Introduction
The process called "climate change" includes a number of atmospheric instability phenomena, among which is the increase in global temperature. According to the Organization for Economic Cooperation and Development (OECD, 2012), the temperature will rise by up to 4 °C before the end of this century.
Agriculture is extremely vulnerable to climate change, so rising temperatures and changes in weather patterns will eventually reduce the production of desired crops; this will increase the likelihood of crop failure in the short term and reduce production in the long term (Food and Agriculture Organization [FAO], 2011). The vulnerability of crops to high temperatures may vary depending on the species and the phenological status of the plant. Some studies carried out on different crops agree that the flowering and seed filling stages are the most sensitive to temperature increase (Agandi et al., 2000; Erickson & Markhart, 2002), since they can cause low quality indices in fruits and jeopardize the availability of quality seeds. However, there is little research on the effect of high temperature on seed development and quality.
In species susceptible to high temperatures, such as tomato (Solanum lycopersicum L.), this factor can affect the physiological processes or modify the development pattern of the plant, negatively altering its morpho-anatomical and biochemical characteristics. Also, under conditions of extreme environmental changes, mainly of temperature and rainfall regimes, plant development, production and quality, both of fruit and seeds, may be affected, even resulting in total losses (Schwarz, Thompson, & Kläring, 2014). In the case of tomato, the optimum temperature for growth and production is between 23 and 26 °C (Adams, Cockshull, & Cave, 2001). At high temperatures, the levels of the enzymes involved in carbohydrate metabolism (glucokinase and fructokinase) may be reduced by an imbalance between their rate of formation and degradation (Biais et al., 2014; Gautier et al., 2008). The FAO (2015) mentions that one way to mitigate the negative effects of high temperatures, inherent in climate change, on crop production is the use of plant genetic resources, as the ability of plants to tolerate climate changes is given by their genetic diversity (FAO, 2015).
Mexico is considered the center of origin and domestication of the tomato, and has a great diversity of plant species. It is estimated that around 50 thousand ha are cultivated each year, and its per capita consumption is 14 kg. The main producing states of this vegetable are Sinaloa, San Luis Potosí and Michoacán (Servicio de Información Agroalimentaria y Pesquera [SIAP], 2017). Wild and native tomato varieties represent an important source of genetic variability that can be included in crop breeding programs. In addition, they have fruit of high nutritional quality, with smaller size and lower weight than commercial varieties, attractive physical characteristics (color and shape) and many fruit per cluster; however, their fruit have a short shelf life or are very soft (Sim, Robbins, Van Deynze, Michel, & Francis, 2011).
The use of native tomato varieties that tolerate environmental changes caused by global warming is feasible, so it is necessary to study in detail the growth and reproduction of these plants. Therefore, the objective of this research was to evaluate the physical and physiological quality of seeds of three native varieties and a commercial one of tomato (Solanum lycopersicum L.), from fruit produced under high temperature conditions.
Materials and methods
Location of the experimental site and plant material
The experiment was carried out during spring 2016, in two greenhouses located in Texcoco, Mexico (19° 27’ 51’’ North latitude and 98° 54’ 15’’ West longitude, at 2,250 masl). The commercial variety used was 'Moneymaker' (MM), considered a worldwide reference in tomato studies (Biais et al., 2014; Luengwilai & Beckles, 2009), and the three native varieties were 'Campeche' (C-40), 'Yucatán' (Y-25) and 'Malinalco' (M-430), which belong to the Colegio de Postgraduados’ breeding program. MM and M-430 are from temperate climates, while Y-25 and C-40 are from warm climates.
Crop management and experimental conditions
Sowing was carried out in 200-cavity polystyrene trays, and the seedlings were transplanted 30 days after sowing (das) in polyethylene bags (40 x 50 cm) containing a mixture of peat moss (Sunshine® No. 3) and tezontle (30:70 v/v). The nutrient solutions used during crop development were: Hoagland and Arnon (1950) in the vegetative stage, Steiner (1984) in the flowering and fruit set stage, and Resh (1981) in the fruit ripening stage. In all cases, the solution was maintained at a pH between 5.5 and 6.0.
In total, 20 plants of each genotype were established in a tunnel-type greenhouse with overhead ventilation and covered with milky white polyethylene, which was subsequently used for the control temperature condition (C). The plants remained in this greenhouse from sowing until anthesis of the fourth cluster; at that time, half of the plants were moved to another similar greenhouse, but equipped with heating to generate the high temperature (HT) condition and obtain a higher daytime (from 7:00 to 19:00 h) temperature. The average maximum temperature was 38 ºC for HT and 34.2 ºC for C (Figure 1). The air temperature inside the greenhouses was recorded with Hobo® sensors (Onset Computer Corporation, USA).
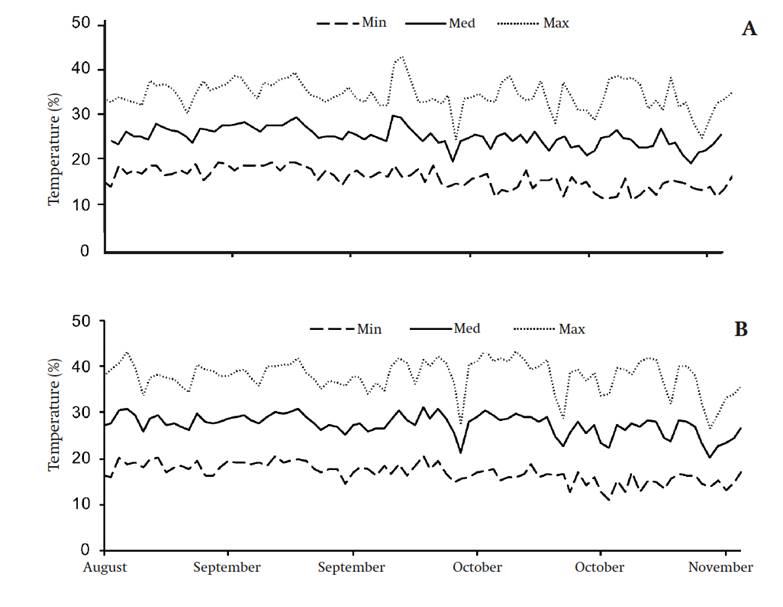
Figure 1 Daytime (7:00 to 19:00 h) temperature behavior within greenhouses for control (A) and high temperature (B) treatments during development of the fourth-cluster fruit.
The flower clusters were pruned to have only six fruit in each plant. The seeds were obtained from the fourth-cluster fruit. Fruit harvesting was carried out at maturity level 6 (where more than 90 % of the fruit surface is red) (Wan, Toudeshki, Tan, & Ehsani, 2018). Seed separation was done manually in 20 fruit of each genotype taken at random, for which the fruit were left to ferment for 48 h, washed with running water to remove mucilage residues and allowed to dry at room temperature (25 °C) until constant weight. They were then safeguarded in hermetically-sealed bags and stored in a jar with silica gel beads at room temperature (25 °C) for two months.
The experiment was carried out based on a factorial arrangement by means of a completely random design. The factors studied were temperature (34.2 and 38 °C) and genotype (MM, M-430, Y-25 and C-40), giving a total of eight treatments, and the variables evaluated were: number of seeds per fruit (NSF), moisture content, weight of 1000 seeds (W1000S), seed length (SL), seed width (SW), germination (G) percentage, rate of radicle emergence after accelerated aging (REAAA), electrical conductivity (EC) and percentage of empty seeds (ES).
Physical seed quality variables
A fruit was taken from the fourth cluster of six randomly-selected plants and the NSF was counted. After the seed storage period, laboratory tests were performed. The moisture content was determined in a sample of 0.5 g. The seeds were dried in a conventional oven (3480, Thelco®, USA) at 103 °C for 17 ± 1 h and the moisture content was determined by the difference between the initial and final weight.
The W1000S was estimated in four replicates consisting of eight samples (corresponding to each treatment) of 100 seeds (International Seed Testing Association [ISTA], 2012). The replicates of each treatment were averaged and the result was multiplied by 10; the result was expressed in g. To determine this variable, an analytical balance (PA2202, Ohaus®, China) with 0.001-g accuracy was used.
The SL and SW were measured in four replicates of 100 seeds by image processing with the Image J® program; for this, the seeds were scanned in a multifunction printer (HP2200, Hewlett Packard, USA), where 1200 dpi images were captured. Both variables were expressed in mm.
Seed physiological quality variables
To determine the G percentage, four replicates of 25 seeds (of each treatment) were sown in plastic boxes (11 x 11 x 3.5 cm) on a layer of paper towels, and placed in a germination chamber (SD8900, Seedburo®, USA) at 25 °C and constant light. After 14 days, the G percentage was calculated, for which the number of normal, healthy seedlings without malformations was taken into account; with this, the ES percentage was also estimated (ISTA, 2012).
The EC was determined from the average of four replicates of 50 previously-weighed seeds, which were immersed in 50 mL of deionized water for 24 h at 25 (C. After imbibition, the EC of the solution was measured with a conductivity meter (72729, Oakton®, Singapore). The measured values were expressed in μS·cm-1·g-1 of seed, to standardize the weight.
The accelerated aging test (Delouche & Baskin, 1973) was established with four replicates of 25 seeds that were placed in plastic boxes (11 x 11 x 3.5 cm) containing 120 mL of distilled water, and then subjected to 45 °C and 100 % relative humidity for 72 h in a conventional oven (3480, Thelco®, USA). After this period, the seeds were sown in sterile river sand. At the beginning of emergence, daily counts were made. With the values obtained, the seedling emergence rate index (seedlings·d-1) was calculated at 6 das (REAAA6) and at 14 das (REAAA14), according to Maguire's formula (1962).
Statistical analysis
With the data obtained, an analysis of variance and Tukey’s test (P ≤ 0.05) were performed for the main factors and their interaction. For this, Statistical Analysis System software (SAS Institute, 2003) was used. Finally, in order to normalize the data of the variables measured in percentage, prior to the analysis of variance, the results were transformed by the arc sine function √X/100.
Results and discussion
Physical characteristics of the seed produced
Seed moisture content averaged 7.3 ± 0.3 % (data not shown) in all varieties, a value that does not interfere with the rest of the physical, physiological and biochemical variables, since in dry seeds the biological processes are slower than in wet ones (Ayala-Villegas, Ayala-Garay, Aguilar-Rincón, & Corona-Torres, 2014). After harvesting, seed moisture content decreases until it is in dynamic equilibrium with the surrounding environment (Rodríguez-Burgos, Ayala-Garay, Hernández-Livera, Leal-León, & Cortez-Mondaca, 2011).
The NSF showed differences (P ≤ 0.01) between genotypes and temperature conditions, as well as in their interaction (P ≤ 0.05). For the other physical quality variables, the results of the analysis of variance showed highly significant effects on the part of the genotypes, temperatures and their interaction (Table 1).
Table 1 Mean squares and level of statistical significance of the analysis of variance of the physical and physiological quality variables of the seeds of three native tomato varieties and one commercial one, produced under two contrasting temperatures.
| SV1 | DF | NSF | W1000S | SL | SW | G | ES | EC | REAAA6 | REAAA14 |
|---|---|---|---|---|---|---|---|---|---|---|
| V | 3 | 22751.8** | 1.46** | 0.17** | 0.29** | 121.8** | 17.8 ns | 110.4** | 1.84 * | 2.9 * |
| T | 1 | 31161.5** | 0.19** | 0.56** | 0.31** | 144.5** | 0.5 ns | 37.4** | 3.42 * | 2.33 ns |
| V*T | 3 | 5054.3* | 0.07** | 0.08** | 0.11** | 92.5** | 5.8 ns | 56.8** | 0.19 ** | 0.34 ns |
| Error | 24 | 1517.2 | 0.004 | 0.01 | 0.01 | 22.5 | 7.5 | 5.9 | 0.17 | 0.64 |
| CV | 38.8 | 1.9 | 2.2 | 2.8 | 5.2 | 115.3 | 14.2 | 39.21 | 22.93 |
1SV = source of variation; DF = degrees of freedom; V = variety; T = temperature; CV = coefficient of variation; NSF = number of seeds per fruit; W1000S = weight of a thousand seeds; SL = seed length; SW = seed width; G = germination; ES = empty seeds; EC = electrical conductivity of the imbibition solution; REAAA = rate of radicle emergence after accelerated aging at 6 and 14 days; ** = highly significant (P ≤ 0.01); * = significant (P ≤ 0.05); ns = not significant.
The MM and M-430 varieties had the highest NSF (145 and 116 seeds·fruit-1, respectively), while Y-25 had only 50 seeds·fruit-1. The HT treatment was statistically higher in the NFS (133 seeds·fruit-1) than the C (77 seeds·fruit-1) (Table 2).
Table 2 Comparison of means by independent study factor for number of seeds per fruit, and physical and physiological quality variables of the seeds of three native tomato varieties (M-430, Y-25 and C-40) and one commercial one (MM), produced in two contrasting temperatures.
| SV1 | NSF (seeds·fruit-1) | W1000S (g) | SL (mm) | SW (mm) | G (%) | EC (µS·cm-1·g-1) | REAAA6 (seedlings· d-1) | REAAA14 (seedlings·d-1) |
|---|---|---|---|---|---|---|---|---|
| Variety | ||||||||
| MM | 145.0 az | 3.12 c | 3.93 a | 2.92 c | 89.0 b | 17.96 a | 0.25 b | 2.76 b |
| M-430 | 115.6 ab | 3.94 a | 3.80 a | 3.12 b | 92.5 ab | 20.13 a | 1.25 a | 3.72 ab |
| Y-25 | 49.7 c | 3.20 b | 3.99 a | 3.26 a | 89.5 b | 18.40 a | 1.52 a | 3.15 ab |
| C-40 | 91.1 b | 2.97 d | 3.68 b | 2.84 c | 97.5 a | 11.64 b | 1.18 a | 4.36 a |
| LSD | 39.3 | 0.087 | 0.119 | 0.118 | 6.54 | 3.337 | 0.681 | 1.324 |
| Temperature | ||||||||
| C | 76.75 b | 3.25 b | 3.76 b | 2.93 b | 94.3 a | 15.96 b | 0.68 b | 3.19 a |
| HT | 132.93 a | 3.41 a | 4.02 a | 3.13 a | 90.0 b | 18.11 a | 1.43 a | 3.81 a |
| LSD | 20.93 | 0.046 | 0.063 | 0.062 | 3.461 | 1.465 | 0.357 | 0.694 |
1SV = source of variation; NSF = Number of seeds per fruit; W1000W = weight of a thousand seeds; SL = seed length; SW = seed width; G = germination; EC = electrical conductivity of the imbibition solution; REAAA = rate of radicle emergence speed after accelerated ageing at 6 and 14 days; C = control temperature; HT = high temperature; LSD = least significant difference.
ZMeans with the same letter within each column do not differ statistically (P ≤ 0.05).
The W1000S was statistically different in all genotypes, where the heaviest seed was that of M-430 (3.94 g) and the lightest was that of MM (3.12 g). As for temperature, the W1000S was 0.16 g higher in the HT condition than in the C. As for SL, the MM (3.93 mm), M-430 (3.80 mm) and Y-25 (3.99 mm) varieties did not differ statistically and were superior (P ≤ 0.05) to C-40 (3.68 mm). This last variety had the smallest seed, and it also had the smallest (P ≤ 0.05) SW (2.84 mm) together with the MM variety (2.92 mm). Also, SW was 0.2 mm longer in the HT condition than in the C (Table 2).
Seeds produced under HT conditions showed, on average, an increase in W1000S in most genotypes. Similarly, under this condition a longer SL (P ≤ 0.05) was observed, with the exception of C-40 (Table 3). Only in the C-40 and Y-25 native varieties did SL not suffer statistically significant effects due to the temperature treatments. In general, this variable is considered to be an important characteristic for assessing seed quality. In some species it can be a parameter to measure vigor, as bigger, bulkier seeds are considered to contain more reserves, so they are likely to express greater germinative power than smaller seeds (Pepper, Corbett, & Kang, 2002).
Table 3 Comparison of means in the varieties x temperature interaction for the physical and physiological quality variables of the seeds of three native tomato varieties (M-430, Y-25 and C-40) and one commercial one (MM), produced under two temperatures contrasting.
| Treatment | NSF1 (seeds·fruit-1) | W1000S (g) | SL (mm) | SW (mm) | EC (µS·cm-1·g-1) | REAAA6 (seedlings·d-1) | REAAA14 (seedlings·d-1) |
|---|---|---|---|---|---|---|---|
| MM C | 123.0 abz | 3.03 de | 3.69 d | 2.68 d | 15.50 bcd | 0.50 bc | 2.50 b |
| MM HT | 167.0 a | 3.22 c | 4.16 a | 3.15 ab | 20.43 ab | 0.0 c | 3.02 ab |
| M-430 C | 97.9 bc | 4.00 a | 3.78 cd | 2.98 bc | 15.98 bcd | 1.47 ab | 3.56 ab |
| M-430 HT | 133.3 ab | 3.89 a | 4.18 a | 3.27 a | 24.28 a | 1.04 ab | 3.89 ab |
| Y-25 C | 46.0 c | 3.11 cd | 3.92 bc | 3.20 a | 19.73 ab | 2.11 a | 2.99 ab |
| Y-25 HT | 53.4 c | 3.46 b | 4.06 ab | 3.31 a | 17.08 bc | 0.93 bc | 3.31 ab |
| C-40 C | 40.1 c | 2.88 e | 3.65 d | 2.88 cd | 12.60 cd | 1.64 a | 3.70 ab |
| C-40 HT | 142.0 ab | 3.07 d | 3.7 d | 2.80 cd | 10.68 d | 0.73 bc | 5.02 a |
| LSD | 65.9 | 0.148 | 0.202 | 0.201 | 5.60 | 1.16 | 2.26 |
1NSF = Number of seeds per fruit; W1000W = weight of a thousand seeds; SL = seed length; SW = seed width; G = germination; EC = electrical conductivity of the imbibition solution; REAAA = rate of radicle emergence after accelerated ageing at 6 and 14 days; MM = Moneymaker; M-430 = Malinalco; Y-25 = Yucatán; C-40 = Campeche; C = control temperature; HT = high temperature; LSD = least significant difference.
ZMeans with the same letter within each column do not differ statistically (P ( 0.05).
Seed size has sometimes been shown to affect germination, emergence rate, and the success of plant establishment, growth, and development (Bewley & Black, 1994). However, larger seeds do not necessarily produce more vigorous seedlings. In general, there are more accumulated reserves in the seed than are necessary to germinate. When the germination process concludes, the cotyledons or endosperm leave reserves that would only be used in stressful or severe germination conditions (Baskin & Baskin, 2014). That is why small seeds can produce seedlings as vigorous as those of large seeds (Ayala-Garay, Pichardo-González, Estrada-Gómez, Carrillo-Salazar, & Hernández-Livera, 2006).
The variables seed weight and seed size are closely related, which could be observed in the HT condition. According to Taiz, Zeiger, Møller, and Murphy (2015), the speed and magnitude of physiological processes in plants increase as the temperature rises to a maximum, after which they tend to decrease; this is called the maximum physiological temperature, and its value may be different for each plant process. It is possible that in the present experiment the temperature was not high enough to exceed the physiological maximum and cause damage to the seed formation and growth processes; therefore, there was no decrease in physical attributes, including NSF, but it caused an increase with respect to the C. On the other hand, according to Gautier et al. (2008) and Florido-Bacallao and Álvarez-Gil (2015), plants exposed to moderately high temperatures in an initial stage acquire a certain tolerance, which is known as acquired thermotolerance. It is possible that in this research there was a period of acclimatization that caused thermotolerance in the plants.
Physiological seed quality
The analysis of variance showed that the G percentage and EC were highly significant due to the effect of genotype, temperature and their combination, whereas in REAAA6 only the effect of the genotype x temperature interaction was highly significant; the opposite occurred in REAAA14, where the effect of this interaction was not significant (Table 1). On the other hand, the effect of the treatments and their interaction was not significant for the ES percentage.
The G percentage was statistically higher under the C than in the HT condition (Table 2). The MM (89 %) and Y-25 (89.5 %) varieties had the lowest values and C-40 had the highest value (97.5 %). The C-40 G value was statistically similar to the M-430 one.
In Figure 2, it can be seen that the MM, M-430 and Y-25 genotypes, produced under HT, had G percentages lower than 90 %, while in the seeds of the C-40 native variety, the HT did not affect germination. This may be due to the fact that the C-40 variety is native to a warm-humid region (Campeche) in southeastern Mexico, so it can adapt to HT conditions, although the same did not happen with the Y-25 native variety, which is also native to tropical regions.
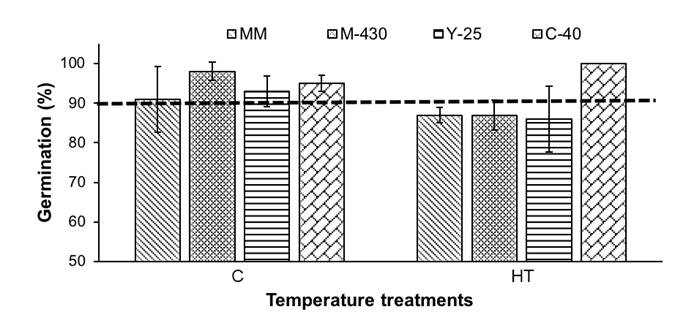
Figure 2 Germination percentage of three native varieties and one commercial one, produced under two temperature conditions. C = control temperature (34.2 °C), HA = high temperature (38 °C). MM = 'Moneymaker', M-430 = 'Malinalco 430', Y-25 = 'Yucatán 25', C-40 = 'Campeche 40'. The vertical bars represent the standard deviation (n = 4).
The above results show that the variability found in plant genetic resources, in this case represented by the three native varieties, can mitigate the effects of global climate change, as indicated by the FAO (2015). The only genotype in which neither its physical characteristics (Table 3) nor its germination were altered was the native variety C-40 (Figure 2), so it would be recommended as a candidate to resist HT.
When considering the effect of HT on the physical quality variables (weight and size) of the seeds and their behavior in G, an opposite effect is shown. In general, the HT increases the value of the physical characteristics of the seed, but decreases that of G. As mentioned earlier, each plant process could have a different maximum physiological temperature (Taiz et al., 2015). Therefore, here it would be shown that the reserve accumulation process, which results in the weight of the seed, is less susceptible to HT than the acquisition of germinative capacity. According to Otho, Stone, and Harada (2007), seed development is divided into three main phases: histodifferentiation, reserve accumulation and acquisition of tolerance to drying, and to achieve the highest seed quality the three phases must proceed optimally (Bewley, Bradford, Hilhorst, & Nonogaki, 2013).
In this work, the value of the correlation between the W1000S and G values was -0.15ns (data not shown), which indicates null correlation between these variables. On the other hand, the EC of the MM and M-430 genotype seeds produced under HT had higher values compared to those obtained with the C (Table 3). This means that these seeds had greater membrane permeability, which is equivalent to a lower physiological potential (Soto-González & Valiengo-Valeri, 2011), and would explain the low germination potential of the other varieties compared to C-40, which showed lower membrane permeability, both in the HT (10.68 µS·cm-1·g-1) and C (12.60 µS·cm-1·g-1) treatments. Likewise, this genotype was one of those with the highest germinative power (Figure 2), revealing the inverse relationship between the seed’s EC and its germinative power.
Low germination values can be explained by the loss of membrane permeability. Dumas, Dadomo, Di Lucca, and Grolier (2003) mention that the antioxidant activity inside fruit developed in HT increases, which affects the stability of cell membranes. As a result of the lower structure and selectivity of these membranes, seeds of lower physiological potential release a greater concentration of leached ions (Filho, Carvalho, Cícero, & Demétrio, 1987). The value of the correlation (r2) between the G and EC values was -0.73*; this could be due to the fact that the HT influenced the structure of the seed membranes, which altered the EC and reduced the germinative capacity (data not shown).
Regarding seed vigor, in the REAAA variable there were differences (P ≤ 0.05) between genotypes (Table 2). The MM variety germinated slower than the rest, and its values re lower at both 6 and 14 days, although on the second date the value obtained is statistically similar to that of Y-25 and M-430. According to the recommendations published by ISTA (2012), the duration of the germination test in tomato should be 14 days; however, in the present experiment, when performing the daily counts, differences were observed that were lost over time. When comparing the results of REAAA6 and REAAA14, it was observed that the HT caused a statistically higher value in the first determination (Table 2).
Thus, the seeds of the genotypes that grew in HT germinated faster, which could indicate that, under this condition, the seeds acquire greater tolerance to extreme temperature conditions, although this aspect will have to be studied in later research. Although the germination process is an event that is highly related to internal (state of maturity) and external seed factors, it is said that a seed is mature when it reaches its full development, both morphological and physiological (Taiz et al., 2015). Relative humidity and temperature are among the external factors involved in germination (Bewley et al., 2013), as was the case in this study.
Conclusions
The average maximum temperature of 38 °C favored the physical characteristics (number of seeds per fruit, weight of 1000 seeds, and seed length and width), as well as the emergence rate at seven days. However, this treatment increased the electrical conductivity by 13 % and showed germination percentages below 90 %; that is, there is an inverse correlation between the latter two.











 text in
text in 


