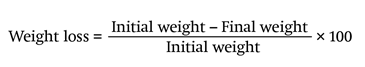Introduction
The rambutan (Nephelium lappaceum L.) is a fruit of tropical origin appreciated for its sweet, juicy pulp, and its high nutritional value (Melvin & Calvo, 2014). In Mexico, it has only recently been cultivated and is mainly produced in Michoacán, Nayarit, Oaxaca, Tabasco and Chiapas, the last being the leading producer in the Soconusco region (Tuxtla Chico, Cacahoatán, Tapachula and Metapa de Domínguez) (Pérez-Romero & Jürgen-Pohlan, 2004). In Chiapas, for 2015, the area sown was 835.96 ha with production totaling 8,730.27 t, with a value of 10.02 million pesos (Sistema de Información Agrícola y Pesquera [SIAP], 2015).
The rambutan is round or ovoid and has either a red or yellow pericarp, long spinterns and a white or translucent edible aril (Arias-Cruz, Velásquez-Ramírez, Mateus-Cagua, Chaparro-Zambrano, & Orduz-Rodríguez, 2016). Its ripening pattern suggests that it is a non-climacteric fruit (Caballero-Pérez et al., 2011), so they are harvested when they are ripe for consumption and have an optimal external appearance (Tindall, Menini, & Hodder, 1994). However, due to its morphological and physiological characteristics, it is a highly perishable fruit, since its shelf life does not exceed seven days after being harvested. This is because the spinterns and pericarp are easily dehydrated and oxidized, resulting in a dark and undesirable appearance that limits its marketing potential for fresh consumption (Hernández-Arenas et al., 2010).
Considering the above, a viable alternative that has proven to be efficient in delaying the degradation processes is the combined use of modified atmospheres and refrigeration, since they allow decreasing the loss of water through transpiration in the fruit (Hernández-Arenas et al., 2012). However, it is possible to increase the quality characteristics of the fruit through proper agronomic management (Melvin & Calvo, 2014).
Agronomic practices that have been found to have an impact on the shelf life of fruit species include: a)girdling or ring-barking of tree branches (promotes greater accumulation of carbohydrates in fruits; Wall, 2006), b) pruning (favors the distribution of photosynthesis products in those trees with a homogeneous shape and structure; Crane et al., 2005) and c) water stress. The last one, in apple tree (Malus domestica L.), mango (Mangifera indica L.) and kiwi (Actinidia deliciosa A. Chev.), induces resistance on the epidermis in relation to the loss of water from the fruits, which is why it prolongs their shelf life (Burdon & Clark, 2001; Osuna-García, Pérez-Barraza, Vázquez-Valdivia, & Urías-López, 2009; Parra-Quezada, Robison, Osborne, & Parra-Bujanda, 2008).
At present, rambutan cultivars are selected considering their content of sugars, total soluble solids (TSS) and titratable acidity at the time the fruit reaches consumption maturity (Hernández-Arenas et al., 2012). However, international standards set out in the Codex Alimentarius for rambutan (CODEX STAN 246-2005) indicate that a commercial quality fruit should be uniformly red, with fruit weight between 18 and 24 g and TSS content between 16 and 18 °Brix.
As is the case for other tropical fruit trees, there is insufficient information to adequately discern the role of cultural practices (pruning, girdling and irrigation) on the postharvest physiological behavior of rambutan fruit. Additionally, in the last decade, the level of acceptance of regional and national markets is positioning the rambutan as an economic alternative in fruit-producing areas where the predominant crop is usually coffee, that is, areas located between 100 and 700 masl (Arias-Cruz et al., 2016; Caballero-Pérez et al., 2011). Therefore, the aim of the present study was to evaluate the effect of agronomic practices such as pruning, girdling and water stress on some postharvest quality parameters in rambutan fruits from ' RJA Clone' trees in Chiapas, Mexico.
Materials and methods
Study area location
Rambutan fruits were obtained from the "La Chinita" commercial orchard, located at km 4.5 on the Huehuetán Estación-Nueva Victoria highway in Huehuetán, Chiapas, Mexico (15° 00' 33'' North latitude and 92° 26' 17'' West longitude, at 19 masl), with average annual precipitation and temperature of 2,326 mm and 28 °C, respectively (Instituto Nacional para el Federalismo y el Desarrollo Municipal [INAFED], 2010). The soil of this orchard has a sandy-loam texture, 1.31 % organic matter, pH 6.1 and 37.2 % porosity.
Study factors
From January to May 2011, 48 'RJA Clone' trees averaging 14 years of age and in their productive stage were selected and subjected to different agronomic practices, individually or in combinations. The treatments were: girdling (G), pruning (P), water stress (WS) and irrigation (I). For G, a razor was used to make incisions of 3 mm in depth and circumference; this activity took place in mid-April. P was carried out in February, removing branches of approximately 30 cm in length, corresponding to the penultimate vegetative flush. WS was implemented in January and concluded at the end of May, coinciding with the beginning of the rainy season. For this practice, the irrigation schedule was suspended until the temporary wilting percentage (TWP), that is, 13 % usable moisture, was reached. Finally, I was applied in the normal way using a drip irrigation system. The irrigation conditions consisted in maintaining the usable moisture content at values higher than 25 % throughout the experiment. Thus, the study factors were water stress (drought), pruning, girdling, irrigation (control) and their combination, resulting in eight treatments (Table 1).
Experimental design
The treatments were established under a split-plot experimental design in randomized complete blocks with a factorial arrangement and six replicates, where each tree was considered as a replicate. In July, 75 fruits at consumption maturity (pericarp 90 % bright red) were harvested from each tree and each treatment. For the evaluation of the different postharvest quality parameters, the fruits were distributed as follows: for 30 fruits of each treatment, fresh weight, weight loss, pericarp browning and shelf life were determined, with three replicates and ten fruits per replicate; for 15 fruits, aril thickness, pericarp thickness, number of spinterns, spintern size and TSS content were quantified, in triplicate with five fruits per experimental unit. The remaining 30 fruits were evaluated in terms of total sugars, vitamin C, total phenols and titratable acidity, with five replicates and six fruits per experimental unit.
The analyses were carried out in the Plant Science Department’s Fruit Physiology Laboratory at the Universidad Autónoma Chapingo under ambient temperature conditions of 30 ± 2 °C and 80-90 % relative humidity.
The postharvest behavior of the fruits was evaluated by recording the following variables:
Fresh weight (FW, g). It was determined at the time of harvest using an electronic scale (Ohaus® model Scout Pro SP2001).
Weight loss (%). Fruits were weighed daily for five days with a balance scale (Ohaus®) and the formula applied was:
Aril thickness (AT, mm) and pericarp thickness (PT, mm). Fruits of each treatment were cut transversely and the thickness of the aril and pericarp were measured with a digital Vernier caliper.
Number of spinterns (NS) and their length (SL, cm). They were evaluated at the time of harvest. Their length was measured with a digital Vernier caliper, from the base to the apex.
Pericarp browning (PD). It was determined daily for five days based on a subjective scale proposed by Caballero-Pérez et al. (2011): 1 = total oxidation (100 %), 2 = intense oxidation (about 75 %), 3 = medium oxidation (about 50 %), 4 = low oxidation (about 25 %) and 5 = no oxidation.
Total soluble solids (TSS, °Brix). The aril was scraped from each fruit and placed in a sieve to extract the juice, which was deposited in a digital refractometer (Atago model Master-BR, Tokyo, Japan).
Total sugars (TS, mg∙100 g -1 ). They were quantified by the anthrone method; for this, 0.5 g of pulp were weighed and mixed with 50 mL of ethyl alcohol (70 %), and then boiled for 15 min. The solution was filtered on Whatman® grade 40 paper and filled to 100 mL with 70 % ethanol. From this, a 1 mL aliquot was taken in a test tube and 6 mL of anthrone reagent (66 % stock [v/v] of H2SO4 + 340 mL of water, 10 g of Thiourea [MERCK®] were dissolved with 0.5 g of Anthrone [MERCK®] and filled to 1 L) were added to it. The tubes were then placed in a water bath for 10 minutes, stained in a container with ice and at the end the absorbance at 630 nm was determined on a UNICO model 1100RS® spectrophotometer using a standard glucose curve (MERCK®) of 30 mg·mL-1.
Titratable acidity (TA, % malic acid). It was determined according to the methodology proposed by the Association of Official Analytical Chemists (AOAC, 1990), with 5 g of pulp which were neutralized with 0.1 N NaOH. Phenolphthalein was used as an indicator.
Vitamin C (VC, mg∙100 g -1 ). It was estimated according to the method of Tillman (AOAC, 1990), known as DFI (2,6 dichlorophenol-indophenol).
Total phenols (TP, mg∙100 g -1 ). The determination was made using the method of Rathjen and Robinson (1992) with some modifications. First, 1 g of pulp was homogenized with 25 mL of distilled water. The solution was filtered, 2 mL were taken, 4 mL of the methanol: chloroform:water (2:1:1) extraction solution were added to it and then the mixture was centrifuged at 2,200 rpm for 15 min. The supernatant was taken, 4 mL of the methanol:chloroform:water (2:1:1) extraction solution were added to it and then the mixture was centrifuged at 2,200 rpm for 15 min. Next, 10 mL of 10 % NaCO3 were added to the mixture, which was then placed at 38 °C for 15 min. Of this solution, 1 mL was taken and 1 mL of the Folin-Ciocalteu® reagent (Sigma-Aldrich®) (1:1 in distilled water) was added to it; it was left to stand for 15 min under dark conditions, where the change of absorbance at 660 nm was evaluated in a spectrophotometer (UNICO model 1100RS®). Quantification was performed using a standard tannic acid curve (Sigma-Aldrich®)
Statistical analysis
Except for the variables percentage of weight loss and pericarp browning, which were plotted as a function of time, the remainder were subjected to an analysis of variance and Tukey’s range test (P ≤ 0.05) using Statistical Analysis System software (SAS, 2002).
Results and discussion
Fresh weight
As shown in Table 2, fruits from trees subjected to water stress showed higher fresh weight (40.2 g, P ≤ 0.05), which could at first be regarded as a rather inconsistent result. However, in this case, it is important to point out that in trees subjected to drought a smaller number of fruits, but with a larger individual size, was frequently observed. In this regard, Tindall et al. (1994) mention that, in rambutan, irrigation is a predominant factor because it is a crop highly susceptible to water scarcity, mainly affecting yield.
Table 2 Comparisons of means of different physical variables and postharvest behavior of fruits of rambutan trees subjected to different agronomic management systems.
| Treatment | FW1 (g) | AT (mm) | PT (mm) | SL (cm) | NS | SL (days) |
|---|---|---|---|---|---|---|
| I | 30.02 bcz | 7.93 b | 2.73 bc | 1.28 c | 314 ± 20.45 | 3.20 c |
| I+P | 21.79 d | 6.63 c | 2.40 c | 1.39 a | 345 ± 23.67 | 3.40 c |
| I+G | 27.79 bc | 7.06 bc | 2.80 bc | 1.39 a | 300 ± 20.10 | 3.93 b |
| I+P+G | 28.81 bc | 7.10 bc | 2.63 bc | 1.32 b | 321 ± 27.80 | 3.13 c |
| WS | 40.20 a | 9.13 a | 3.66 a | 1.01 f | 357 ± 22.90 | 4.93 a |
| WS+P | 27.58 bc | 6.70 c | 3.03 b | 1.12 e | 368 ± 29.67 | 4.46 a |
| WS+G | 26.30 c | 7.96 b | 2.66 bc | 0.98 g | 357 ± 20.45 | 4.73 a |
| WS+P+G | 26.90 c | 7.33 bc | 2.73 bc | 1.16 d | 368 ± 28.67 | 4.56 a |
| CV (%) | 17.38 | 11.66 | 15.64 | 10.36 | - | 15.38 |
1FW = fresh weight, AT = aril thickness, PT = pericarp thickness, SL = spintern length, NS = number of spinterns, SL = shelf life, CV = coefficient of variation, I = irrigation, WS = water stress, P = pruning and G = girdling.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
By contrast, Ferreyra, Selles, and Lemus (2002) indicate that possibly the water contained in the soil after the winter rains was sufficient and managed to meet the plant’s water supply needs. In this study, it could also be correlated with the presence of favorable soil characteristics at the tree growth site, such as organic matter content and percentage of soil porosity. Arias-Cruz et al. (2016) indicate that, as in other tropical fruit trees, floral induction in rambutan is directly related to water supply, as well as a decrease in temperature, as occurs during the winter period. Additionally, Parra-Quezada et al. (2008) mention that the effect of water stress on fruits depends on the severity and time to which the plant is subjected to this condition.
Weight loss
Weight loss in rambutan fruits after harvest is gradual and constant, losing up to 7 % of their weight daily, depending on temperature and relative humidity conditions (Landrigan, Morris, Eamus, & Mcglasson, 1996; Nakano, Ogura, Kubo, & Inaba, 2003). Figures 1 and 2 show the weight loss in fruits of rambutan trees subjected to different agronomic management systems, with fruits with water stress and its respective combinations, with pruning and girdling, having greater resistance to weight loss (up to 38 % in the case of water stress with pruning and 33 % only with water stress). On the other hand, fruits of trees subjected to constant irrigation lost up to 43 % of their weight during the five days of evaluation, at a temperature and relative humidity of 30 ± 2 °C and 80 to 90 %, respectively.
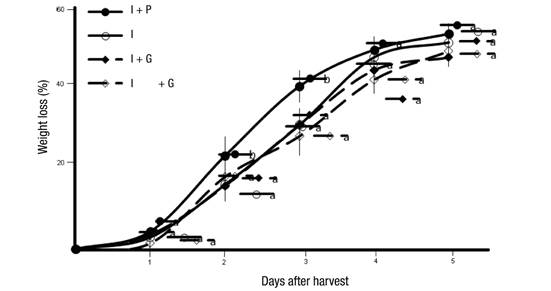
Figure 1 Weight loss in fruits of rambutan trees subjected to different agronomic management systems. I = irrigation, P = pruning and G = girdling. Means with the same letter within each series do not differ statistically (Tukey, P ≤ 0.05).

Figure 2 Weight loss in fruits of rambutan trees subjected to different agronomic management systems. WS = water stress, P = pruning and G = girdling. Means with the same letter within each series do not differ statistically (Tukey, P ≤ 0.05).
Kaewchana, Niyomlao, and Kanlayanarat (2006) and Landrigan et al. (1996) found that the greatest weight loss (23 %) in rambutan occurs six days after harvest (dah), at 22 °C and 95 % relative humidity. However, the results of this research show that the loss was much greater, which could be due to the temperature (30 °C) and relative humidity (from 80 to 90 %) conditions to which the fruits were subjected during the five days of storage. The results are consistent with those reported by Burdon and Clark (2001), who observed that kiwifruit fruits that were well hydrated during the harvest had greater postharvest water loss than those that had been subjected to water stress during cultivation.
Pericarp and aril thickness
The I+P treatment showed the least thickness (2.4 mm), while WS+P and WS only presented the greatest pericarp thickness with 3.0 and 3.6 mm, respectively (Table 2). According to Caballero-Pérez et al. (2011) and van Welzen and Verheij (1991), in rambutan, the thickness of the pericarp is 2 to 4 mm, depending on the cultivar; in addition, some authors state that size, composition, and color are important marketing characteristics and that they define shelf life behavior (Aparecida-de Andrade, de Macedo-Lemos, Geraldo-Martins, de Paula, & Pitta-Junios, 2008; Hernández-Arenas et al., 2010). On the other hand, Huang et al. (2004) showed that litchi cv. Huaizhi fruits with thicker spongy tissue in the pericarp showed minor dehydration.
On the other hand, aril thickness is a very important characteristic for the commercialization and acceptance of rambutan fruits in the market, since it is the edible part of the fruit. This variable had significant differences between treatments with water stress and with constant irrigation. Table 2 shows that fruits subjected to drought produced greater aril thickness, while with the other management methods there was no significant statistical difference (P ≤ 0.05) in relation to this variable.
Number and length of spinterns
The NS varied from 300 to 368, without showing significant statistical differences (P ≤ 0.05) among treatments (Table 2). Their size varied greatly, which is associated with the heterogeneity of the crop in the production area. Previous studies have shown that the fruit's morphology affects the loss of the characteristic red color, due to the presence of stomata in the spinterns that allows water to escape from the fruit (Avendaño-Arrazate, Arévalo-Galarza, Sandoval-Esquivez, & Caballero- Pérez, 2011; Caballero-Pérez et al., 2011; Wongs & Kanlayanarat, 2005). These results contrast with those results obtained in the present study, since the fruits subjected to water stress have practically the same number of spinterns and, nevertheless, a smaller weight loss was obtained during the five days of storage (Figures 3 and 4). On the other hand, the length of the spinterns was greater for the I+P and I+G treatments (1.39 cm in both cases, Table 2), values that exceeded those obtained by Caballero-Pérez et al. (2011), who report lengths ranging from 1.07 to 1.19 cm.
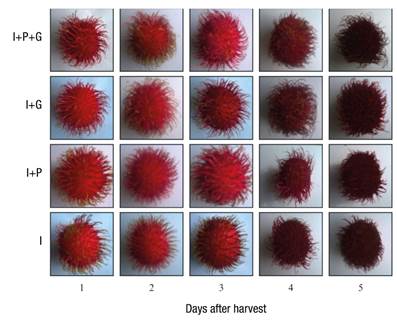
Figure 3 Pericarp browning in fruits of rambutan trees subjected to different agronomic management systems, stored at 30 ± 3 °C and 80-90 % relative humidity. I = irrigation, P = pruning and G = girdling.
Pericarp browning and shelf life
With the irrigation treatment, pericarp browning started from the second dah, while in the fruits subjected to water stress it was noticed on the third dah. On the other hand, shelf life was prolonged one day when stored at 30 ± 3 °C and 80-90 % relative humidity (Table 2; Figures 3 and 4). Caballero-Pérez et al. (2011) showed that the oxidation of rambutan fruits can vary according to the variety. Among their studied selections, RI-115 presented 30 % oxidation at two dah, RI-148 at three dah and RI-104 at four dah, this under conditions of 95 % relative humidity, 22 °C and 1 atm.
Although the edible part of the fruit is not affected, oxidation causes it to lose quality and commercial acceptance. The results of the present study suggest that rambutan fruits subjected to water deficit have a higher number of solutes that allow them to increase the pressure of the solutes and to counteract the effects of the vapor pressure caused by the environment, thus allowing the dehydration to occur gradually (del Ángel-Pérez, Adame-García, & Villagómez-del Ángel, 2014; Yingsanga, Srilaong, & Kanlayanarat, 2006). This is consistent with what was indicated by Kaewchana et al. (2006), who mention that weight loss in rambutan and litchi is closely related to the dehydration of the fruit; similarly, they indicate that pericarp browning is proportional to weight loss (Figures 3 and 4).
Total soluble solids
The concentration of TSS had significant statistical differences (P ≤ 0.05) between the constant irrigation and water stress treatments, and even between the combinations with other agronomic practices (Table 3). The rambutan fruits subjected to water stress had more TSS (between 20 and 22 °Brix) than fruits with constant irrigation (between 18 and 19 °Brix). The results agree with those reported by Caballero-Pérez et al. (2011), del Ángel-Pérez et al. (2014) and Hernández-Arenas et al. (2010), who found, depending on the cultivar, a concentration of TSS in the range of 17 to 21 °Brix at maturity.
Table 3 Comparisons of means of the biochemical composition of rambutan fruits from trees subjected to different agronomic management systems.
| Treatment | TSS1 (°Brix) | TS (mg·100 g -1 ) | TA (% malic acid) | VC (mg·100 g -1 ) | TP (mg·100 g -1 ) |
|---|---|---|---|---|---|
| I | 19.25 bcz | 246.66 cd | 0.417 b | 13.90 b | 1.39 c |
| I+P | 18.41 c | 203.13 d | 0.296 cd | 19.43 b | 1.39 c |
| I+G | 19.80 bc | 234.16 dc | 0.379 bc | 17.13 b | 1.42 c |
| I+P+G | 18.88 bc | 189.90 d | 0.577 a | 12.68 b | 1.22 c |
| WS | 22.62 a | 372.46 ab | 0.306 bc | 37.79 a | 2.31 a |
| WS+P | 20.48 b | 307.28 bc | 0.355 bc | 12.63 b | 1.85 b |
| WS+G | 21.39 a | 423.93 a | 0.196 d | 39.90 a | 2.33 a |
| WS+P+G | 20.33 b | 343.60 ab | 0.27 c | 14.23 b | 1.98 b |
| CV (%) | 8.31 | 30.54 | 15.87 | 29.28 | 6.49 |
1TSS = total soluble solids, TS = total sugars, TA = titratable acidity, VC = vitamin C, TP = total phenols, CV = coefficient of variation, I = irrigation, WS = water stress, P = pruning and G = girdling.
zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
Total sugars
Significant statistical differences (P ≤ 0.05) were also recorded in this variable. Fruits subjected to water stress had values between 343.60 and 423.93 mg·100 g-1, while fruits with constant irrigation had values from 189.90 to 246.66 mg·100 g-1 (Table 3). The I+P and I+P+G treatments had the least amount of TS (203.13 and 189.90 mg·100 g-1, respectively); however, these values were similar to those reported in rambutan by Paull and Chen (1987), with 201 mg·100 g-1 at the time of harvest.
Titratable acidity
TA showed significant statistical differences (P ≤ 0.05) between treatments with constant irrigation and with water stress, specifically between I+P+G and WS+G, which had the maximum (0.577 %) and minimum (0.196 %) titratable acidity, respectively (Table 3). In the other treatments, TA was between 0.27 and 0.41 %, similar to that reported in rambutan cv. 'Rongrien' (0.3 to 0.4 %) (Caballero-López et al., 2011; Harjadi & Tahitoe, 1992).
Vitamin C
Fruits from trees with constant irrigation had VC values ranging from 12.68 to 19.43 mg·100 g-1 and those treated with I+P and WS+P+G obtained 12.63 and 14.23 mg·100 g-1, respectively. However, the fruits of the treatment with only water stress had 37.79 mg·100 g-1 and with WS+G 39.90 mg·100 g-1 (Table 3). In general, VC results were low.
The ascorbic acid content has been reported for different cultivars such as 'R9' (22.02 mg·100 g-1), characterized by being one of the cultivars with a lower VC content. Values of 38.12 and 39.34 mg·100 g-1 have been found in 'Jitlee' and 'Rongrien' respectively (Wall, 2006). The latter two coincide with the results obtained in the fruits with WS+G.
Total phenols
All trees subjected to constant irrigation had from 1.22 to 1.42 mg·100 g-1 of TP. These results agree with those reported by Gorinstein et al. (1999), who demonstrated that the amount of TP in fruits of rambutan var. 'Rongrien' was 1.66 mg∙100 g-1. On the contrary, fruits subjected to WS, WS+P and WS+G presented almost double that amount (between 1.85 and 2.33 mg∙100 g-1, Table 3).
Conclusions
The temporary lack of water (water stress) combined with pruning and girdling favored fruit size and shelf life. It also increased the concentration of bioactive compounds such as vitamin C and total phenols in fruits, which had a lower percentage of acidity and greater sweetness (°Brix). These physical and biochemical characteristics are considered important for the marketing of rambutan fruit as a product for fresh consumption.











 text in
text in