Introduction
Citrus tristeza is a disease that has had a great impact in all the citrus regions of the world due to the death of almost 100 million trees grafted onto sour orange (Bar-Joseph, Batuman, & Roistacher, 2010). The pathogen responsible is the citrus tristeza virus (CTV), a 20 kb positive-sense, single-stranded RNA Closterovirus with 12 open reading frames and nontranslatable regions 5’ and 3’ terminus (Karasev, 2000). The CTV species is made up of a collection of homologous sequences that differ from each other by up to 4 %. Within the CTV population there are isolates with sequence variants or haplotypes (Ayllón et al., 2006; Rubio et al., 2001). Each isolate can induce different symptoms (Herrera-Isidrón, Ochoa-Sánchez, Rivera-Bustamante, & Martínez-Soriano, 2009; Weng et al., 2007), or even be asymptomatic (Bové, Vogel, Albertini, & Bové, 1988; Rivas-Valencia et al., 2010).
CTV is transmitted semi-persistently by Aphis gossypii and Toxoptera citricida, among other aphids (Yokomi et al., 1994). Transmissibility may also be specific (Hermoso-de Mendoza, Ballester-Olmos, & Pina-Lorea, 1984; Roistacher & Bar-Joseph, 1987), as well the ability to interfere with other isolates of different virulence (Costa & Müller, 1980; van Vuuren, Collins, & da Graça, 1993). As in other biological systems, vector abundance, viral replication rate and viral transmission are significantly modified by environmental conditions (Hermoso-de Mendoza et al., 1984; Raccah, Loebenstein, & Bar-Joseph, 1976). Among the most studied climatic elements are temperature, relative humidity and rainfall (Piyaratne et al., 2014; Wallin & Loonan, 1971). The ideal temperature for the biological cycle of the vectors, and the replication and transmission of the virus is between 20 and 26 °C (Garnsey et al., 2005; Raccah et al., 1976; Yokomi & de Borde, 2005).
In Mexico, the first CTV detections were in Tamaulipas (1983) and Veracruz (1986) (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación [SAGARPA], 2001). Until 2007, the Dirección General de Sanidad Vegetal (DGSV) detected and eliminated infected plants (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria [SENASICA], 2014). However, despite the eradication of the initial foci, the disease has continued to spread (Góngora-Canul et al., 2005; Rivas-Valencia et al., 2010; Ruiz-García et al., 2009; Silva-Vara et al., 2001). Currently, T. citricida is found in 18 citrus-producing states (SENASICA, 2014), putting them at risk of having severe citrus tristeza epidemics. The prevalent CTV isolates in Yucatán have been the moderate type (Rivas-Valencia et al., 2010), while severe infections have been detected in Nuevo León, Colima, Baja California, Veracruz and Tamaulipas (Herrera-Isidrón et al., 2009; Loeza-Kuk et al., 2005; Mendoza, Salazar, Alvarado, Cruz, & Barrera, 2003; Silva-Vara et al., 2001).
In Yucatán, with the arrival of T. citricida in 2000 (Michaud & Álvarez, 2000), an increase in isolates and severe symptoms of CTV was predicted as in other countries (Halbert et al., 2004; Matos et al., 2013); however, these have not been detected. It is unknown whether the absence of severe symptoms is linked to local climatic conditions that mask severe isolates. In this sense, it was necessary to carry out a long-term study to detect changes in the population structure linked to virus replication, productive effects on the host, vector survival, dispersion and the climatic component that offer relevant information for the management, control and prediction of the epidemic expression of CTV.
Therefore, the aim of this study was to temporally characterize CTV behavior, considering its population structure (PS), vigor, concentration in tissues and availability of vectors, this on the basis of an analysis of the thermal conditions in Yucatán.
Materials and methods
Location of study area
In October 2009 and November 2010 and 2014, two commercial citrus orchards were sampled: Cabaché 2 (20° 20’ 25.8’’ North latitude and 89° 26’ 48.2’’ West longitude) and Unión 2 (20° 20’ 26.2’’ North latitude and 89° 26’ 21’’ West longitude), located in Ticul, municipality of Yucatán, Mexico, both orchards with CTV incidence since 2002 and 25-year-old trees.
CTV sampling and detection
The sampling carried out in Cabaché 2 was restricted to 24 rows with 24 trees each and in Unión 2 to 9 rows with 24 trees each, in order to detect new CTV-positive trees and update the registry initiated in 2003 (Rivas-Valencia et al., 2010). The material consisted of young shoots collected from the four cardinal points of the tree at 1.50 m in height and later stored at 4 °C until processing in the laboratory. Detection of CTV-positive trees was performed with direct-ELISA immunoprinting (Plant Print Diagnostics SL. and Agdia S.C.), according to the procedure of Ruiz-García et al. (2009).
Molecular characterization and population structure of CTV
RNA extraction
First, 100 mg of fresh leaf midribs from each sample were used for the extraction of total RNA with Trizol® Reagent (Invitrogen™), according to the manufacturer's protocol.
For the polymerase chain reaction (PCR), cDNA was obtained by reverse transcription with hexamers, and a portion of the p25 gene encoding the coat protein (CP) (273 bp; Table 1) was amplified and analyzed on agarose gel (Kong, Rubio, Polek, & Falk, 2000). The PCR products were subjected to single-strand conformation polymorphism (SSCP), which allows detecting most sequence variants without having to use cloning procedures to separate them, especially when there is a dominant sequence (Iglesias et al., 2008). Afterwards, 5 µl of the amplification product were denatured for 10 min at 99 °C in a solution containing 95 % formamide, 20 mM EDTA, blue bromophenol and xylene cianol. The fragments were separated by 12 % polyacrylamide gel electrophoresis in a Bio-Rad Protean II® chamber with TBE buffer 1x at 200 v for 2 h 30 min at room temperature. The gel was stained with silver according to the protocol of Beidler, Hilliard, and Rill (1982).
Table 1 Primers used for detection and characterization of citrus tristeza virus isolates in Yucatán.
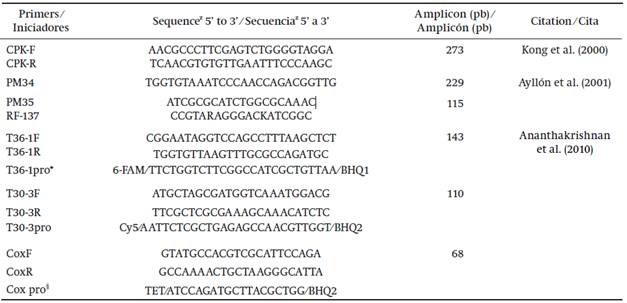
z R = A or G, K = T or G, FAM = 6-carboxyfluorescein), Cy5 = cyanine-5, TET = tetraclorofluorescein.
* Prefix pro indicates probes labelled for identification of isolates in real-time PCR.
§Cytochrome oxidase, internal reaction control
Analysis of electrophoretic patterns
To compare the differences between the electrophoretic patterns, a matrix with presence vectors (1) and absence (0) of band signal SSCP was constructed from Nei and Li’s (Nei & Li, 1979) coefficient. A dendrogram was generated by the unweighted pair group method with arithmetic mean (UPGMA) using the software NTSYSpc ver. 2.10L and an analysis of molecular variance was performed with the software GenAlEx 6.5 (Peakall & Smouse, 2012).
Selective PCR
Additionally, the cDNA of the samples was processed to differentiate T-385 (moderate: PM34/RF137 primers) and T-36 (severe; PM35/RF137 primers) isolates (Table 1; Ayllón et al., 2001).
The same cDNA obtained was subjected to real-time PCR to differentiate T-30 and T-36 isolates (Table 1, Ananthakrishnan, Venkataprassana, Roy, & Brlansky, 2010) and to determine the relative concentration of the isolates present in the plant. The final reaction volume was 25 μl containing 1X PCR buffer, 2.5 mM MgCl2, 100 nM dNTPs, 200 nM of primers and probe for T-30 (100 nM were used for T-36), 100 nM of primers for citrus cytochrome oxidase (COX), 50 nM COX probe (internal control) and 1 U Taq DNA polymerase (Invitrogen™). The reactions were carried out in a Cepheid-brand SmartCycler® thermocycler with a profile of 90 s, 40x (94 oC, 30 s and 60 oC, 90 s).
Photographic record and vigor evaluation
The digital photographic record of positive trees was carried out in 2004, 2006, 2009 and 2015 (with Samsung® L200). Tree vigor assessment was performed using a diagrammatic scale that takes into account the vigor of the primary and secondary branches based on leaf density, appearance and coloring to relate it to the presence of pathogens, with an emphasis on CTV (Figure 1).
Climate analysis
Maximum and minimum temperatures and accumulated precipitation were analyzed. A daily thermal oscillation index and an index of days with favorable conditions for the citrus tristeza pathosystem were generated using daily data from 1966 to 2014 from the Dzán conventional weather station (Comisión Nacional del Agua - Servicio Meteorológico Nacional [CONAGUA-SMN]), located 4.3 km from the orchard; also, data from an automated station in Maní belonging to the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) weather station network was used.
Results and discussion
Sampling and detection of CTV-positive trees
In 2009 and 2010, 3,712 tissue prints were made in Cabaché 2 and 1,728 in Unión 2 for the detection of CTV. All trees sampled were grafted onto sour orange (Citrus aurantium L.). In Cabaché 2, a 40 % increase in positive trees (83 trees) was detected, compared to the number found in 2006 by Rivas-Valencia et al. (2010). In Unión 2, seven positive trees were detected that were not consistent between dates (data not shown). In 2014, 95 trees were sampled, including those with a negative historical record to CTV as controls, of which 81 were positive and 14 negative (Table 2). In orchards located in Nuevo León with the same variety/rootstock combination, an annual dispersion of 0.27 and 0.19 % was observed (Silva-Vara et al., 2001), which is comparatively lower than estimated in Yucatán (10 %).
Table 2 Number of trees positive to citrus tristeza virus by immunoprinting and percentage of incidence in two commercial orchards in Ticul, Yucatán, Mexico.
| Orchard § | 2002 | 2003 | 2004 | 2006 | 2009-2010 | 2014 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTV z | CTV | Y (%) | CTV | Y (%) | CTV | Y (%) | CTV | Y (%) | CTV | |
| Cabaché 2 | 9 | 11 | 4.04 | 46 | 16.91 | 49 | 18.01 | 83 | 17.62 | 81 |
| Unión 2 | 1 | 0.2 | - | - | - | - | - | 7 | 3.24 | - |
§ Mixed orchard (sapodilla, mamey, avocado, banana and coconut) with irregular topological arrangement.
zCTV = citrus tristeza virus, Y = restrictive absolute incidence.
For 2014, only positive trees detected in 2009 and 2010 were sampled.
Molecular characterization and population structure
All positive samples by immunoprinting amplified part of the p25 gene of the CTV’s CP (81 samples; Figure 2A). Isolates subjected to selective PCR were moderate (81). Real-time PCR confirmed the prevalence of moderate T-30 isolates and they had a low viral particle concentration, with an average Ct (threshold cycle) of 25.6 ± 2.94 (Table 3). This variation is dependent on the type of isolate, time of infection, species of citrus and vigor of the trees, in addition to the temperature that limits the replication of the virus. In warm conditions, CTV reduces its rate of replication; however, the effect is lower in moderate isolates (Targon et al., 2005), so they are able to overcome, in the occupation of infection niches, the severe isolates.
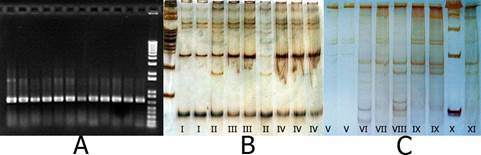
Figure 2 A) Electrophoresis in agarose (1 %) of p25 partial amplicons of citrus tristeza virus (CTV) (273 bp), B) and C) single-strand conformation polymorphism (SSCP) in 12 % polyacrylamide with fragments of the p25 gene of CTV isolates from Cabaché 2 (2010 and 2014), Yucatán, Mexico.Roman numerals indicate the type of electrophoretic pattern.
Table 3 Historical vigor of trees positive to the citrus tristeza virus and concentration of T-30 copies in Cabaché 2, Ticul, Yucatán, Mexico.
| Tree | Vigor | Ct z | Tree | Vigor | Ct | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2006 | 2010 | 2015 | 2004 | 2006 | 2010 | 2015 | ||||
| L1-A11 | 2a | 2 | 2 | 2 | 25.95§ | L6-A4 | 1 | 1 | 1 | - | 24.90 |
| L1-A12 | 3 | 3 | 4 | 4 | 25.28 | L6-A5 | 1 | 1 | 1 | - | 25.95 |
| L1-A14 | 2 | 3 | 3 | 4 | 23.86 | L6-A17 | 1 | 1 | - | 1 | 27.50 |
| L1-A17 | 1 | 1 | 1 | 1 | 26.76 | L7-A12 | - | - | - | - | 32.91 |
| L1-A18 | 2 | 2 | 1 | * | 24.65 | L8-A3 | 2 | 2 | 2 | - | 29.66 |
| L2-A11 | 2 | 2 | 2 | * | 31.99 | L8-A4 | 2 | 2 | 2 | - | 28.27 |
| L2-A17 | 1 | 2 | 2 | 2 | 27.91 | L9-A2 | 2 | 2 | 2 | - | 28.61 |
| L3-A9 | 1 | 1 | 1 | 2 | 26.57 | L9-A3 | 2 | - | - | - | 26.56 |
| L3-A12 | 2 | 2 | 2 | 3 | 28.90 | L9-A5 | - | 2 | 2 | - | 23.56 |
| L3-A14 | 2 | 2 | 2 | 2 | 25.60 | L9-A6 | 1 | 1 | 1 | 1 | 25.99 |
| L3-A15 | 2 | 2 | 2 | 3 | 25.94 | L11-A5 | - | 1 | 1 | - | 28.44 |
| L3-A16 | 1 | 1 | 1 | 1 | 25.70 | L11-A14 | - | 1 | 1 | 1 | 22.50 |
| L4-A9 | 2 | - | - | - | 27.74 | L12-A11 | 1 | 1 | 1 | 1 | 23.23 |
| L6-A3 | 2 | 2 | 2 | - | 23.60 | L12-A14 | 1 | 1 | 1 | 1 | 20.90 |
z CT = threshold cycle.
*Only live rootstock.
§Values < 22 high concentration and > 25 low concentration.
a1 = excellent, 2 = good, 3 = bad, 4 = very bad and 5 = dead.
The PS in 2009-2010 with SSCP showed electrophoretic patterns (EPs) with a greater number of bands (12), representing at least six haplotypes in the same tree (Figure 2B). In 2014, up to 10 bands were detected, represented in 11 EPs with at least five haplotypes (Figure 2C). This shows the dynamics in the pathosystem with temporal changes in PS, but not in prevalence. Rivas-Valencia et al. (2010) found, in this same orchard in 2006, three patterns of up to three bands; that is, a combination of up to two haplotypes per isolate. The existence of dominant T-30 sequences was determined by the SSCP analysis.
The dendrogram constructed from the absence and presence of bands separated the isolates into four groups, with a similarity coefficient of 0.80 (Figure 3). The proportion of isolates for groups I, II, III and IV was 13.6, 39.7, 13.63 and 32.95 %, respectively. Isolates from another geographical area of the peninsula were located in group IV, which indicates a common origin for the two populations.
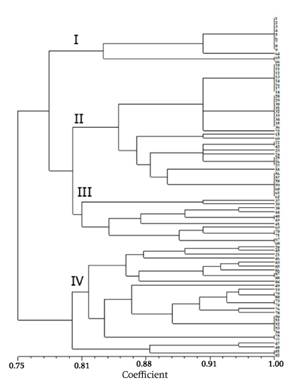
Figure 3 Dendrogram obtained from 88 electrophoretic profiles of citrus tristeza virus (CTV) isolates, Yucatán, Mexico. Seven isolates from a different geographical origin were included.
The analysis of variance showed that there is greater diversity within the populations (65 %) than among them (35 %, Table 4); this is in accordance with the number of electrophoretic patterns (11) and haplotypes (five) found. Previous studies have revealed that genomic diversity within and among CTV isolates is attributed to a certain pathogenicity (Albiach-Martí et al., 2010; Papaylannis, Santos, Kyriakou, Kapari, & Nolasco, 2007), which depends on the structure of the CTV population present (Kong et al., 2000).
Table 4 Analysis of molecular variance of electrophoretic patterns obtained from a fragment of the p25 gene (273 bp) from Yucatán isolates, Mexico (2014).
| Sources of variation | DF z | Variance estimators | Variation (%) | Significance* |
|---|---|---|---|---|
| Among populations | 1 | 1.50 | 35 | 0.35 |
| Within populations | 86 | 2.78 | 65 | -- |
z DF = degrees of freedom.
*Probability of occurrence of statistical probability variance values more extreme than those observed (P ≤ 0.05).
Evaluation of vigor of trees with chronic infection
The photographic record in 2004, 2006, 2009 and 2015 was 46, 49, 83 and 37, respectively. Throughout the 12 years, the vigor of the infected trees presented different degrees of deterioration (Figure 4).

Figure 4 Vigor of trees positive to the citrus tristeza virus (CTV). A) 2004, B) 2009 and C) 2015. Commercial orchard in Ticul, Yucatán, Mexico.
The interaction between citrus species and isolates with different severity has been extensively studied (Albiach-Martí et al., 2000). In this study, tree response to CTV infection showed few changes, consistent with infection by moderate isolates. Also, trees with a vigorous and healthy appearance showed a higher relative concentration of CTV. The concentration of viral particles in trees with vigor 1 was similar (Table 3) to young C. aurantifolia plants confined in a greenhouse (Ct = 23), while in less vigorous trees the viral concentration was lower (Ct = 27). In general, the viral concentration detected was low when compared to C. aurantifolia plants maintained at 28 °C (Ananthakrishnan et al., 2010).
Regional climatic characterization
The graph of temperature and precipitation (Figure 5A) indicated that the study area has an Aw1(w’’)(i’)g climate type (García, 1998), which corresponds to warm intermediate subhumid with midsummer drought, little thermal oscillation and a Ganges-type annual temperature march. This is not a common climate in which severe isolates are developed and expressed, except in Florida, U.S.A. (Table 5). In Tamaulipas, even though the incidence of severe isolates was recorded, in subsequent years it was not possible to detect them again (Rivas-Valencia et al., 2010), indicating the effect of temperature on severe isolates.
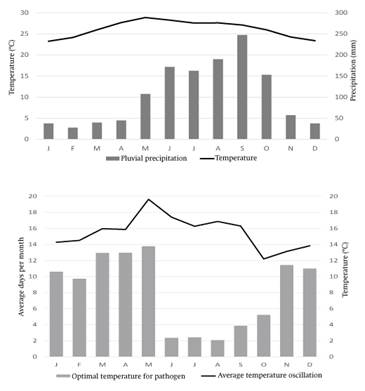
Figure 5 A) Graph of temperature and pluvial precipitation of the study area (1966-2014) and B) daily thermal oscillation (line) and appropriate daily hours (bars) for the citrus tristeza virus (CTV).Generated with data from the 31009-Dzán conventional weather station, CONAGUA-SMN and EMA-Maní-INIFAP.
Table 5 Climate and thermal condition of sites with incidence of severe isolates of the citrus tristeza virus.
| Site | Type of CTV z isolate | Climate § | Annual TO | Monthly TO | Classification of TO | Citation |
|---|---|---|---|---|---|---|
| Dzán, Yucatán, Mexico | NS* | Aw1w’’(i’)g | 5.6 | 14.5 | VE | |
| Mante, Tamaulipas, Mexico | S | Aw0w’’(e’) | 10.8 | 13.5 | E | Loeza-Kuk et al. (2005) |
| Cundinamarca, Bogota, Colombia | S | Cb’(w2)(x’)w’’ig | 1.45 | 8.5 | E | Oliveros-Garay, Martínez-Salazar, Torres-Ruiz, and Acosta (2009) |
| Meta, Villavicencio, Colombia | S | Am(i)g | 2.7 | 9.4 | E | |
| Ora, Salta, Argentina | S | (A)Ca(w2)(w)w’’(e) | 11.6 | 11.3 | E | Iglesias et al. (2008) |
| Montecasero, Corrientes, Argentina | S | (A)Ca f w’’(e) | 13.5 | 10.7 | E | |
| Entre Rios, Concordia, Argentina | S | (A)Ca(fm)w’’(e’) | 14.1 | 11.7 | E | |
| Tucuman, Alta Gracia, Argentina | S | (A)Ca(w2)(w)(e) | 13.2 | 11.6 | E | |
| Jujuy, Calilegua, Argentina | S | (A)Ca(w0)(w)(e) | 12.2 | 12.6 | E | |
| Sharon plain, Tel Aviv, Israel | S | (A)Ca s(e) | 13 | 7.8 | E | Marcus, Salomon, and Bar-Joseph (1987) |
| Seferihisar, Izmir, Turkey | S | Ca s(e’) | 18.9 | 9.2 | E | Çevik, Yardimci, and Korkmaz (2013) |
| Kastela, Split, Croatia | S | Ca s(e’) | 18 | 6.4 | E | Černi et al. (2005) |
| Immokalee, Florida, United States | S | Aw1(x’)w’’(e) | 10.5 | 10.1 | E | Halbert et al. (2004) |
Data obtained from the World Meteorological Organization (WMO, 2016).
zCTV = citrus tristeza virus, TO = thermal oscillation.
*NS = non-severe isolate, S = severe isolate, I = isothermal, E = extreme, VE = very extreme.
§Climates classified according to García (1998).
In the annual temperature march, January has the lowest average monthly minimum temperature (ammt) with 15.7 °C, while in June the ammt reaches its maximum value with 21.4 °C. Similarly, January shows the lowest average monthly maximum temperature (AMMT) at 30.8 ° C, while in May it reaches its maximum value with 36.8 °C. This variation frequently translates into a very extreme daily thermal oscillation (TO), which in May reaches 19.6 °C (Figure 5B). These conditions are restrictive during half the year for the components involved in the studied pathosystem. Although Florida shares a similar climate with Yucatan, in sites with severe isolate expression, the monthly thermal oscillation is defined as extreme (Table 5).
The CTV depends on the aphids involved for transmission; however, they lose their reproductive capacity at 30 °C. The values of higher fecundity and net reproductive rate of T. citricida, A. spiraecola and A. gossypii are recorded at 20 °C (Komasaki, 1982). Toxoptera citricida shows a similar behavior; at 20 °C a female can generate 52 individuals, while at 32 °C it generates seven and survival plummets to 29 % (Tsai & Wang 1999).
In Yucatán, favorable temperatures for the pathosystem are partially reached from October to December and from January to March, which coincides with the findings previously reported by Patiño-Arellano, Rodríguez-Leyva, Mora-Aguilera, Lomelí-Flores, and Díaz-Gómez (2012), where the abundance of T. citricida, T. aurantii, A. spiraecola and A. gossypii populations is concentrated in those months. Even so, in this interval the TO is extreme (10 to 14 °C), or eventually very extreme (> 14 °C, Figure 5B). These values are a stress factor, even more important than the minimum and maximum te mperatures per se. Studies on the impact of extreme TO reveal an increase in mortality of other insects (Ma, Hoffmann, & Ma, 2015), especially when maximum environmental temperatures exceed lethal levels for the insect (Chanthy, Martin, Gunning, & Andrew, 2012).
In Yucatán, the months with the highest number of hours conducive to aphids and the virus (>22 <31 °C) are from November to May, and may suggest their presence during this whole period. However, TO also increases from March to September with values above 16 °C. Citruses in the region (especially C. latifolia) show high sprouting flows in February, May, August, December and a small one in November (Lomas-Barrié, Loeza-Kuk, Cicero-Jurado, Sanchez-Borja, & Arredondo-Bernal, 2015). That is, there is sufficient food and colonization niches for the aphids, but they are not able to take advantage of the sproutings due to climatic factors.
The thermal regime of the region also modifies the transmission of the virus, which is maximized at 22 ± 2 °C (60.8 %) and reduced at 31 °C (12.2 %) (Bar-Joseph & Loebenstein, 1973; Raccah et al., 1976).
This study shows that the CTV population structure is composed of moderate T-30 isolates. This prevalence allows the coexistence of the virus with the infected trees, even with the presence of T. citricida, which has not occurred in other producing areas (Matos et al., 2013). However, this does not exempt the structure from being modified by severe isolates such as T-36 or VT, as in other producing areas (da Graça & van Vuuren, 2010; Roistacher, da Graça, & Müller, 2010). In the Dominican Republic over a 10-year period, T-30 isolates were displaced by T-36 and VT, introduced by the exchange of infected propagating material (Matos et al., 2013). In Yucatán, the use of sour orange as a rootstock is a risk factor in two ways: 1) for its susceptibility to CTV and 2) for the unknown origin and quality of the propagating material used, which sometimes does not comply with NOM-079-FITO-2002 (SAGARPA 2002).
Conclusions
Changes in the pathogen’s population structure were identified in the trees of the orchards studied; however, no severe CTV symptoms or isolates were found, nor was there a significant detriment to the vigor of the CTV-positive trees. Nonetheless, CTV dispersion increased by 40 % in four years. Among the climatic variables, the daily thermal oscillation and the number of hours conducive to the pathosystem operate to its detriment and reduce its impact, making this a restrictive thermal regime. These conditions have allowed the sour orange rootstock to persist as the producers’ preferred choice. However, it is necessary to provide alternative rootstocks, due to the risk that exists in the commercialization of propagating material that does not comply with the regulations.











 texto en
texto en 



