Introduction
Stevia rebaudiana, known as the sweet herb of the family Asteraceae, produces a natural sweetener in its leaves due to the presence of steviol glycosides (stevioside and rebaudioside) that account for 10 to 20 % of the leaf’s dry weight (Shivanna, Mahadev, Khanum, & Kaul, 2013). In addition to being a calorie-free sweetener, the medicinal use of steviosides as a hypoglycemic agent, antibiotic, antioxidant, renal protector and skin care product has been documented (Shivanna et al., 2013; Thiyagarajan & Venkatachalam, 2012). In veterinary medicine, leaves are used in animal feeds to improve their development (Atteh et al., 2011).
Among the most used stevia cultivars are 'Morita II' and 'Silvestre', both with sweet characteristics. The former has about 10 to 12 % rebaudioside "A" and 2 to 4 % steviosides, which gives the plant a softer and sweeter taste than other species. In 'Silvestre' the leaves have 12 % steviol glycoside yields (Thiyagarajan & Venkatachalam, 2012).
There is a high worldwide demand for stevia raw material for use in the treatment of mainly obesity and diabetes. Therefore, it is necessary to have a methodology that allows increasing its multiplication rate for commercial production, since the planting density in the field is approximately 70,000 plants∙ha-1 (Casaccia & Álvarez, 2006). Although there are different ways to propagate the species, the process of reproduction by seed is not viable due to its low germination percentages and the high variability of the results (Alvarenga-Venutolo & Salazar-Aguilar, 2015; Goettemoeller & Ching, 1999).
Another method is asexual reproduction, which is effective, cheap and produces homogeneous material that preserves the characteristics of the mother plant (Vilchez & Albany, 2014). However, if mass propagation is required, the use of in vitro tissue culture is recommended, since it allows plants to be regenerated on a larger scale by embryogenesis or organogenesis, but the production cost is higher and requires intensive manual labor (Ziv, 2005).
On the other hand, liquid media reduce production costs by not using gelling agent in the multiplication protocols (Cabrera-Jova et al., 2008). In many species, greater propagule production has been reported with the use of liquid media, since in this system the explants are in direct contact with the culture medium, which makes the taking of nutrients more effective by the explant (Berthouly & Etienne, 2005; Preil, 2005; Watt, 2012).
Among the in vitro propagation techniques, temporary immersion systems (TIS) allow overcoming the limitations of the methods described above, because they are semi-automated (Ziv, 2005). These systems are based on the contact of the explants with the liquid culture medium for short periods of time and the renewal of the gaseous atmosphere to avoid the hyperhydricity of the tissues and the accumulation of toxic gases (Basail-Pérez et al., 2012).
It has been documented that the longer contact time, between the micro-cuttings and the culture medium, increases the supply of nutrients and growth regulators to the explants (Santos-Pino et al., 2011), which can maximize their development (Preil, 2005). In addition, with each immersion, the environment of the culture container is renewed in order to eliminate volatile compounds, such as ethylene (Roels et al., 2006), and promote the recirculation of carbon dioxide necessary for photosynthesis, further improving the autotrophic metabolism of the carbon in the leaves (Aragón et al., 2014).
Some researchers point out that, from a commercial point of view, conventional micropropagation has ceased to be an economically efficient process; in addition, they agree that the root cause is the use of gelling agents as supports for the explants and the high number of manual operations, which imply a high labor cost (Quiala et al., 2012). Based on these considerations, the present study aimed to scale up the in vitro production of two Stevia rebaudiana cultivars using RITA®, BIT® and SETIS®, and to evaluate their biomass production in the field.
Materials and methods
Plant material
Two Stevia rebaudiana cultivars were used: ‘Morita II’ and ‘Silvestre’. The former was a donation from the Centro de Productos Bióticos of the Instituto Politécnico Nacional, Yautepec, Morelos, Mexico. The latter was acquired in a nursery in the Costa Rican Central Valley. Both cultivars were worked with in the greenhouse of the Centro de Investigación en Biotecnología (CIB) at the Instituto Tecnológico de Costa Rica. These materials were used as mother plants for micropropagation due to their quality and health.
Although both stevia cultivars have sweet characteristics, there are physical and chemical differences between them (Figure 1). 'Morita II' has oval, light green leaves, grows better in tropical climate areas and is more demanding in terms of crop management. On the other hand, 'Silvestre' has elongated, serrated leaves and shades of darker green and adapts to different agro-environmental conditions (Thiyagarajan & Venkatachalam, 2012).
Introduction and multiplication
For its in vitro propagation, nodal segments containing axillary shoots (2 to 3 cm) of the mother plants were used as explants. For this, the explants were washed with water and antibacterial soap, then immersed in a solution of Agrimycin® (5 g∙L-1) and Benlate® (5 g∙L-1) for 60 min. After this period, they were disinfected with calcium hypochlorite (1.8 % i.a.) for 10 min and taken to the laminar flow transfer chamber.
Under aseptic conditions, calcium hypochlorite was removed and three washes were performed with sterile distilled water. Finally, the explants were placed in a sterile solution with antioxidants (citric acid and ascorbic acid, 100 g∙L-1 of each one) before being placed in the culture medium (Gerber®-type flasks of 5.5 x 5.5 cm). The material was multiplied every three or four weeks by passing each explant to a test tube (14 x 2.2 cm) with 10 mL of fresh semi-solid medium containing the base medium described by Murashige and Skoog (1962) enriched with 30 g∙L-1 sucrose, 2 mg∙L-1 calcium pantothenate (CaPa) and 0.5 mg∙L-1 gibberellic acid (GA3).
Liquid medium test
For growth in liquid medium, a medium test was carried out using the RITA® TIS (Alvard, Cote, & Teisson, 1993) with 200 mL of culture medium. For this test the cultivar 'Morita II' was used with one month of in vitro subculture. Each RITA® was inoculated at a density of 10 mL per explant. Different concentrations of sucrose, kinetin (K, as growth regulator) and a microbiological suppressant (methylisothiazolone [2-methyl-4-isothiazolin-3-one, MIT] and methylchloroisothiazolinone [5-chloro-2-methyl-4- isothiazolin-3-one, CIT], Chemical Reagents Gamma, Laboratorios ARVI S.A.) were added to the base medium (Table 1). Each trial was performed in triplicate and evaluated after three weeks. The variables studied were elongation (cm), greenhouse survival and morphological response of the plant to each culture medium (number of leaves and number of shoots).
Temporary immersion systems
For the massive propagation of stevia, three TIS were evaluated: RITA® (Alvard et al., 1993), BIT® (Escalona et al., 1999a) and SETIS® (Duchefa Biochemie B.V.) (Figure 2). The BIT® system consists of two, 2-L culture flasks, one where the explants were placed and the other as a reservoir for the culture medium to be used. The flasks are connected to each other by a hose that goes from the lid to the bottom of the container, which allows the recirculation of the culture medium from one flask to another. In the container lid there is a hydrophobic filter (0.22 μm) to ensure the sterilization of the incoming air. In general, the BIT system is easy to operate and build, and it can be maintained for long periods allowing good ventilation and explant contact with the culture medium (Georgiev, Schumann, Pavlov, & Bley, 2014).
On the other hand, RITA® consists of a single polysulfone container with a maximum capacity of 500 mL. The lid has two holes with a hose; in one of the holes there is a hydrophobic filter (0.22 μm) attached and in the other an air opening and closing valve. A mesh is placed inside the container where the explants are established.
The SETIS® system consists of two horizontal containers, coupled one on top of the other with a capacity of 3 L. Each one has an 80-mm opening with silicone gaskets and screw caps; they are connected to each other by a hose at the bottom of the container. At their upper ends, an orifice connected to a filter allows the entry of sterile air.
The three TIS were connected to solenoid valves, which allow the entry or not of air, and these to a programmable timer with which the immersion frequency and time are defined, given the regulation by a manometer.
All systems were operated at half capacity, at a density of 10 mL per explant (Alvarenga-Venutolo & Salazar-Aguilar, 2015). The material maintained in vitro was inoculated with one month of subculture, for which explants of 0.5 to 1 cm in length (no apical buds) were introduced.
The culture conditions of each system are detailed in Table 2. The immersion time and frequency were run with the program already established in the laboratories of the Centro Nacional de Innovaciones Biotecnológicas (CENIBiot-CENAT), San José, Costa Rica, where these tests were carried out.
Table 2 Culture conditions in the different temporary immersion systems evaluated.
| RITA ® | BIT ® | SETIS ® | |
|---|---|---|---|
| Maximum capacity (L) | 0.500 | 2 | 3 |
| Work volume (L) | 0.200 | 1 | 1.5 |
| Number of explants | 20 | 100 | 150 |
| Volume of medium per explant (mL) | 10 | 10 | 10 |
| Filling time (s) | 30 | 100 | 90 |
| Immersion time (min) | 2 | 2 | 2 |
| Emptying time (s) | 0 | 170 | 135 |
| Air flow (L∙min-1) | 10 | 15 | 18 |
| Immersion frequency (h) | 12 | 12 | 12 |
Each system was evaluated in triplicate for each cultivar of Stevia rebaudiana: ‘Morita II’ and ‘Silvestre’.
Evaluation of plants obtained in temporary immersion systems
After one month of cultivation in the different systems, the plant material was removed and in each treatment the number of shoots and elongation (cm) per explant were evaluated considering that all the explants had 10 mL of medium and the same immersion time (2 min).
Acclimatization in greenhouse
Immediately after the plants obtained from the TIS were evaluated, they were taken to a greenhouse and cultivated in humidity chambers constructed with disposable cups. The substrate used was soil and rice husk (60:40), and three drops of a rooting solution (5 mg∙L-1 indolbutyric acid) were added before sowing. At the end of the acclimatization period, the survival percentage of the plants was measured based on the TIS used.
Material handling in the field
After a month of greenhouse cultivation, when the plants reached 10 to 15 cm in length, they were transported to the field. Field planting was carried out in conjunction with commercial farmers in the area of San Ramón de la Virgen de Sarapiquí, Costa Rica (10° 21’ 16.9’’ North latitude and 84° 07’ 00.79’’ West latitude, 200-400 masl). The zone has soil described as Inceptisol Dystrandep, with temperatures of 24 to 30 °C and annual precipitation of 5,000 mm.
In the field, the spacing between plants and rows was 30 and 80 cm, respectively, which resulted in a density of 65,000 plants∙ha-1. At the time of planting, the material was fertilized with organic fertilizer (cattle manure, 100 g∙plant-1); after eight days an application of the NPK formula (10:30:10, 5 g∙plant-1) was made, and after two months a second application of organic fertilizer was made. Additionally, 2 mL∙L-1 foliar fertilizer (Nitrofoska®) and 2 mL∙L-1 low potassium humic acids (Agroroots® 10-30-10) were applied.
Fifteen days after transplanting, the survival percentage was evaluated, and after three months the biomass was harvested 15 cm from the soil (aerial part) to allow the plant to re-sprout. In total, samples of 10 plants were collected by cultivating stevia (in triplicate) of the same age and origin. Subsequently, the biomass produced (stems and leaves) was determined based on fresh weight and dry weight.
Statistical analysis
The Minitab 17 program (2010), with the Kruskal-Wallis test (P ≤ 0.05), was used for the statistical analysis and comparison of means of the TIS results. The data obtained in the field were analyzed statistically with the Infostat program (di Rienzo et al., 2017) and a one-way analysis of variance was performed (P ≤ 0.05).
Results and discussion
Introduction and multiplication of in vitro material
The axillary buds proliferated after two to three weeks of culture in the base medium. Some researchers have enriched the medium with kinetin to increase the number of shoots, but at a very low concentration (Espinal-de Rueda, Delvalle, Cifuentes, & Ramia, 2006); however, like Alvarenga-Venutolo and Salazar-Aguilar (2015), in this study GA3 was used as the sole growth regulator. GA3 induces a wide variety of physiological effects, including stem elongation due to the activation of intercalary meristems (George, Hall, & de Klerk, 2008). The results obtained seem to confirm this effect, since the plants showed rapid vertical growth, taking advantage of the cultivation space. This characteristic is evident when changing the container (from flask to test tube) during the multiplication, observing greater shoot elongation and thickness. These results could be explained based on the height of the test tube, which allowed the plants to direct their growth vertically, since the culture medium was the same in both cases. These results agree with those observed by Sánchez-Chiang and Jiménez (2010), who recommend selecting the container depending on the subculture frequency and time.
Liquid medium test
Regarding the culture media evaluated using RITA®, significant statistical differences were observed among the media (P ≤ 0.002). Culture medium 1 (base medium, 30 g∙L-1 sucrose, without microbial suppressant and without cytokinin) allowed for greater elongation of the explants, followed by medium 3 (same as above but with 1 mL∙L-1 suppressant) (Figure 3). However, when the plants obtained in vitro were taken to the greenhouse, those from medium 3 had a higher survival percentage during acclimatization (92 %) than those from medium 1 (80 %), which presented the best growth in vitro (Figure 4). In addition, it was observed that the microbial suppressant allowed maintaining low bacterial contamination levels throughout the micropropation process, which, according to the results, was an advantage when it came to taking the plants to the greenhouse.
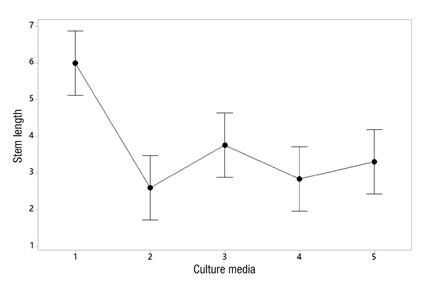
Figure 3 Effect of the culture medium (sucrose, calcium pantothenate and gibberellic acid; Murashige & Skoog, 1962) on stem length of explants of Stevia rebaudiana cv. ‘Morita II’. Medium 1: 30 g∙L-1 sucrose; medium 2: 40 g∙L-1 sucrose; medium 3: 30 g∙L-1 sucrose + microbial suppressant; medium 4: 30 g∙L-1 sucrose + kinetin; medium 5: 30 g∙L-1 sucrose + suppressant + kinetin.
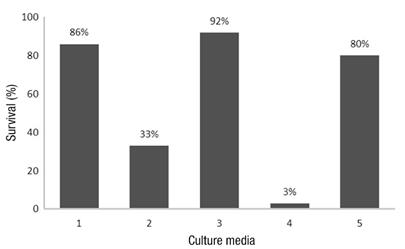
Figure 4 Survival percentages of seedlings of Stevia rebaudiana cv. ‘Morita II’ in the greenhouse. Medium 1: 30 g∙L -1 sucrose, medium 2: 40 g∙L -1 sucrose, medium 3: 30 g∙L -1 sucrose + microbial suppressant, medium 4: 30 g∙L -1 sucrose + kinetin and medium 5: 30 g∙L -1 sucrose + suppressant + kinetin.
The microbial suppressant was used because in both Stevia rebaudiana materials a gram-negative endogenous bacterium appeared in the third week, identified by DNA sequencing as Burkholderia fungorum (data not shown). This suppressant delays the appearance of contaminants in the in vitro culture, which allows not discarding a large amount of plant material and being able to take more plants to the greenhouse.
Bacteria are the most common in vitro contaminants and cause serious problems, and are also difficult to detect and eliminate (Orlikowska, Nowak, & Reed, 2017). Their distribution can be localized or systemic, by xylem or phloem, and they usually do not manifest themselves in the first subcultures, since the high osmotic pressure, pH and certain hormones of the culture media can inhibit their growth. Due to these inhibitory factors, many microorganisms require a period of adaptation to the new conditions before manifesting their presence, which usually occurs in the multiplication phase (Hernández & González, 2010).
The results of this study show that Stevia rebaudiana is not a demanding plant in terms of culture medium, since the base medium enriched with 2 mg∙L-1 CaPa and 0.5 mg∙L-1 GA3 allowed micropropagation of the explants, which coincides with the findings reported by Alvarenga-Venutolo and Salazar-Aguilar (2015). However, these results are contrary to those obtained by Sivaram and Mukundan (2003), who found the highest multiplication and growth rates using cytokinins and auxins in the culture medium. In the present study, the addition of kinetin to the culture medium caused heterogeneous growth (in rosette, hyperhydrated explants, glassy and translucent appearance of both the stems and leaves) (Figure 5), being characteristic of this growth regulator when found in high concentrations (George et al., 2008).
Temporary immersion systems
All treatments evaluated in the TIS (BIT®, SETIS® y RITA®) produced vigorous plants with low levels of hyperhydricity, a higher average number of leaves and shoots, and, in general, a higher rate of multiplication than those explants cultured in semi-solid medium.
The TIS are automated systems that base their operation on the contact of the culture medium with the explants at regular time intervals and the pumping of clean air through a filter. This method allows excess liquid in the explants to be drained by gravity at the end of the pumping and they are kept moist (Santos-Pino et al., 2011; Steinmacher, Guerra, Saare-Surminski, & Lieberei, 2011). The air flow is regulated by electronic timers that control the immersion frequency and times, and solenoid valves that allow the system to turn on or off
According to the comparison of means, 'Morita II' showed greater stem length in the plants grown in the BIT® system (11.65 cm), compared to SETIS® (9.00 cm) and RITA® (2.85 cm). Also, in ‘Silvestre’, the longest stem length was observed with BIT® (13.50 cm). In both cases, the differences were statistically significant (P ≤ 0.05) (Figure 6).
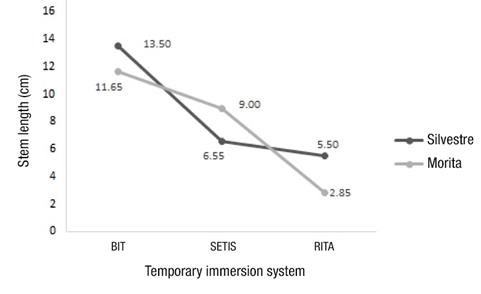
Figure 6 Stem length of two cultivars of Stevia rebaudiana, ‘Morita II’ and ‘Silvestre’, based on the temporary immersion systems evaluated.
In general, the composition of the gases in the culture vessel is influenced by the volume of the container, the volume of the medium and the ventilation (Georgiev et al., 2014). In TIS, the control of the gas phase depends on the volume of the container, the gases produced by the explants and the frequency of immersion through the air flow (Basail-Pérez et al., 2012). Additionally, Ziv (2005), by measuring the concentration of gases within TIS, showed that the concentration of O2 and CO2 in the culture container is as important.
The BIT container, which showed the best results in terms of plant elongation, presented a higher relative volume for the exchange and accumulation of gases. This is due to the fact that a vertical container is used, and the space between the explants and the upper part of the container is greater compared to that available in the other systems evaluated, allowing a greater availability of gases and space for the elongation of the explants (Figure 2).
Sprouting
In ‘Morita II’, the BIT® median was eight shoots per plant, whereas in SETIS® it was three shoots, with significant differences between the two systems evaluated (P ≤ 0.05). For ‘Silvestre’, the number of shoots in both cases was two (P = 0.42). Both cultivars presented similar seedling elongation in BIT®; however, according to the sprouting results, ‘Morita II’ produces more shoots than ‘Silvestre’.
These results seem to indicate that 'Silvestre' is more sensitive to the presence of GA3, since it induces stem elongation (George et al., 2008). Similar results were reported by Escalona et al. (1999b), who observed that GA3 promoted better shoot elongation and uniformity in pineapple (Ananas comosus).
According to Thiyagarajan and Venkatachalam (2012), the greater sprouting of stevia in solid medium is due to the use of growth regulators such as kinetin (0.5 mg∙L-1) and benzylaminopurine (1.0 mg∙L-1), achieving up to 85.7 % regeneration. The culture medium used in this research was only supplemented with GA3, and in the initial medium tests the presence of cytokinins caused negative effects, so the results seem to indicate that the sprouting effect is due to the TIS. Vilchez and Albany (2014) attribute the increase in the number of guava shoots to the type of TIS evaluated (RITA® and BIT®).
Immersion time and frequency
Immersion time and frequency are two key factors to achieve the highest multiplication coefficient and the best quality of plants. Although in this study they were not analyzed, the parameters used are recommended by certain researchers for magnophyte plants. Castro and González (2002) evaluated different immersion frequencies and times. In their case, the frequency of 12 h and 3 min of immersion showed a greater number of shoots in relation to lower frequencies and 1 min of immersion.
On the other hand, in banana cultivation under TIS, the immersion times were longer, since the morphology of the species is very different from that of stevia. In banana, immersion times of 5, 10 and 15 min were evaluated, demonstrating after 21 days that this significantly influenced all the evaluated variables, with 0 min being the time that allowed a higher multiplication coefficient, a larger pseudostem diameter and a lower degree of oxidation (Basail-Pérez et al., 2011).
At the end of this study, differences could be observed among the TIS evaluated, among which some advantages stand out, but there are also some disadvantages in terms of the use of the equipment, which can be useful for the search for improvements. The capacity of RITA® is lower compared to the other two systems evaluated, although its design allows easier introduction of the explants to the culture flask. On the other hand, the mesh where the explants are placed in both RITA® and SETIS® makes it difficult to recover the material if it has roots at the end of the multiplication period. The distribution of the culture medium is more homogeneous in RITA® and BIT®; however, the acquisition cost of BIT® is much lower. Vilchez and Albany (2014) report similar results.
Greenhouse acclimatization
After a month of cultivation in the TIS, the plants were taken to the greenhouse for acclimatization. It is important to mention that in vitro growth is heterotrophic and in vivo autotrophic. The in vitro atmosphere presents high relative humidity, low or no gas exchange, a CO2 shortage during almost the entire period, ethylene production and low photosynthetic density, which induces disturbances in plants developed under these conditions.
The plants obtained in vitro have thin leaves, weak stems, weak and little functional roots, incomplete stem-root connection and a low photosynthetic rate (George et al., 2008). Therefore, acclimatization is an important factor in the subsequent survival of the plant, since it is a critical stage in the process and is where the greatest loss of plants occurs. It is important to start the acclimatization process by gradually reducing the relative humidity to allow, in addition to stomatal closure, better cuticle formation and reduced water loss (Nava et al., 2011).
In this work, the highest plant mortality in the greenhouse occurred during the first acclimatization stage, due, in part, to the characteristics of the plants recently released from in vitro culture and the conditions of the greenhouse, whose infrastructure does not allow properly regulating relative humidity, temperature or luminosity. Another major constraint during this period was an attack by whitefly (Trialeurodes vaporariorum), which was controlled with yellow chromatic traps and the insecticide Actara® (Tiametoxam), of which 2.5 mg∙L-1 were applied every 15 days. The high sugar content in this species makes it very attractive to these insects. Once past this first stage, the plants that survived were transferred to the field. The results of this stage obtained in this research seem to indicate that the initial acclimatization conditions were not the most appropriate for this species, and that in future tests attention must be paid to the conditions given in the initial greenhouse period.
For both cultivars, regardless of the TIS used, the greenhouse survival percentages were very similar (Figure 7), although low due to the aforementioned problems. Based on the results obtained, it can be deduced that the quality of the plants obtained in vitro is good and similar in both cultivars, and that the management of the newly sown plants in the greenhouse will determine the survival percentage.
Field cultivation
Once the plants reached a length of 10 to 15 cm in the greenhouse, they were taken to the field. During field cultivation there were some problems with pests such as the leaf-cutter ant (Atta spp) and may beatles (Phyllophaga spp); however, 95 % plant survival was observed 15 days after planting. In this stage the plants showed vigorous growth and the characteristic coloration of the species (Figure 8).
Foliage production was high in both cultivars, although with statistically significant differences (P ≤ 0.05) between them, with ‘Morita II’ producing greater fresh weight (260.7 g) than ‘Silvestre’ (214.4 g). By contrast, in dry weight there were no statistically significant differences (P ≤ 0.05), both producing about 80 g of dry matter per sample (10 plants of the same age and origin, in triplicate) (Figure 9).
Conclusions
All the immersion systems evaluated allowed scaling-up and, therefore, obtaining a greater number of plants compared with the conventional semi-solid system. With the BIT system there was greater sprouting and shoot length in the cultivar 'Morita II'. In 'Silvestre' there was no significant difference in the number of shoots between the different systems, but there was greater shoot length in BIT.
It is important to pay attention to the conditions given at the beginning of the greenhouse acclimatization stage, as this is critical to ensure the survival of the plant and, therefore, obtain a greater volume of material for planting in the field. The plants in the field produced a lot of biomass, confirming not only the quality of the material planted but also the suitability of the planting site.











 texto en
texto en 








