Introduction
Lettuce (Lactuca sativa L.) is one of the most used vegetables for food preparation due to its content of water, polyphenols, carotenoids, fiber, Ca, Fe, K, and vitamins A, C and E (Vargas-Arcila et al., 2017). According to data from the Food and Agriculture Organization Corporate Statistical Database (FAOSTAT, 2014), global production of lettuce in 2014 was 24,976,317 t, with China being the main producer with 13,657,570 t, followed by the United States with 3,791,140 t; Mexico and Chile rank 9th and 19th with 406,678 and 89,544 t, respectively. The Ministry of Agriculture of Colombia recorded that lettuce production in this country went from 23,038 t in 2007 to 83,643 t in 2014, increasing the planted area three times.
The lettuce crop is severely damaged by attacks by pests such as Trialeurodes vaporariorum (whitefly) and Milax gagates (slugs), which affect production and quality, causing great economic losses. M. gagates and other gastropods are vectors of parasites such as Angiostrongylus costaricensis, A. cantonensis, Angiostoma margaretae and Criptosporidium parvum, which cause various diseases in humans (Neira, Muñoz, Stanley, Gosh, & Rosales, 2010).
The methods used to manage slugs in lettuce crops include cultural, biological and chemical control, which partially or totally eliminate the pest. As a biological control, the Phamarhabditis hermaphrodita nematode is mainly used, and the most-used chemical options are molluscicides (metaldehydes and carbamates) that cause environmental damage due to their high toxicity (Garavano et al., 2013).
Biopesticides offer useful alternatives for pest control, such as their specificity and harmlessness to the environment and human beings. Bacillus thuringiensis is the most widely used biocontroller and accounts for approximately 90 % of the biopesticide market. During sporulation, B. thuringiensis produces proteic-type parasporal crystalline inclusion with insecticidal activity called Cry proteins (Hung et al., 2016). This bacterium acts mainly on the orders Lepidoptera, Diptera, Coleoptera, Hymenoptera, Homoptera, Orthoptera and Mallophaga, although activity has also been reported against nematodes, mites and some tumor lines (Rojas-Arias, López-Pazos, & Chaparro-Giraldo, 2013; Bravo et al., 2017).
In general, about 950 different genes have been identified for toxins, distributed in different groups: 74 of Cry proteins, 3 of Cyt proteins and 3 of Vip proteins (Crickmore, 2017). Cry proteins are classified according to the primary amino acid sequence (Crickmore, Bone, Williams, & Ellar, 1995). Several studies have shown the insecticidal activity of Cry1 proteins on Lepidoptera, and other orders of insects and gastropods (Gao, Li, Dai, Wu, & Yi, 2008; van Frankenhuyzen, 2013).
Due to the negative environmental impact caused by the use of chemicals for the management of M. gagates in the cultivation of L. sativa, the implementation of other control alternatives is sought; therefore, the aim of this research was to evaluate the toxic effect of B. thuringiensis isolates, identified with Cry1 genes, on M. gagates through a bioassay.
Materials and methods
Biological material
Seventy-five soil samples were taken from the municipalities of Cúcuta, El Zulia, Los Patios, San Cayetano and Villa del Rosario, Norte de Santander, Colombia, which presented a clay-sandy texture and a pH between 4.3 and 9.8. From the soil samples, 236 colonies were isolated that showed morphological characteristics corresponding to B. thuringiensis: flattened, circular colonies of 6 to 8 mm in diameter, with an irregular, lobed and arborescent border, soft consistency, opaque/matt appearance, creamy white pigmentation and bacteria with morphology pertaining to straight bacilli with round or blunt ends (Galvis-Serrano, 2013).
Of the total isolates, 172 colonies were selected as Gram positive, of which 58 had non-deforming cylindrical or ovoid spores of the body of the bacillus in terminal or subterminal position and with the presence of parasporal crystal. The 58 isolated colonies were identified as B. cereus by the BBL™ CRYSTAL™ Gram positive/GP system. B. cereus and B. thuringiensis are biochemically identical and differ only in the production of parasporal crista, so we used T3 medium, which contains per liter 3 g of tryptone, 2 g of tryptose, 1.5 g of yeast extract, 6.9 g of sodium phosphate and 0.005 g of MnCl (Khodabandeh, Safaralizadeh, Safavi, & Aramideh, 2014), for the release of spores and crystals of all isolates and controls used in the bioassay.
For the bioassay, 1,000 M. gagates individuals were collected and the 58 isolated colonies of B. thuringiensis (Bt1 to Bt58) identified microbiologically and biochemically were used. As a positive control, the B. thuringiensis var. Kurstaki strain isolated from the commercial product Turilav® was used.
Isolation of B. thuringiensis
To isolate the bacterium, 500 mg of soil were added to 10 mL of sterile distilled water and incubated at 65 °C for 30 min with vigorous stirring. Subsequently, 100 μL of each sample were inoculated in solid LB medium (10 g of tryptone, 10 g of NaCl, 5 g of yeast extract and 15 g of agar per liter) and incubated at 28-30 °C for 24 h (Galvis-Serrano, 2013). The grown colonies were examined and selected by their appearance and morphology by observation under a stereoscopic microscope. The bacillary shape, distention of the sporangium, location, morphology of the spore and presence of parasporal crystal were determined by differential staining with malachite green.
Molecular identification
The DNA of the isolates and the positive control was obtained with the UltraClean® Microbial DNA Isolation kit from the MoBio company. For the molecular identification of Cry1 genes by means of polymerase chain reaction (PCR), the primers proposed by Djenane et al. (2017) were used: Un1(f) CATGATTCATGCGGCAGATAAAC and Un1(r) TTGTGACACTTCTGCTTCCCATT. The final volume of the mixture for PCR was 50 μL, and contained 1X of DNA polymerase buffer, 200 μM of dNTP, 1 U of DNA polymerase, 1 μM of each primer and 2 μL of DNA. The amplification conditions were: 95 °C for 5 min for initial denaturation, followed by 30 cycles of 95 °C for 1 min, 60 °C for 30 s and 72 ° C for 1 min, and a final extension at 72 °C for 7 min. The PCR products were visualized on 1 % agarose gels stained with ethidium bromide.
Bioassay
Maintenance of M. gagates. Twenty-five polypropylene boxes (17 x 10 x 10 cm) with perforated lids covered with mesh cloth were used. Each box contained 70 g of sterile moist soil and 30 g of fresh lettuce (L. sativa var Capitata) replaced every 48 h. Between 30 to 50 slugs were placed in each box. The average temperature of each box was 25 °C.
Daily diet. A test was conducted to determine the daily lettuce intake of M. gagates, for which 10 polystyrene (icopor) containers with perforated lids covered with mesh cloth were used. In each container, 20 g of sterile moist soil, 4 g of lettuce per day and one individual of M. gagates, weighing approximately 800 mg, were added; each container was kept at 25 °C on average. The lettuce was weighed every 24 h for seven days to establish the amount consumed.
Bacterial formulation. To obtain the spore-crystal complex, the PCR-identified isolates of the Cry1 genes and the control B. thuringiensis var Kurstakii were used, inoculated in 100 mL of T3 medium (Khodabandeh et al., 2014) and placed under stirring for 10 days at 30 °C. Subsequently, the culture was centrifuged at 3,500 g for 15 min and three consecutive washes were carried out with 1.5 M NaCl, eliminating the supernatant at each opportunity. The final precipitate was frozen and lyophilized. To carry out the bioassays, the lyophilisate was resuspended in sterile distilled water until reaching a concentration of 500 μg∙mL-1 (Carmona, 2002).
Toxicity test. There are reports with different concentrations of product from B. thuringiensis isolates with Cry1 genes, where the most widely used ranges are from 2 to 2,000 μg∙mL-1 and from 0.5 to 2,000 μg∙cm-2 of spore-crystal complex (Galvis-Serrano, 2013; Ibarra et al., 2003; Monnerat et al., 2007; Peña et al., 2006; Pitre, Hernández-Fernández, & Bernal, 2008; Schünemann, Knaak, & Fiuza, 2014). According to the previous information, four concentrations of B. thuringiensis lyophilisate (125, 250, 375 and 500 μg∙mL-1) were tested in this work to evaluate their toxic effect on M. gagates. Two bioassays were performed at different times for each isolate and control.
No published data proving the toxic effect of B. thuringiensis on M. gagates were found; however, there are studies on the toxicity of B. thuringiensis on other slug species and snails using commercial preparations at concentrations of 0.2 to 2 g∙L-1 with lethality between 0 and 100 % (Abd-El-Ghany & Abd-El-Ghany, 2017; Kienlen, Gertz, Briard, Hommay, & Chaufaux, 1996; Osman & Mohamed, 1991; Zurbrügg & Nentwig, 2009).
For each isolate, at each of the four concentrations, 20 M. gagates individuals (10 to determine the toxic effect and 10 as controls), each weighing approximately 800 mg, were placed individually in polystyrene containers with perforated lids covered with mesh cloth. Each box contained 20 g of sterile moist soil and 4 g of lettuce, impregnated with 4 mL of suspension of the lyophilisate or 4 mL of water for the control. Mortality was recorded every 12 hours for seven days and a Probit regression was performed (Finney, 1971) using the IBM SPSS version 23 statistical package (2014) to determine the dose that would produce 50 (LD50) and 99 % (LD99) mortality.
Results and discussion
PCR and gene sequencing are effective tools for identifying and finding new Cry genes in B. thuringiensis that could be used as potential biopesticides (Vázquez-Ramírez, Rangel-Núñez, Ibarra, & del Rincón-Castro, 2015). Therefore, the determination of Cry1 genes in the isolates and the B. thuringiensis control was carried out using the specific primers Un1(f) and Un1(r) (Djenane et al., 2017; Gorashi, Tripathi, Kalia, & Gujar, 2014; Salama, Abd-El-Ghany, & Saker, 2015; Zothansanga, Kumar, & Gurusubramanian, 2011), and the expected product was observed in five isolates (Bt2, Bt23, Bt35, Bt39 and Bt42) and in the commercial strain B. thuringiensis var Kurstaki (Figure 1). The difference in the concentration of the amplified product is probably due to the amount of template used in the PCR or to the number of copies of Cry1 genes present in the bacteria, since the primers used generate different fragments with a size between 274 to 277 bp.
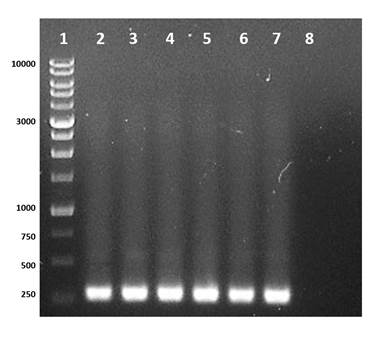
Figure 1 Identification of the Cry1 gene in Bacillus thuringiensis with primers Un1(f) and Un1(r). Line 1: molecular weight marker 1Kb DNA Ladder RTU (range between 250 to 10,000 bp); line 2-6: isolates Bt2, Bt23, Bt35, Bt39 and Bt42 of B. thuringiensis where the expected DNA fragment is observed between 274 to 277 bp; line 7: control B. thuringiensis var. Kurstaki; line 8: negative control.
The buccal apparatus of M. gagates has well-developed jaws that allow it to destroy large amounts of plant material. They feed on all kinds of crops, such as corn, wheat, soybean, sunflower, lettuce, cabbage and celery. During the day they remain hidden underground protecting themselves from drying out and at night they go out in search of food, moving up to 5 m and managing to consume up to 50 % of their live weight (Serre, 2005). For this reason, in the establishment of the M. gagates hatchery, a daily intake of 400 mg per individual was expected; however, an average consumption of 250 mg of lettuce per day was obtained. This could be because they were not in their natural habitat and the characteristics of the hatchery did not fully recreate the temperature, moisture and light required for the development of M. gagates, which shows its maximum activity at night under high moisture concentrations (Córdoba-Vargas & León-Sicard, 2010).
In the bioassay, using the 500 μg∙mL-1 concentration, the isolates Bt23 and Bt39 and the control B. thuringiensis var. Kurstaki presented 100 % lethality in M. gagates with an exposure of five to seven days. With the rest of the concentrations, the observed lethality results were: with 375 μg∙mL-1 from 70 to 80 %, with 250 μg∙mL-1 from 50 to 60 % and with 125 μg∙mL-1 from 15 to 30 % (Figure 2). All records were taken seven days after exposure of M. gagates to the toxin. Death was evidenced by the blackening of the lower abdomen and the bending of the slug (Figure 3). No mortality was observed in the negative controls used.
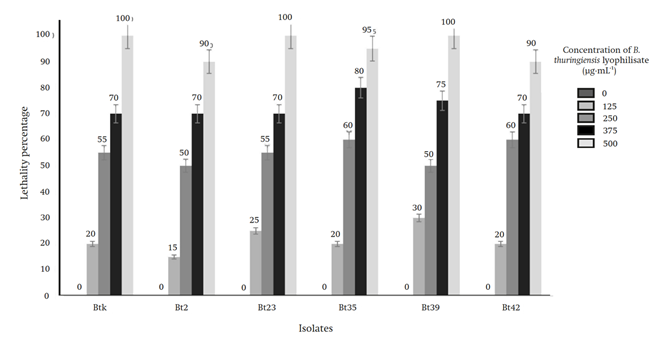
Figure 2 Lethality results of the isolates (Bt2, Bt23, Bt35, Bt39 and Bt42) and the control B. thuringiensis (Btk) at different concentrations on M. gagates after seven days of exposure.

Figure 3 Toxic effect of B. thuringiensis on M. gagates at five days of exposure using 500 μg∙mL-1 of lyophilizate from isolate Bt39.
The Probit analysis allowed establishing the LD50 and LD99 of the isolates and control at 310 and 820 μg∙mL-1, respectively (Table 1). No significant differences were observed between the isolates and the positive control at each concentration used. This suggests the possible relationship between the molluscicidal effect and the presence of Cry1 genes in the isolates of B. thuringiensis and the control B. thuringiensis var. Kurstaki.
Table 1 Probit analysis to determine the LD50 and LD99 of the results obtained in the bioassays using isolates and the control Bacillus thuringiensis var. Kurstaki on M. gagates.
| B. thuringiensis (Bt) | 95 % confidence limits for concentration | |||||
| LD50 | LD99 | |||||
| Estimate (μg∙mL -1 ) | Lower limit | Upper limit | Estimate (μg∙mL -1 ) | Lower limit | Upper limit | |
| Bt var. Kurstaki | 260 | 200 | 320 | 690 | 550 | 1040 |
| Bt2 | 310 | 240 | 370 | 820 | 650 | 1260 |
| Bt23 | 280 | 210 | 340 | 740 | 580 | 1090 |
| Bt35 | 260 | 200 | 320 | 710 | 560 | 1050 |
| Bt39 | 270 | 200 | 330 | 730 | 580 | 1070 |
| Bt42 | 290 | 220 | 350 | 780 | 620 | 1170 |
Efficient control strategies are necessary if one considers that slugs cause damage to the foliage and roots of lettuce plants as high soil moisture, high organic matter content, low brightness and high planting density favor their populations and cause crop losses of up to 100 % (France, Gerding, Céspedes, & Cortez, 2002). Chemical management of this pest uses toxic baits composed of a food attractant and an active ingredient (carbamates or metaldehydes), which are fast and effective in the control of slugs. Godan (1983) reported that the genus Milax sp. can present resistance to molluscicides. Additionally, the use of these chemicals can be toxic to other organisms such as mammals, birds, fish and insects (France et al., 2002). In mammals, it is known that the intake of metaldehyde can cause depression of the central nervous system and, depending on the amount ingested, death (Córdoba-Vargas & León-Sicard, 2010). Consequently, control methods less harmful to the environment are required, such as the use of natural enemies of slugs (insects and nematodes) and the study of traditional biopreparates such as B. thuringiensis.
Gao et al. (2008) report pesticidal activity against the snail (Oncomelania hupensis) of a B. thuringiensis isolate, containing the Cry1A and Cry1C genes, with a lethality percentage above 90 %. The Cry1 genes are the most common in nature, being the most abundant in various molecularly characterized strains of B. thuringiensis (Arrieta, Hernández, & Espinoza, 2004; Gao et al., 2008; Jain, Sunda, Sanadhya, Nath, & Khandelwal, 2017). Van Frankenhuyzen (2013) reports the activity of Cry1 proteins across different orders and found toxicity in Lepidoptera, Coleptera and Diptera; in this review, the activity across classes and phyla of Cry proteins is also presented. This could explain the toxic effect observed in this study, where the B. thuringiensis isolates and the positive control with Cry1 genes showed over 90 % mortality against M. gagates, at a concentration of 500 μg∙mL-1 of lyophilisate from the sporulated culture. The LD99 calculated in this study suggests the use of 820 μg∙mL-1 to achieve 100 % lethality with any of the isolates tested.
In the field, greater slug voracity in the lettuce crop is expected since they are in their natural habitat; however, with a correct formulation and dose of the biopreparate, an effective biological control of this pest would be expected.
Conclusions
This study allows us to conclude that B. thuringiensis, by presenting Cry1 genes, not only has an insecticidal effect, but also causes lethality in gastropods such as M. gagates, thus offering a biological alternative, harmless to humans, for the control of this type of pest that considerably affects lettuce crops.











 texto en
texto en 


