Introduction
Ficus L. is a very broad genus that groups many different species together; in addition, it is native to tropical and subtropical regions (Serrato, Ibarra-Manrriquez, & Oyama, 2004). Many Ficus species are common in different biogeographic regions, and Ficus is considered one of the most diverse genera with regard to its habits and life forms (Chaudhary et al., 2012). In Mexico, Ficus benjamina L. is mainly used as an ornamental and has been widely accepted because of its low production cost, rapid growth and dense, glossy foliage (Alanís-Flores, 2005). Like many plant species, the growth and development of F. benjamina is affected by several phytopathogenic fungi, where ascomycetes and their anamorphs predominate.
The fungi of the family Botryosphaeriaceae (Ascomycetes) infect plants such as Acacia karroo (South Africa), Hylocereus polyrhizus (Malaysia), Eucalyptus camaldulensis and Populus nigra (Iraq), Citrus paradisi (Israel), Ficus spp. (Sudan), Manihot esculenta (Brazil), Vitis vinifera (California), among many other tree and shrub plants in the world (El Atta & Aref, 2013; Hassan, Haleem, & Hassan, 2011; Jami, Slippers, Wingfield, & Gryzenhout, 2013; Machado, Pinho, Oliveira, & Pereira, 2014; Mohd, Salleh, & Zakaria, 2013; Sadowsky, Solel, & Sztejnberg, 2007; Úrbez-Torres, Leavitt, Voegel, & Gubler, 2006).
Identification of species of the family Botryosphaeriaceae has been inconsistent and poor for many years (Slippers & Wingfield, 2007). However, with the aid of molecular tools, differences in ribosomal DNA sequences, necessary for a more reliable characterization, have been identified (Machado et al., 2014).
In 2014, during the hottest season (June-September), F. bejamina plants with symptoms of branch dieback were detected in private gardens and avenues in public areas in Hermosillo, Sonora, Mexico, but without knowing the cause of the organism(s) associated with the disease. Therefore, the aim of this study was to morphologically and molecularly identify the causal agent of branch dieback in F. benjamina plants.
Materials and methods
Collection and isolation
From gardens and urban areas in Hermosillo, Sonora, samples of tissue with dieback symptoms were collected from F. benjamina trees, disinfested with 1 % sodium hypochlorite for 1 min, rinsed three times with abundant sterile distilled water and placed on sterile blotting paper. The tissue fragments were seeded in PDA medium (Difco®) and incubated at 28 °C for three days.
For the morphological identification, tissue assemblages were made from 10-day-old cultures and genus and species classification was performed using the keys of Crous et al. (2006) and Phillips et al. (2013).
Pathogenicity tests
Four isolates from F. benjamina plants with dieback symptoms were used. For this, five F. benjamina plants, of approximately one year of age, were inoculated with each of the isolates. In addition, five plants were used as the control. For the inoculation of the plants, superficial cuts were made in the bark of approximately 2 x 5 mm and 2 mm deep with a razor disinfested with alcohol. In the cut made, pieces of the PDA medium with mycelium of the fungus were placed. Similar wounds were made to the control plants, but culture medium without the fungus was placed in them. To avoid drying and contamination, wounds were covered with parafilm tape.
Molecular identification and phylogenetic analysis
The DNA extracted from the four inoculated isolates was partially amplified with primers: ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’) / ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) (White, Bruns, Lee, & Taylor, 1990), RPB2-5F (5’-GAY GAY MGW GAT CAY TTY-3’) / fRPB2-7cR (5’-GGC CCA TWG CYT GCT TMC CC AT-3’) (Liu, Whelen, & Hall, 1999) and LROR (5’-ACC CGC TGA ACT TAA GC-3’) / LR5 (5’-TCC TGA GGG AAA CTT CG-3’) (Vilgalys & Hester, 1990), which amplify the ITS1-5.8S-ITS2, RPB2 and 28S regions, respectively. The purified amplicons were sequenced in both directions, using the respective primer pair separately. The sequencing equipment used was ABI PRISM® 3700, Genetic-Analyser - Applied Biosystems. The sequences of two representative N. dimidiatum isolates, together with other sequences of the family Botryosphaeriaceae, were annealed using Clustal W, edited and analyzed using the Maximum Likelihood method with MEGA 7 software (Kumar, Stecher, & Tamura, 2016). A total of 1,345 positions were analyzed. The robustness of the nodes was examined with a bootstrap analysis with 2,000 replicates. Aplosporella hesperidica CBS:732.79 was used as the external group.
Results and discussion
Isolation and identification
Neoscytalidium dimidiatum was isolated with 100 % frequency from F. benjamina plants with symptoms of branch dieback (Figure 1A), bark peeling and cankers in the trunk stem, which resulted in the emergence of adventitious roots. Neoscytalidium belongs to the family Botryosphaeriaceae, being a recently created genus (Crous et al., 2006). The fungi of the family Botryosphaeriaceae are typically opportunistic pathogenic organisms, causing diseases only when the plants are stressed and with a cosmopolitan distribution; they are also commonly associated with dieback and cankers in woody plants and shrubs (Bush, 2015). Neoscytalidium dimidiatum presented the following morphological features: light green to olive-green mycelium when young (three days) and gray-dark to black when old (seven to ten days), dark pycnidia in affected trunks and conidia in chain, ellipsoid and globose, with 0-2 septa at maturity of approximately 3 to 5.5 and 6 to 9.5 µm (Figure 1B).

Figure 1 Characteristics of Neocytalidium dimidiatum. A) Dieback in F. benjamina plants; B) arthroconidia (above) and mycelial growth in PDA medium (below).
The sequences of the internal transcribed spacer region (ITS; KU141333 and KU141334), large subunit (LSU; MF508739 and MF508740) and RNA polymerase II subunit (RPB2; MF508741 and MF508742) of the DNA from the representative isolates NDFB002 and NDFB003 were deposited in the National Center for Biotechnology Information GenBank (NCBI; http://www.ncbi.nlm.nih.gov/). These sequences were compared using the NCBI BLAST tool, showing 99 to 100 % similarity to Neoscytalidium dimidiatum.
Pathogenicity tests
The Ficus benjamina plants inoculated with fungal isolates showed necrosis and slight bark peeling in the inoculated area at 40 days after inoculation (dai) (Figure 2D, E, G and H):, whereas in the control plants these symptoms were not observed (Figure 2 C and F):. The morphological characteristics of the isolates obtained from the inoculated plants coincided with the inoculated isolates, confirming Koch’s postulates.

Figura 2 Symptoms in Ficus benjamina at 40 days after inoculation. C) and F) control plants, without and with a cut, respectively; D) and G) necrosis induced by N. dimidiatum (isolated NDFB002), without and with a cut, respectively; E) and H) necrosis induced by N. dimidiatum (isolate NDFB003), without and with a cut, respectively.
Diseases caused by Botryosphaeriaceae spp. are greater when the affected plants are under stress conditions. Under the environmental conditions of Hermosillo, Sonora, namely extreme heat and low relative humidity, Ficus plants are more likely to become infected with N. dimidiatum. The greater predisposition of the plants to infections by species of the family Botryosphaeriaceae due to specific environmental conditions was reported by Hassan et al. (2011). They exposed eucalyptus (Eucalyptus camaldulensis) and poplar (Populus nigra) plants to warm (32 °C) and very warm (40 °C) temperatures and to N. dimidiatum, observing that extreme temperatures predisposed the plants to greater infection by the fungus. Similarly, Sadowsky et al. (2007) reported the effect of three temperatures (25, 32 and 40 °C) on grapefruit plants, indicating that extreme temperatures (40 °C) are a crucial factor in predisposing plants to infection by Scytalidium lignicola. In this regard, Jami et al. (2013) and Slippers and Wingfield (2007) note that several members of the family Botryosphaeriaceae are latent pathogens that live as endophytes in their asymptomatic hosts for extended periods and that cause diseases under stress conditions. In particular, N. dimidiatum has been reported as a pathogen of woody plants such as English walnut (Juglans regia), mango (Mangifera indica), grapeine (Vitis vinifera L.), Ficus carica and Ficus spp., in which it produces symptoms such as dieback, branch necrosis and cankers in the stem, as well as death of the graft union in English walnut plants in nurseries, among other symptoms (Al-Saadoon, Ameen, Hameed, Al-Badran, & Ali, 2012; Chen, Fichtner, Morgan, & Michailides, 2013; El Atta & Aref, 2013; Ray, Burgess, & Lanoiselet, 2010).
Phylogenetic analysis
The concatenated sequences of the ITS, RPB2 and LSU regions were compared with those of fungal isolates from species belonging to the family Botryosphaeriaceae.The multilocus analysis indicated that the N. dimidiatum isolates (CBS: 204.33, CBS: 125616 and CBS: 125695) are grouped in the same phylogenetic branch as the fungi obtained in the present study. By contrast, the species of Botryosphaeria, Macrophomina, Neofusicoccum, Dothiorella, Lasiodiplodia and Diplodia are grouped in phylogenetically different nodes (Figure 3).
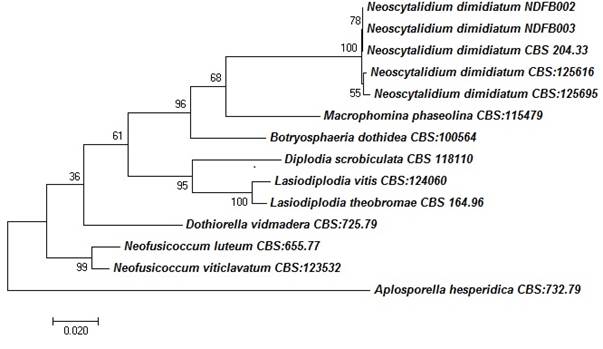
Figure 3 A phylogenetic tree of species of the family Botryosphaeriaceae using the Maximum Likelihood method with combined sequences of the ITS, LSU and RPB2 regions. Only bootstrap values ≥ 60 % are shown. The length of the branches is proportional to the genetic distances. All related sequences were taken from the CBS-KNAW culture collection database of the Westerdijk Institute in Holland.
Conclusions
Based on the morphological and molecular characteristics of the ITS, RPB2 and LSU regions, Neoscytalidium dimidiatum was identified as the causal agent of branch dieback and cankers in Ficus benjamina plants in Hermosillo, Sonora. To our knowledge, this is the first report of Neoscytalidium dimidiatum affecting Ficus benjamina plants in Mexico.











 texto en
texto en 


