Introduction
The potato (Solanum tuberosum L.) is the fourth most important food crop in the world, surpassed by rice (Oryza sativa L.), wheat (Triticum aestivum L.) and corn (Zea mays L.), and plays a crucial role in food security and the economic development of humanity (Zhang, Hou, Liu, Xie, & Song, 2014). Tubers are rich in carbohydrates, proteins, minerals and vitamins, as well as having certain antioxidant properties (Suttle, 2008).
Dry matter production in the potato crop is determined by a variety of different factors; one of the most influential is the duration of its growth cycle, expressed through a set of phases and phenological stages, which in turn depend on the environment and the cultivar (Kooman, Fahem, Tegera, & Haverkort, 1996). Under non-limiting conditions, temperature can drastically affect biomass production, especially when it exceeds 20 °C during the tuber bulking stage (Rykaczewska, 2015); therefore, the regulation of this factor is crucial, especially in protected agriculture.
The yield, defined as the product of the number of tubers per plant and their weight (Lynch, Kozub, & Kawchuk, 2001), is determined by the genotype, the environment and their interaction (Milton & Allen, 1995). Thus, the formation of tubers in the potato plant is a complex process that involves different biological systems induced by environmental signals, such as a short photoperiod, low temperatures and high moisture content in the soil (Cenzano, Abdala, & Hause, 2007).
The induction of tuberization occurs in the leaves through the phytochrome. Initially, gibberellic acid activates a transmissible signal to the subapical region of the stolon; this causes cellular division to generate, instead of longitudinal growth, a radial expansion (Cenzano et al., 2007; Hannapel, Chen, Rosin, Banerjee, & Davies, 2004; Viola et al., 2007) by apoplastic transport towards the apical meristem and the subapical region (Hancock, Roberts, & Viola, 2008). In this way, the development of the tuber occurs thanks to the discharge of photoassimilates in cells of the lateral parenchyma that produces widening of the subapical region due to the accumulation of proteins and carbohydrates, which are later transformed into starches (Cenzano et al., 2007; Hancock et al., 2008).
During the tuberization stage, massive starch accumulation occurs in the tuber, which represents a great demand for carbohydrates (Sabba et al., 2007); this causes the highest levels of sucrose, glucose and fructose to be recorded in young or immature tubers (Stark & Love, 2003), and as they reach physiological maturity these concentrations tend to decrease (Knowles, Driskill, & Knowles, 2009). Some research reports values of 0.2 to 1.5 % sucrose and 0.01 to 0.7 % reducing sugars in immature tubers (Kolbe & Stephan-Beckmann, 1997; Kumar & Ezekiel, 2006), while during physiological maturity, 0.1 to 0.6 % sucrose and 0.04 to 0.4 % reducing sugars have been found (Knowles et al., 2009; Kumar & Ezekiel, 2006; Sabba et al., 2007).
The exact quantification of sugars in potatoes is important for scientific and commercial purposes. The main sugars present in this tuber are sucrose, glucose, fructose (Morales-Fernández et al., 2015) and small amounts of maltose during sprouting (Ilin, Durovka, & Markovic, 1997), whose contents are affected by the genotype, phenological status, crop management, environmental factors and storage conditions (Thompson, Love, Sowokinos, Thornton, & Shock, 2008).
Based on the above, the aim of this research was to evaluate the growth and yield of four potato varieties under greenhouse conditions and to determine the soluble sugar content of this tuber at different maturity stages.
Materials and methods
The research was conducted at the Universidad Autónoma Chapingo Institute of Horticulture (19° 29’ North latitude and 98° 53’ West longitude, at 2,250 masl), between October (2008) and February (2009), under greenhouse conditions.
The commercial potato varieties studied were Alpha, Atlantic, Mondial and Vivaldi. At planting, minitubers of 23 to 32 mm in diameter in a physiological state of apical dominance were used. Two minitubers of each variety were established in black, 6-L polyethylene bags, with a substrate composed of a peat moss-perlite mixture (1:1 v/v), at a depth of 10 cm. The bags were placed with a separation of 30 and 10 cm between rows and lines, respectively.
At planting, 1.2 g of the mixture of N, P, K, Ca and Mg, with 34, 31, 27, 4 and 4 %, respectively, were applied to the substrate of each bag. During the crop cycle, irrigation was applied manually to field capacity, according to the crop's water needs. From tuber emergence to physiological maturity, the maximum and minimum air temperature was recorded daily (Figure 1) with a Taylor® model 5458 mercury column thermometer; with these data the average temperature was obtained. The maximum temperature fluctuated between 27 and 45 °C, and the minimum temperature between 1 and 11 °C (Figure 1). Temperatures above 30 °C and below 10 °C were observed in the growing season, but had a short duration through one day.
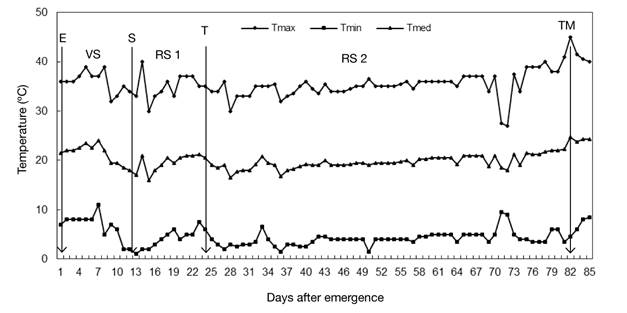
Figure 1 Air temperature recorded during the growing season under greenhouse conditions. Chapingo, Mexico Autumn-winter cycle, 2008-2009. The arrows indicate the emergence (E), stolon (S), tuberization (T) and physiological maturity of the tuber (TM) initiation phases, and the intervals between them the vegetative (VS), initial reproductive (RS 1) and final reproductive (RS 2) stages. Average values of four varieties.
With the classical residual method, and by considering a base temperature of 6 °C (Cao & Tibbits, 1995), the number of cumulative degree days (DD) from emergence to the beginning of each phenological phase was determined. From this information, the duration of the phenological stage in each genotype studied was determined.
The emergence (E) phase was determined when 50 % of the plants in the experimental unit had emerged, and the start of the stolon stage (S) when the first stolon was observed in the main stem. The initiation of tuberization or tuber growth (T) was considered when the apex of the stolon thickened. The physiological maturity of the tuber (TM) was established visually based on the loss of the green color of the foliage or the beginning of its senescence.
The vegetative stage (VS) was the period from emergence to the onset of the stolon stage, during which the growth of the aerial part and establishment of the root system in the underground part occur. The initial reproductive stage (RS 1), characterized by the generation and growth of stolons, occurred between the onset of the stolon stage and tuberization. The final reproductive stage (RS 2) occurred from the beginning of tuberization until the physiological maturity of the tuber, and in it the growth of the tuber occurred.
At harvest, the number of tubers per plant (NTP) with a diameter greater than 5 mm and tuber weight per plant (TWP, in g) were quantified. Average tuber weight per plant (ATWP, in g) was obtained from the TWP/NTP ratio.
The dry matter of the aerial part (ADM, in g), which included leaves and stems, as well as the dry matter of the underground part (RDM, in g), which included the root and part of the stolon, were determined in the harvest from two plants of the experimental unit. The structures were dried for 72 h at 75 °C in a Blue M® model POM-326F stove.
Quantification of soluble carbohydrates by high-performance liquid chromatography (HPLC)
Obtaining samples
During tuber development, four samplings were carried out; the first 21 days after the start of tuberization and the other three at intervals of 14 ± 2 days, where the last coincided with physiological maturity. The samples were made from two plants established in an experimental unit. For the sugar content determinations, the largest tuber was selected, of which two duplicates were considered (Table 1). Each sample consisted of 5 ± 1 g of the central part of the tuber, which was placed in liquid nitrogen (-196 °C) until analysis.
Table 1 Weight of tubers of four potato varieties with a different maturity stage. Chapingo, Mexico.
| Variety | Tuber maturity stage | |||||||
|---|---|---|---|---|---|---|---|---|
| One | Two | Three | Four | |||||
| DAT z | Weight y (g) | DAT | Weight (g) | DAT | Weight (g) | DAT | Weight (g) | |
| Alpha | 25 | 14 | 40 | 32 | 54 | 45 | 67 | 69 |
| Atlantic | 9 | 14 | 23 | 29 | 37 | 39 | 50 | 66 |
| Mondial | 26 | 21 | 40 | 47 | 54 | 67 | 67 | 84 |
| Vivaldi | 24 | 8 | 38 | 9 | 52 | 26 | 64 | 41 |
| Media | 21 | 14 | 35 | 29 | 49 | 44 | 62 | 65 |
zDAT: days after the start of tuberization; y average of 2 tubers.
Extraction of soluble carbohydrates
The frozen tuber samples were individually ground in a blender, with 20 mL of 70 % HPLC-grade ethanol, and brought to boiling temperature for 5 min. The extract was decanted and the residue was subjected to a second extraction with 10 mL of ethanol. At the end, the extracts were mixed. Subsequently, the mixture was centrifuged for 15 min at 4,000 x g in a HERLE model Z230A centrifuge (Labnet International, Inc. Edison, NJ. U.S.A), filtered with Whatman number 4 paper and mannitol was added at a concentration of 4 mg·mL-1 as internal standard. Finally, the extract was adjusted to 25 mL with the same solvent used for the extraction.
From the graduated extract, 10 mL were taken to concentrate to dryness under reduced pressure at 40 °C, in a BUCHI R-215 rotoevaporator (Switzerland). The residue was resuspended in 2 mL of HPLC-grade water to pass it through a column containing ion exchange resins, one basic (0.5 mL of Dowex - 1 x 8 - Fluka) and the other acid (0.5 mL of Dowex - 50W X8 - Fluka), at a ratio of 1:1 (w/w). The purified sample was passed through a 0.45-μm nylon acrodisk in order to collect the sample in a 1.5-mL vial.
Analysis of sugars in HPLC
A Perkin Elmer series 200 high-performance liquid chromatography system (Boston Ma. U.S.A), consisting of an autosampler, quaternary pump, degasifier, refractive index detector and column oven, was used. The system was operated with TotalChrom version 6.2.1 software. A Rezex, RCM-monosaccharide Ca+2 (8 %) column 30 cm long and 7.8 mm in diameter was used. HPLC-grade water was used as the mobile phase. The flow rate was 0.6 mL·min-1, the injected sample volume was 20 μL and the running time was 25 min. The temperature of the column was maintained at 85 °C. Standard curves were developed with commercial standards for sucrose, glucose and fructose (Sigma, MN), at concentrations of 0.5, 1.0, 2.5 and 5 mg·mL-1 (Morales-Fernández et al., 2015; Rodríguez-Saona & Wrolstad, 1997). Each point of the curves was injected at least three times to obtain the data of the area associated with each concentration; with this information the regression equations for each sugar were obtained.
A randomized complete block experimental design was used with three replicates, and the experimental unit consisted of four containers (bags) filled with substrate. With the obtained data an analysis of variance and regression was made. In addition, Tukey's range test was applied (P ≤ 0.05); for this, Statistical Analysis System software (SAS, 2004) was used.
Results and discussion
Crop phenology
The crop’s phenological development was adapted to greenhouse conditions. The observed mean temperature of 20 °C coincided with the optimum for potato cultivation (Rykaczewska, 2015), which allowed the development and accumulation of adequate tuber dry matter, without observing secondary tuberization and physiological defects in the tuber, characteristic of crops grown at high temperatures (Rykaczewska, 2015).
The potato varieties had differential behavior (P ≤ 0.05) with respect to the growth cycle. Alpha and Vivaldi were contrasting, since the latter required 11 % fewer DD to the physiological maturity of the tuber, while Atlantic and Mondial had intermediate duration (1194 DD). Except in Atlantic, the varieties started the stolonage stage (200 DD) and tuberization (309 DD) at the same time, and had statistically similar behavior in the vegetative (200 DD) and initial reproductive (109 DD) stages. However, greater variations (P ≤ 0.05) occurred in the final reproductive phase, which had an impact on the decrease in the growth cycle of the varieties (Table 2), a situation similar to that reported by Morales-Fernández et al. (2011), due to genotypic and environmental differences in crop development (Rykaczewska, 2015).
Table 2 Degree day requirement to reach the main phases and phenological stages of four potato varieties grown under greenhouse conditions. Chapingo, Mexico.
| Variety | Phases (DD 1 ) | Stages (DD) | ||||
|---|---|---|---|---|---|---|
| S | T | TM | VS | RS 1 | RS 2 | |
| Alpha | 198 az | 307 b | 1,230 a | 198 a | 109 b | 923 a |
| Atlantic | 122 b | 429 a | 1,189 b | 122 b | 307 a | 760 c |
| Mondial | 203 a | 299 b | 1,199 b | 203 a | 96 b | 900 b |
| Vivaldi | 199 a | 321 b | 1,093 c | 199 a | 123 b | 771 c |
| HSD | 23 | 34 | 23 | 23 | 40 | 17 |
1DD: degree days; S: start of stolonage; T: start of tuberization; TM: physiological maturity of tuber; VS: vegetative stage; RS 1: initial reproductive stage; RS 2: final reproductive stage; HSD: honest significant difference.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
Yield and its components, and dry matter content
High temperatures (Figure 1) occurred in the initial reproductive stage (RS 1), which, according to Struik, Haverkort, Vreugdengil, Bus, and Dankert (1990), could be beneficial to promote branching and increase the number of stolons, a condition that directly increases the number of tubers and these, in turn, have an impact on yield (Haverkort, van de Waart, & Bodlaender, 1990).
The Mondial variety had higher yield per plant and exceeded (P ≤ 0.05) Vivaldi, Alpha and Atlantic by 75, 31 and 31%, respectively (Table 3). This parameter is associated with the greater average weight of the tuber, since, together with the number of tubers per plant, they are the main components that define potato yield (Zvomuya & Rosen, 2002).
The Alpha and Atlantic varieties produced the highest (P ≤ 0.05) number of tubers per plant, 36 % more than the rest of the varieties, although its yield was lower (P ≤ 0.05) than Mondial’s, due to the lower average weight of tubers per plant. These results agree with those of Walworth and Carling (2002), who detected that the increase in the number of tubers per plant diminishes their final size.
Table 3 Yield and its components, and dry matter accumulation in four potato varieties grown under greenhouse conditions. Chapingo, Mexico.
| Variety | NTP 1 | TWP (g) | ATWP (g) | ADM (g) | RDM (g) |
|---|---|---|---|---|---|
| Alpha | 10 az | 247 b | 24 b | 22 a | 3 b |
| Atlantic | 12 a | 277 b | 22 b | 28 a | 9 a |
| Mondial | 7 b | 380 a | 61 a | 29 a | 7 a |
| Vivaldi | 7 b | 95 c | 11 c | 7 b | 1 b |
| HSD | 2 | 44 | 7 | 8 | 3 |
1NTP: number of tubers per plant; TWP: tuber fresh weight per plant; ATWP: average tuber weight per plant; ADM: dry matter of the aerial part; RDM: dry matter of the underground part; HSD: honest significant difference.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
The biomass content of the aerial part was similar (P ≤ 0.05) in the Alpha, Atlantic and Mondial varieties, which surpassed Vivaldi by 73 %, while the biomass of the underground part was 75 % higher in Atlantic and Mondial compared to Alpha and Vivaldi (Table 3). The high aerial and subterranean biomass of Mondial was associated with greater tuber weight per plant. Similar results were reported by Morales-Fernández et al. (2011), who indicate the importance of both characters in potato yield.
Although Alpha and Atlantic had a high aerial dry matter content, the behavior of their yield was different from that of Mondial, which could be due to an imbalance in the competition between the growth of both parts and the greater number of tubers per plant, which caused less tuber development and size (Walworth & Carling, 2002) and, therefore, generated lower yield.
Sugar content in potato tubers
During its growth and development, the tuber is the main demand site for photoassimilates, which are translocated from the synthesis sites (leaves), mainly in the form of sucrose (Fernie, Willmitzer, & Trethewey, 2002). In the present research, the average content of sucrose, glucose, fructose and total sugars was, respectively, 63, 65, 38 and 63 % higher at 21 than 62 days after the start of tuberization and, in general, the amount of soluble sugars decreased as the growth of the tubers proceeded (Table 4).
Table 4 Sugar content in potato tubers at different maturity stages. Chapingo, Mexico.
| Maturity stage | DAT 1 | Concentration of sugars (mg·g -1 FW) | |||
|---|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Total | ||
| MS 1 | 21 | 7.65 az | 2.22 a | 0.37 a | 10.25 a |
| MS 2 | 35 | 5.44 b | 1.19 b | 0.25 b | 6.89 b |
| MS 3 | 49 | 3.29 c | 0.47 c | 0.20 b | 3.96 c |
| MS 4 | 62 | 2.81 c | 0.77 c | 0.23 b | 3.82 c |
| HSD | 1.01 | 0.31 | 0.10 | 1.26 | |
1DAT: days after the start of tuberization; FW: fresh weight; HSD: honest significant difference.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
Other studies coincide with the present one in detecting that the highest concentrations of sucrose, glucose and fructose occur in immature tubers (Knowles, Pavek, Knowles, & Holden, 2008; Kumar & Ezekiel, 2006), and that the lowest sucrose concentration occurs during maturity (Knowles et al., 2009; Sabba et al., 2007), as was the case in the present study in MS 4 due to the reduced supply of photosynthates as a result of the senescence of the foliage of the plants, as suggested by Kumar and Ezekiel (2006). Thus, the low concentration of this sugar can be used as a harvest indicator (Chen, Zhang, Miao, & Asakura, 2010); however, due to the complexity of measuring it and the cost involved, it is difficult to consider its use in conventional production systems, given the possibility of detecting this stage through the senescence of the foliage.
When analyzing the behavior of soluble sugars on the basis of the tuber maturity stage (Figure 2), a higher concentration (P ≤ 0.05) was observed at the start of growth compared to physiological maturity, both in sucrose (10.44 to 1.81 mg·g-1) and in total sugars (13.40 to 2.16 mg·g-1). In this period, glucose and fructose concentrations were lower than 3.30 and 0.60 mg·g-1, respectively. The tubers in maturity stage one (MS 1) had a higher content of soluble sugars that those harvested at physiological maturity (MS 4).
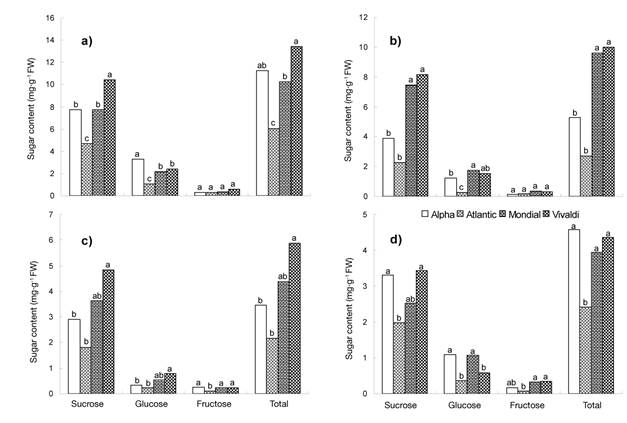
Figure 2 Soluble sugar content in potato tubers with different maturity stages: a) one (21 DAT), b) two (35 DAT), c) three (49 DAT) and d) four (62 DAT). Chapingo, Mexico Autumn-winter cycle, 2008-2009. Means with the same letter within each type of sugar do not differ statistically (Tukey, P ≤ 0.05).
Tubers of the Vivaldi variety accumulated more sucrose (10.44 mg·g-1) and total sugars (13.40 mg·g-1) than the rest of the genotypes studied (Figure 2a). Despite these differences, it was found that, as maturity was reached, the amounts of sucrose, glucose, fructose and total sugars in the tuber were less than 5 mg·g-1 (Figure 2d). These values are within the range reported by Knowles et al. (2009) for processing and handling conditions during storage.
The interaction between varieties and tuber maturity stages indicated that Vivaldi exhibited greater variation (P ≤ 0.05) in the amount of sugars when changing from one maturity stage to another (Table 5); likewise, in MS 1 and MS 2, it had 80 and 81 % more sucrose and total sugar content than Atlantic. In the case of Alpha, MS 1 had a greater amount of glucose (92 %) than Atlantic in MS 2 and MS 3. The Vivaldi variety, in MS 1, had a higher fructose content (88 %) than Atlantic in MS 4, which is explained by the specific development of the genotypes.
Table 5 Comparison of means of the variety by potato tuber maturity stage interaction, under greenhouse conditions, on sugar content. Chapingo, Mexico.
| Variety | MS 1 | Concentration of sugars (mg·g -1 FW) | ||||
|---|---|---|---|---|---|---|
| DAT | Sucrose | Glucose | Fructose | Total | ||
| Alpha | 1 | 25 | 7.71 abz | 3.26 a | 0.30 bcd | 11.27 ab |
| Alpha | 2 | 40 | 3.90 de | 1.22 def | 0.15 def | 5.27 de |
| Alpha | 3 | 54 | 2.89 de | 0.32 hi | 0.25 bcde | 3.47 de |
| Alpha | 4 | 67 | 3.31 de | 1.09 defg | 0.17 cdef | 4.58 de |
| Atlantic | 1 | 9 | 4.71 cde | 1.05 defgh | 0.29 bcd | 6.06 cd |
| Atlantic | 2 | 23 | 2.26 de | 0.26 i | 0.19 bcdef | 2.72 de |
| Atlantic | 3 | 37 | 1.81 e | 0.24 i | 0.10 ef | 2.16 e |
| Atlantic | 4 | 50 | 1.98 de | 0.36 ghi | 0.07 f | 2.41 de |
| Mondial | 1 | 26 | 7.73 ab | 2.17 bc | 0.35 bc | 10.26 ab |
| Mondial | 2 | 40 | 7.48 abc | 1.75 bcd | 0.36 b | 9.60 bc |
| Mondial | 3 | 54 | 3.63 de | 0.53 fghi | 0.22 bcdef | 4.37 de |
| Mondial | 4 | 67 | 2.53 de | 1.07 defgh | 0.33 bcd | 3.94 de |
| Vivaldi | 1 | 24 | 10.44 a | 2.39 b | 0.57 a | 13.40 a |
| Vivaldi | 2 | 38 | 8.15 a | 1.53 cde | 0.31 bcd | 9.99 ab |
| Vivaldi | 3 | 52 | 4.84 bcd | 0.80 efghi | 0.23 bcdef | 5.87 cde |
| Vivaldi | 4 | 64 | 3.44 de | 0.58 fghi | 0.35 bc | 4.37 de |
| HSD | 2.97 | 0.75 | 0.18 | 3.76 | ||
1MS: maturity stage; DAT: days after tuberization; HSD: honest significant difference.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
The variation shown in the sugar content among varieties, even at the same maturity stage, reflects the ability of some genotypes to accumulate carbohydrates during crop development, although this behavior depends on the genotype (Park et al., 2009), water stress (Bethke, Sabba, & Bussan, 2009), nutrition (Ilin et al., 1997), and temperature (Rykaczewska, 2015) during growth.
In the present study, extreme isolated temperatures with short duration during the day were observed; these temperatures did not manage to generate a generalized stress that would limit the development of the cultivars, with the exception of the Atlantic variety that produced low concentrations of soluble sugars at the different maturity stages, even though its yield was lower (P ≤ 0.05) than that of the most outstanding variety. These results are similar to those reported by Fernández et al. (2015) during tuber sprouting.
The presence of genotypic variation, demonstrated especially through the behavior of Atlantic, suggests the possibility of selecting varieties according to production conditions. Also, when considering the origin of the varieties used in the present study, which were not developed for establishment in the greenhouse, it is observed that the environmental factor influenced their development and, therefore, their yield (Rykaczewska, 2015).
Low levels of total sugars, especially of sucrose, shown in some varieties with MS 3 suggest that they could be harvested in this condition. According to Knowles et al. (2009) and Sabba et al. (2007), the lowest values of sucrose are found during the physiological maturity of the tuber, a criterion that could be used as a harvest indicator, independently of the phenotypic characteristics of the foliage of the plants.
The soluble sugar content showed a high significant association with tuber weight (Figure 3). On average in the varieties, sucrose (Figure 3a), reducing sugars (glucose and fructose; Figure 3b) and total sugars (Figure 3c) decreased as tuber weight increased, while individually the varieties studied showed the same tendency to associate the three sugars with tuber growth. These results agree with those of Kumar and Ezekiel (2006) in detecting a negative association between soluble sugar content and tuber weight.
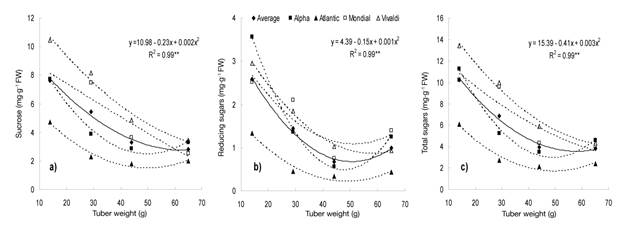
Figure 3 Relationship between the content of sucrose (a), reducing sugars (b) and total sugars (c) and tuber size in four potato varieties under greenhouse conditions. Chapingo, Mexico. Autumn-winter cycle, 2008-2009. The regression model represents the average of the varieties.
Stark and Love (2003) indicate that the highest content of soluble sugars in the tubers occurs when they are of low weight or have a high degree of immaturity, as was the case with MS 1 in this work, because the transport rate of sugars from the leaves to the tuber exceeds that of conversion to starch. This condition can change with the growth of the tuber or when it reaches physiological maturity and the environment is not a conditioning factor (Knowles et al., 2009).
The concentrations of sugars (sucrose, glucose, fructose and total) recorded in the physiological maturity of the tubers produced under greenhouse conditions were lower than 5 mg·g-1 (Table 5), values that are within the range reported by Knowles et al. (2009) for processing and handling conditions during storage. For reducing sugars at maturity, the values obtained were found in the range reported by Kumar and Ezekiel (2006) and Sabba et al. (2007), which was from 0.04 to 0.40 %. However, according to Vázquez-Carrillo, Santiago-Ramos, Ybarra-Moncada, Rubio-Covarrubias, and Cadena-Hinojosa (2013), these tubers should be consumed either fresh or cooked. This is due to the fact that, during the processing of fritters, the temperature-amino acid-sugar reducing reaction of the potato produces darkening.
Conclusions
The longest growth cycle of the varieties showed no association with a higher tuber yield in terms of weight. The yield component that contributed the most to production was the average tuber weight per plant, expressed with greater magnitude in an intermediate-cycle variety.
The soluble sugar content was modified by the maturity stage and tuber weight. The highest concentration occurred 21 days after the start of tuberization (MS 1). This variation was also affected by the genetic component, since there was differentiation in the concentrations of sucrose, glucose, fructose and total sugars, which was independent of the tuber maturity stage.
An inverse relation was detected between tuber weight and sucrose, reducing sugar (glucose and fructose) and total sugar contents.
The low concentrations of sugars (sucrose, glucose and fructose) in the tuber can be considered as an indicator of maturity, although their values cannot be generalized. However, the difficulty and cost involved in such determinations make this procedure impractical.











 texto em
texto em 


