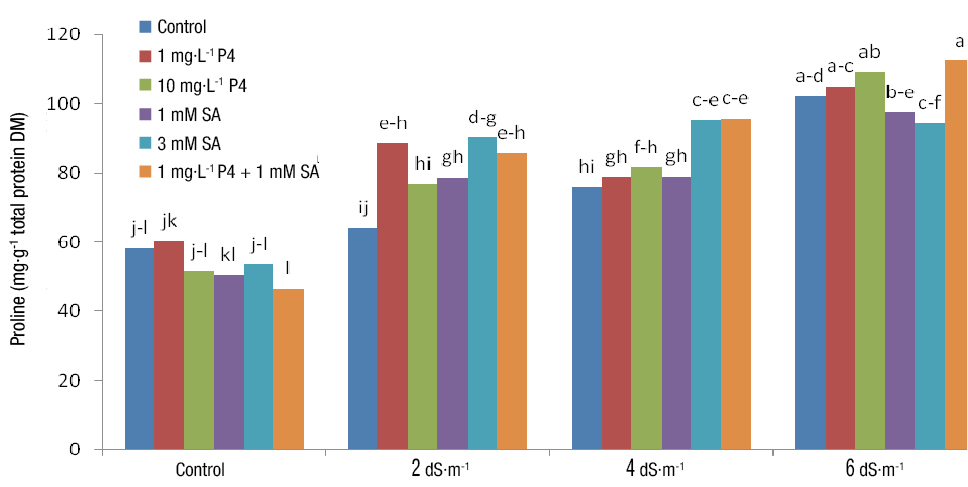
Proline
Analysis of variance showed a highly significant effect in the simple and interaction treatments of salinity and PGRs on proline content (Table 3). Means comparison implied that 6 dS·m-1 salinity and SA + P4 application resulted in the highest proline content and no salinity stress (control) and SA + P4 application resulted in the lowest one (Figure 4). Leaf proline content was increased with salinity level; however, slight differences existed between different levels of PGRs. The increase in proline level under stress is related to the fact that proline is a compatible osmolyte that scavenges free oxygen synthesized during environmental stress and protects macromolecules (Rahdari, Tavakoli, & Hosseini, 2012). The results of the present study showed that at high salinity levels, simultaneous application of SA and P4 was most effective in proline content, which is in agreement with other research (Abdul-Qados, 2015; Agamy, Hafez, & Taha, 2013). The impact of PGRs on proline accumulation may increase proline metabolism. It has been observed that under salinity conditions, there is an increase in the activity of proline biosynthetic enzymes, pyrrholine-5-carboxylate reductase, and gamma-glutamyl kinase, resulting in high proline content (Misra, Misra, & Singh, 2010).
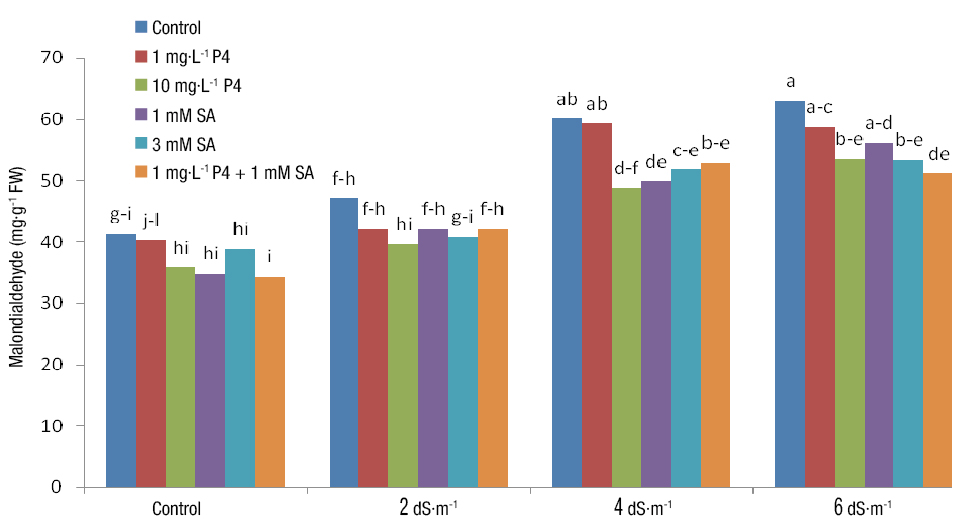
Malondialdehyde
According to the results of the analysis of variance, the simple effect of salinity and PGRs, as well as their interaction, had a significant effect on MDA (Table 3). The highest MDA was obtained from 6 dS·m-1 salinity and no PGR application and the lowest MDA was related to no salinity (control) and simultaneous application of SA and P4. Different levels of salinity stress increased MDA by 12-50 % (Figure 5). Li et al. (2014) reported a 13-45 % increase in MDA with the increase in salinity. As well, the results showed that PGRs reduced the MDA of grasses exposed to salinity (Figure 5); in other words, PGRs alleviated the damage caused by salinity in the cells, which may arise from the reduction of destructive effects of free radicals by SA. These results are consistent with those reported by Wang, Yang, Zhang, and Li (2009). At all salinity stress levels, MDA content was lower with 10 mM P4 than with 1 mM P4, indicating the desirable effect of P4 on reducing the destructive effects of salt stress (Figure 5). It seems that P4 hinders fat oxidation by scavenging free radicals, thereby inhibiting the increase in MDA. Erdal et al. (2012) showed that P4 application under salinity conditions reduced MDA content in beans.
Superoxide dismutase
Analysis of variance showed that the simple and interaction effects of salinity and PGRs were significant for SOD activity (Table 3). The highest SOD activity (15.14 mg·DM-1 protein on a dry basis) was obtained from plants exposed to 4 dS·m-1 salinity and 1 mg·L-1 P4 and the lowest SOD activity was related to the exposure to 4 dS·m-1 salinity and 3 mM SA (Figure 6). Superoxide anions synthesized by plants under salinity increase cell respiration and the production of destructive ions in mitochondria. In these conditions, the SOD enzyme as a superoxide ion destruction enzyme exhibits an increase (Fikret, Manar, Şebnem, Şebnem, & Ozlem, 2013). In the present work it was observed that foliar application of SA and P4 increased SOD activity, which results in increased detoxification of superoxides and alleviates plant damage. It seems that P4 and SA are involved in reducing the destructive effects of salinity so that they improve salinity resistance by increasing the activity of enzymes. Shahmoradi and Naderi (2018) reported that SA application moderated the negative effects of salinity by reducing oxidative stress generated by increased antioxidant enzyme activity. Erdal and Dumlupinar (2010) reported that P4 application resulted in high SOD activity in peas.
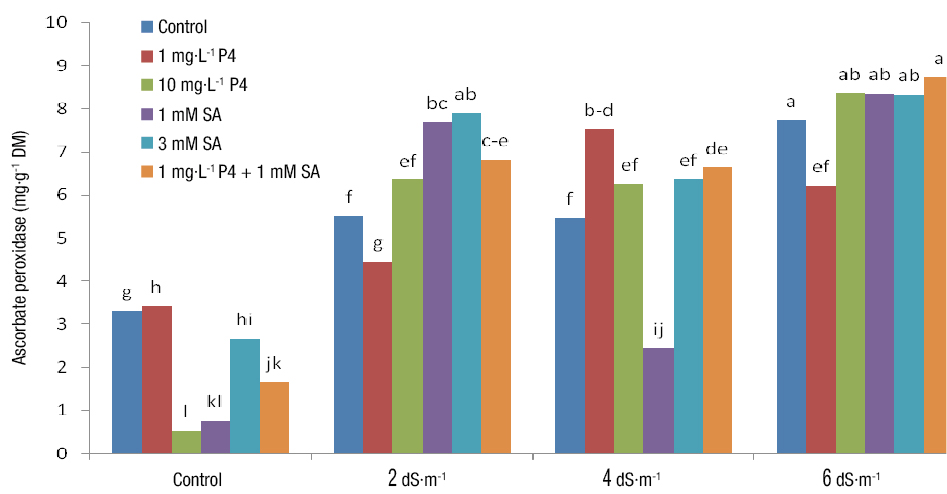
Ascorbate peroxidase
The simple and interaction effects of salinity and PGRs were significant on APX (Table 3). The highest APX was obtained from the simultaneous use of SA and P4 under 6 dS·m-1 salinity, while the lowest activity was related to control treatment and 1 mg P4 (Figure 7). SA and P4 directly or indirectly stimulate the synthesis of antioxidant enzymes like peroxidase, contributing to the scavenging of ROS synthesized during stress. This is because these enzymes can act as the electron donor substrate for peroxidase and play a role in mitigating the stress (Erdal & Dumlupinar, 2010; Shahmoradi & Naderi, 2018). It is evident from our results that salinity stress leads to oxidative stress which is responsible for the increased activity of antioxidant enzymes. These detoxifying enzymes mitigate stress and remove excessive amounts of H2O2. Likewise, by stimulating antioxidant enzymes under stressful conditions, SA creates a stress alleviation system in plants (Morsi, Abdelmigid, & Aljoudi, 2018). Salinity stress resulted in the increased activity of APX, which is in agreement with the findings reported by Ma, Zheng, Zhang, Hu, and Qian (2017), who obtained an 8-50 % increase in APX activity under salinity.
Conclusions
Salinity stress in the first week had no significant effect on the qualitative traits of Kentucky bluegrass; however, a visible impact on the apparent quality of the grass was observed in subsequent weeks. Higher salinity reduced grass quality by 60 % compared to the first week. The increase in salinity (6 dS·m-1) entailed a marked loss of grass quality, which was decreased by 10-55 % versus control. Nonetheless, the application of SA and P4 was useful for grass survival and its quality improvement under salt stress. To cope with salinity, grasses increase their proline content and the activity of APX, MDA, and SOD enzymes. Most recorded physiological traits were influenced by the simultaneous application of SA and P4, which have distinct effects. Based on these results, Kentucky bluegrass can be grown in saline conditions, but its growth and development will be decreased. However, the detrimental effects can be alleviated to a great extent by the application of P4 and SA. Applying these compounds does not entail a heavy financial burden.











 texto en
texto en