Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Chapingo. Serie horticultura
versión On-line ISSN 2007-4034versión impresa ISSN 1027-152X
Rev. Chapingo Ser.Hortic vol.21 no.3 Chapingo sep./dic. 2015
https://doi.org/10.5154/r.rchsh.2014.11.050
Artículo de revisión
Mechanisms of resistance in postharvest fruit-pathogen interaction
Mecanismos de resistencia en la interacción fruto-patógeno en poscosecha
Rocío Aurora Sandoval-Chávez1; Ramón Álvar Martínez-Peniche1*; Montserrat Hernández-Iturriaga1; Neus Teixidó-Espasa2; Josep Usall-Rodié2; Inmaculada Viñas-Almenar3; Rosario Torres-Sanchis2
1 Cuerpo Académico de Inocuidad Microbiana de los Alimentos, DIPA, Facultad de Química, Universidad Autónoma de Querétaro. Cerro de las Campanas s/n, col. las Campanas, Querétaro, Querétaro, C.P. 76010, MÉXICO. Correo-e: alvar@uaq.mx, teléfono: 442 192 13 04 (*Autor para correspondencia).
2 Institut de Recerca i Tecnologia Agroalimentàries, XaRTA-Postharvest, Rovira Roure 191, 25198 Lleida, Catalonia, ESPAÑA.
3 Food Technology Department, Lleida University, XaRTA-Postharvest, Agrotecnio Center. Rovira Roure 191, 25198 Lleida, Catalonia, ESPAÑA.
Received: November 28, 2014.
Accepted: August 12, 2015.
Abstract
The objective of this review was to bring together concepts related to studies aimed at elucidating defense mechanisms against disease-causing agents, mainly in postharvest. Like plants, fruits are exposed to attack by pathogens that cause rot during postharvest storage, resulting in considerable losses. To control these pathogens, synthetic chemicals are used; however, since they are toxic, genetic resistance is regarded as a viable alternative. Fruits can withstand pathogens by means of physical barriers (presence of thick cuticular or trichome layers) and chemical ones, or through induced defenses that are activated once the host detects the presence of the pathogen, triggering the oxidative burst during the early hours of interaction. This burst entails the generation of reactive oxygen species (ROS), such as superoxide (O2–), hydroxyl radical (OH–) or hydrogen peroxide (H2O2), and the activation of genes involved in several metabolic pathways. The study of such mechanisms may allow detecting disease-resistant genetic materials, thus reducing the use of toxic products.
Keywords: constitutive defenses, induced defenses, postharvest diseases, phytopathogen, defense mechanisms.
Resumen
El objetivo de esta revisión fue conjuntar conceptos relacionados con estudios dirigidos a elucidar los mecanismos de defensa contra agentes causantes de enfermedades, principalmente en poscosecha. Al igual que las plantas, los frutos se encuentran expuestos al ataque por patógenos que producen podredumbres durante su almacenamiento en poscosecha, causando considerables pérdidas. Para el control de dichos patógenos, se emplean productos químicos de síntesis que son tóxicos, y la resistencia genética se considera una alternativa viable. Los frutos pueden tolerar a los patógenos mediante barreras físicas (presencia de capas gruesas de cutícula o de tricomas) y químicas, o bien, a través de defensas inducidas que se activan una vez que el huésped detecta la presencia del patógeno, desencadenando la explosión oxidativa durante las primeras horas de la interacción. Esta explosión conlleva la generación de especies reactivas de oxígeno (ROS) como el superóxido (O2–), el radical hidroxilo (OH–) o el peróxido de hidrógeno (H2O2), y la activación de genes involucrados en diversas rutas metabólicas. El estudio de tales mecanismos puede permitir detectar materiales genéticos resistentes a enfermedades, reduciendo así el uso de productos tóxicos.
Palabras clave: defensas constitutivas, defensas inducidas, enfermedades de poscosecha, fitopatógeno, mecanismos de defensa.
INTRODUCTION
Plants carry out physiological processes necessary for their survival, including cell division and elongation, differentiation and development, absorption and translocation of water and minerals from the soil, synthesis, degradation and storage of organic compounds and reproduction. When one of these functions is disrupted, either by phytopathogens or certain environmental conditions, a disease is generated (Agrios, 2007).
As in plants, the presence of pathogens in fruits causes considerable postharvest losses. However, they have different protective barriers, which may be intrinsic or develop once they detect the presence of a pathogen (Ferreira et al., 2006).
Phytopathogenic fungi are a very diverse group of heterotrophic nutrition organisms, which require a number of virulence factors that enable them to cause infection, such as possessing penetration structures (appresoria) and producing hydrolytic enzymes that degrade the cell wall of fruits, among others (Prusky, Mcevoy, Saftner, Conway, & Jones, 2004). The main method of controlling these fungi in postharvest has been the use of fungicides, which have been restricted because of their toxicity, so alternative methods such as the use of resistant varieties are being sought (Janisiewicz, Saftner, Conway, & Forsline, 2008; Jurick et al., 2011).
In this sense, the study of fruit defense mechanisms could allow the detection of resistant genotypes, as well as the exogenous application or induction of compounds that naturally reduce postharvest diseases. Therefore, the aim of this review was to bring together concepts related to research aimed at elucidating the mechanisms of protection against disease-causing agents, especially in post-harvest, which is of great importance because it is an emerging area of knowledge.
Postharvest fruit diseases
In Plant Pathology, diseases produced in fruits are called "rot" and the fungi which cause them are called "pathogens." In the postharvest period, these alterations cause deterioration of the fruit from harvesting until being consumed or processed (Viñas, Abadias, Teixidó, Usall, & Torres, 2013). Losses due to postharvest diseases have been estimated at around 20 % in developed countries, and up to 50 % in developing countries (Janisiewicz & Korsten, 2002).
Disease development
Like plants, fruits are in contact with a myriad of microorganisms found in the environment; however, in order for the disease to develop, the host must be susceptible to a virulent pathogen and the environment must be conducive to the infection (Ferreira et al., 2006).
Listed below are the stages of postharvest disease development (Figure 1) according to Viñas et al. (2013): 1) contamination, the inoculum reaches the host, usually by the spreading of spores; 2) penetration, the pathogen enters through the tissue of the host by means of penetration structures, specialized enzymes or wounds; 3) infection, the process by which the pathogen comes in contact with the cells of the host which it later feeds on; 4) incubation, the time between infection and the appearance of disease symptoms; 5) diffusion or invasion, the pathogen extends beyond the colonized tissues; 6) reproduction and dissemination, the pathogen colonizes new tissues and forms resistance structures, and 7) survival, the resistance structures formed remain in the environment until finding conditions conducive to infect new hosts.
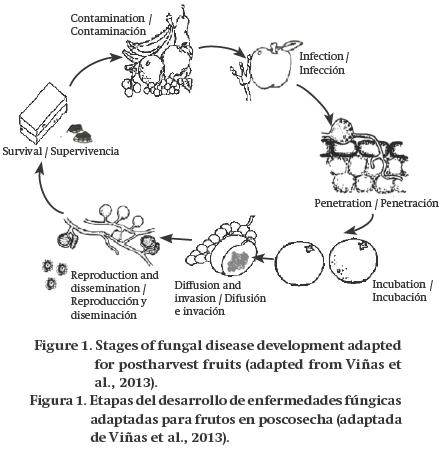
As mentioned above, not all pathogens cause disease. This inability is because, in many cases, fruits have different defense mechanisms: a) constitutive or non-induced, which involve intrinsic factors that can foster a hostile environment for pathogens, and b) activated or induced, including pathogen recognition by the fruit and activation of different biochemical pathways to counteract the infection (Wood, 2012).
Constitutive or non-induced defenses
These defenses can be divided into two categories: a) structural, involving physical barriers able to stop the spread of pathogens, such as the presence of thick epidermal layers composed of cutin and waxes, or trichomes, among others (Wood, 2012); b) chemical, consisting of the presence of toxic compounds found in their active form, such as alkaloids, phenols, polyphenols, essential oils, terpenes, etc. Examples include the tannin concentration in the 'Wichita' pecan nut, which makes it more resistant to Aspergillus flavus and A parasiticus (Vázquez, Martínez, & Fernández, 2001), 5-n-pentadecylresorcinol and 5-n-heptadecenylresorcinol found in varieties of mango as resistance factors to anthracnose caused by Colletotrichum gloeosporioides (Hassan, Dann, Irving, & Coates, 2007), or the presence of a greater amount of phenolic compounds in immature chilli (Capsicum annuum L.) fruits compared with ripe fruits, which makes them less susceptible to C. capsici and Alternaria alternata (Anand, Bhaskaran, Raguchander, Samiyappan, Prakasam, & Gopalakrishnan, 2009).
In the case of physical barriers, it is assumed that fruits can be more or less susceptible to both biotic and abiotic stresses depending on factors such as size, shape, firmness, epicarp resistance, presence of stomata, osmotic concentration or growth stage of the fruit (Khadivi-Khub, 2014). In olive, differences in the thickness of the cuticular membrane were observed among different varieties (Gentile di Chieti with 17.04 μm vs Intosso with 8.46 um); it was also noted that epicarp thickness decreased during the ripening process (Lanza & Di Serio, 2015). Guan et al. (2015) conducted a study with different varieties of apple inoculated with Botryosphaeria dothidea, and found a negative correlation between lesion size and the cuticular thickness of the fruit, meaning the greater the cuticular thickness the lesser the pathogen development. They also noted that natural openings are a determining factor in the susceptibility of apples to B. dothidea. Despite these important factors, pathogens are, in most cases, able to overcome such barriers, which is why alternative control methods are used.
Activated or induced defenses
The most common form of activated defense is called horizontal or non-specific resistance (also known as "nonhost," polygenic or quantitative); in this, the pathogen is unable to produce disease in a host because the latter is immune to all races pathogen, and the resistance is controlled by several genes (Nürberger & Lipka, 2005). This type of resistance has been classified into two types (Mysore & Ryu, 2004). In Type 1, necrosis does not occur in the cells, since the pathogen is unable to overcome the defense mechanisms such as the expression of genes encoding pathogenesis-related proteins (PRs), and components of systemic acquired response (SAR) that are induced by the pathogen elicitors (Figure 2A). In Type 2, the pathogen is able to overcome the host's protective responses, probably due to the production of detoxification enzymes, resulting in localized necrosis (Figure 2B). For its part, the host recognizes specific pathogen elicitors and activates defenses that lead to the hypersensitive response (HR) (Figure 2B).
Another type of resistance is the vertical or specific kind (gene-gene model), based on the genetic recognition and interaction between the product of an avirulence (AVR) gene in the pathogen and a resistance (R) gene in the plant or fruit. When this event occurs, it is called an incompatible interaction. It is important to note that the host will only be resistant to pathogens that are carriers of the AVR gene. On the contrary, when the pathogen is capable of causing infection in the host it is known as a compatible interaction (Elvira, Molina, Gilardi, García-Luque, & Serra, 2008). The resistance or susceptibility depends on the time it takes for the host to recognize the pathogen and the speed with which it activates its defense mechanisms (Boller & Yang, 2009) (Figure 3).
Another more recent theory includes a "zigzag" model, which holds that the amplitude of the resistance or susceptibility is proportional to PTI - ETS + ETI, and is carried out in four phases: 1) in the first, the plant detects a series of PAMPs (pathogen-associated molecular patterns) through PRRs (pattern recognition receptors) that leads to PAMP-triggered immunity (PTI); 2) at this stage, some pathogens manage to overcome this resistance by means of substances that interfere with PTI, resulting in effector-triggered susceptibility (ETS); 3) one of the effectors is recognized by an R protein (resistance-associated protein), activating effector-triggered immunity (ETI) that amplifies PTI, and in turn is related to hypersensitive response (HR); 4) during this phase, natural selection makes some of the pathogen's diversified genes lose the recognized effector and gain new effectors through horizontal gene flow, enabling them to overcome ETI. Finally, selection favors the appearance of new R proteins, enabling plants to recognize other effectors, again resulting in activation of ETI (Jones & Dangl, 2006).
In the case of beans, it has been observed that the pathogen Phytophthora sojae suppresses the positive regulator of programmed cell death (PCD) (Dou et al., 2008). In addition, P. infestans uses the expression of effectors SNE1 and PiNPP1.1 to optimize its growth (Kelley et al., 2010).
In fruits it is known that softening promotes pathogen development, and that the cell wall plays an important role in the recognition of this and in the deployment of defense responses. However, due to the degradation of the cell wall by the pathogen there is enzyme depolymerization, a process linked to the PTI molecules (Mengiste, 2012).
In tomato, the simultaneous suppression of the LePG and LeExp1 genes (associated with the fruit ripening process) reduces their sensitivity to B. cinerea. On the other hand, this pathogen also induces the expression of these genes, suggesting that there are aspects that are induced by the fungus and that they are similar to those that occur during fruit ripening, which contributes to their greater susceptibility (Cantu et al., 2008).
The behavior of PAMPs in plant-pathogen interactions has been widely studied, and it is thought to have certain similarities with fruit responses to pathogens; however, the details of how they are regulated and how they interact in these cases have yet to be elucidated; therefore, more research in this area is needed.
Hypersensitive response (HR)
HR takes place during the first few minutes of interaction, at the pathogen's point of entry and in adjacent cells. This occurs as a "suicide" program, active and organized, and not because of a passive cell collapse produced by the pathogen attack or toxins resulting from it (Torres, Jones, & Dangl, 2006). In this cellular process, protein kinase and phosphatase enzymes are activated, and there are also changes in membrane permeability and intracellular ion concentration (Zhao, Davis, & Verpoorte, 2005). At the same time, rapid activation of reactive oxygen species (ROS) occurs; this process includes the production of substances such as superoxide (O2–), hydroxyl radical (OH–) and hydrogen peroxide (H2O2), which are responsible for the oxidative burst that indicates that the host has successfully recognized the pathogen. This takes place in two phases: the first is transient and nonspecific and occurs in the first few minutes of interaction, while the second appears hours after the pathogen attack and is associated with the establishment of defenses (Grant & Loake, 2000) (Figure 4).
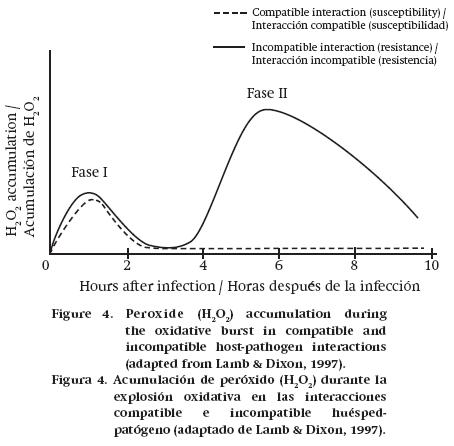
ROS are associated with defense responses, playing a varied and complex role, since they can directly cause toxicity to pathogens, such as the radical OH– which is more reactive than the other oxidative species (H2O2, O2–) (Torres, 2010). It was observed that unripe avocado fruits were able to activate ROS production in response to infection by C. gloeosporioides, resulting in greater resistance to the pathogen, unlike what happened with ripe fruits (Beno-Moualem & Prusky, 2000).
Plasma membrane NADPH oxidases and cell wall peroxidases (POXs), both of which are enzymes, are probably the main biochemical sources of ROS production (Torres et al., 2006). The NADPH oxidase enzyme subunit (gp91phox) is responsible for transferring electrons to molecular oxygen for the generation of superoxide (O2–) (Alkan, Davydov, Sagi, Fluhr, & Prusky, 2009). Meanwhile, the peroxidases catalyze the oxidation-reduction (redox) of different substrates using H2O2, and depending on the pH they can also be a source of H2O2 in the presence of a free reductant (O'Brien, Daudi, Butt, & Bolwell, 2012). In unripe Golden Delicious apples, increased H2O2 levels in response to infection by Penicillium expansum were observed (Torres, Valentines, Usall, Viñas, & Larrigaudiere, 2003). On the other hand, Macarisin et al. (2007) found that inoculation with Penicillium digitatum suppressed H2O2 production in citrus fruits (compatible interaction), while inoculation with P. expansum increased H2O2 accumulation by 63-fold over the control (nonhost interaction).
ROS can also form physical barriers at the infection site by cross-linking ofcell wall glycoproteins or via oxidative cross-linking of precursors during biosynthesis of polymers such as lignin and suberin (Torres, 2010). These polymers can also be directly toxic, degrading the wall of the fungi and bacteria (Treutter, 2006). In this regard, Vilanova, Teixidó, Torres, Usall, and Viñas (2012) observed lignin production in unripe apples infected with P. expansum (compatible interaction) and P. digitatum (nonhost interaction) one day after inoculation; on the other hand, the lignin increase in unripe oranges occurred after 48 hours with both pathogens, decreasing in ripe fruits (Vilanova, Viñas, Torres, Usall, Jauset, & Teixidó, 2012). Apples treated with ROS-inducing compounds, such as guaiacol, also showed increased lignin production, which reduced the incidence of P. expansum (Valentines, Vilaplana, Torres, Usall, & Larrigaudiere, 2005).
Because the oxidative burst occurs as an active and organized program, fruits have various ROS detoxification systems involving enzymes such as ascorbate peroxidases (APX), glutathione reductase (GR), superoxide dismutases (SOD) and catalases (CAT), which maintain homeostasis in different cell compartments, restricting the ROS-induced damage and activating transduction signals (Mittler, Vanderauwera, Gollery, & Breusegem, 2004). Valentines et al. (2005) observed in immature apples infected with P. expansum higher oxidation potential, and in turn they were less susceptible than mature fruits, suggesting that POX is involved in pathogen resistance. It was also observed that oranges infected with P. digitatum reduced the levels of H2O2, SOD and CAT, after 72 hours, confirming the role of the pathogen in the deactivation of the fruit's ROS production mechanisms (Torres et al., 2011).
It is evident that ROS are also important defense gene activators. They can encode for enzymes involved in the synthesis of phytoalexins, which are secondary metabolites with antimicrobial properties that can accumulate in response to pathogens (Torres et al., 2006). Most of these metabolites are from the phenylpropanoid pathways, such as flavonoids, isoflavones, coumarins, stilbenes, dihydrophenanthrenes and other phenols. In unripe avocados a significant increase in epicatechin levels occurs six hours after being infected with C. gloeosporioides (Beno-Moualem & Prusky, 2000).
Another phytoalexin, the scoparone, was found in citrus fruits inoculated with P. digitatum and treated with hot water or UV radiation (Ben-Yehoshua & Mercier, 2005). It is known that scoparone levels increase in response to stress, resulting in a hostile environment for the pathogen, which decreases the incidence and severity of disease (Ballester, Izquierdo, Lafuente, & González-Candelas, 2010). An increase in polymethoxylated flavones (PMFs), chlorogenic acid (CGA), and scoparone in oranges infected with P. digitatum was also observed (Ballester, Lafuente, & González-Candelas, 2013).
Caffeic acid (CA) and CGA have also been proposed as resistance factors in peach against brown rot caused by Monilinia fructicola (Lee & Bostock, 2007). CA is able to affect the virulence of this pathogen through cellular redox regulation, increasing the activity of glutathione reductase, which in turn can maintain a high proportion of reduced glutathione (GSH) to oxidized glutathione (GSSG) in the cell (Chiu et al., 2012). These compounds are also capable of inhibiting the expression of virulence genes such as mfcut1 and mfpg1, which encode for a cutinase and a polygalacturonase, respectively, and they also inhibit the formation of appressoria (Lee & Bostock, 2007).
Also, the production of phytoalexins has been associated with the expression of a series of specific genes that encode specific enzymes responsible for their synthesis, such as phenylalanine ammonia lyase (PAL), chalcone synthase (CHS) and chalcone isomerase (CHI) (Yu & Jez, 2008). In oranges it was observed that pal1 and pox1 gene expression, as well as PAL and POX enzyme activities, increased after infection with P. digitatum and a subsequent heat treatment used as a control method (3 d, 37 °C) (Ballester et al., 2010).
This same behavior of the pal1 and pox1 genes was observed in oranges infected with P. digitatum and P. expansum after 24 hours of incubation, whereas, at 48 hours, the expression of pal1, pox1, comt1 and sad increased in response to the wound, and decreased against P. digitatum, demonstrating that this pathogen has the ability to suppress defense gene expression (Vilanova et al., 2013). For their part, Ballester et al. (2013) observed in the flavedo of oranges an increase in the level of expression of 10 genes related to the phenylpropanoid pathway, 48 hours after being infected with P. digitatum.
Systemic acquired resistance (SAR)
It is a broad spectrum response that provides the plant or fruit with long-lasting protection, even in areas far from the pathogen penetration site, from a second infection by the same or another agent (Glazebrook, 2005). The manifestation of SAR implies the existence of a system capable of transmitting signals through local and systemic tissues, including processes involving the synthesis of salicylic acid (SA) (Durrant & Dong, 2004), as well as jasmonic acid (JA) and ethylene (van-Loon, Geraats, & Linthorst, 2006) (Figure 5).
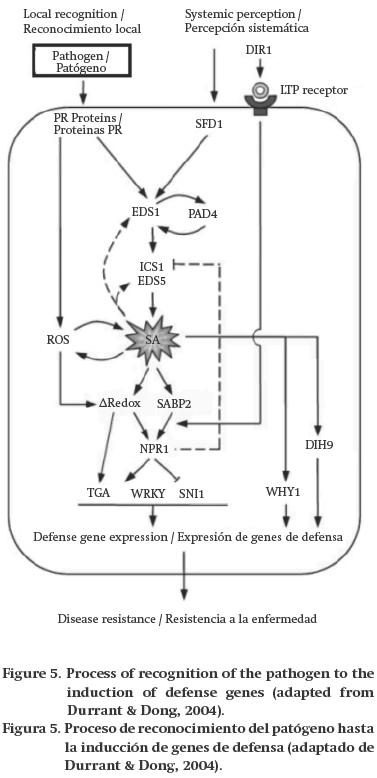
Zeng, Cao, and Jiang (2006) observed increased levels of POX, PPO, PAL, β-1,3-glucanase and ROS (namely H2O2 and O2– in mangoes treated with SA and subsequently inoculated with C. gloeosporioides, with a consequent reduction in the damage caused by the fungus. SAR is also associated with the accumulation of low molecular weight, protease-resistant PR proteins (Durrant & Dong, 2004), which are located extracellularly and are stable at low pH (Liu & Ekramoddoullah, 2006). Since the cell wall of fungi consists of glucans, chitins, fatty acids, etc., the fruit can present mechanisms to degrade these compounds with proteins such as PR-2 (β-1,3-glucanases) and PR-3 (chitinases); therefore, the high expression of these genes is important for defense against pathogens containing these substrates. The expression of pdpr5-1, which encodes for thaumatin, increases during plum (Prunus domestica) ripening and is over-expressed in cultivars less susceptible to M. fructicola. Also, the PR-5 proteins may indirectly contribute to other protection mechanisms such as in the phenylpropanoid and phytoalexin pathways (El-Kereamy et al., 2011). Ballester et al. (2010) observed increased activity of the gns1 and chi1 genes that encode for β-1,3-glucanase and chitinase enzymes in orange fruits inoculated with P. digitatum and heat treated (3 d, 37 °C). Casado et al. (2006) detected increased expression of various genes encoding PR proteins, ROS, leucine-rich proteins, glucanases and chitinases, in strawberries infected with C. acutatum that are less susceptible to the pathogen.
On the other hand, the induction of ethylene production and the expression of genes involved with their synthesis in interactions with citrus fruits and P. digitatum were reported (Marcos, González-Candelas, & Zechariah, 2005). Similarly, increased ethylene levels in oranges heat-treated and inoculated with P. digitatum were observed (Ballester et al., 2011; González-Candelas, Alamar, Sánchez-Torres, Zacarias, & Marcos, 2010). However, the role of ethylene in defense responses is complex, depending on the type of interaction (Berrocal-Lobo, Molina, & Solano, 2002).
In the case of JA, defense responses are not associated with cell death, making it an alternative in the application of treatments against necrotrophic fungi (McDowell & Dangl, 2000). It was observed that the application of methyl jasmonate in guava (Psidium guajava) is effective for controlling C. gloeosporioides through stimulation of the enzyme activities of lipoxygenase (LOX) and PAL (González-Aguilar, Tiznado-Hernández, Zavaleta-Gatica, & Martínez-Tellez, 2004).
CONCLUSIONS
Studies to date indicate that compounds such as ROS, phenylpropanoid pathway metabolites, ethylene, jasmonic acid and PR proteins, among others, or the expression of genes encoding them, are related to defense responses that fruits trigger once they detect the presence of a pathogen.
Although disease response processes in plants have been extensively studied, in the case of postharvest fruits many of them are still unknown, and also the results are different for each pathosystem, making it difficult to generalize them. Therefore, it is necessary to elucidate fruit protection mechanisms against diseases in comparison with other systems, metabolic pathways or other compounds and, on the other hand, deepen our knowledge of the virulence factors developed by pathogens to counteract such defenses. Generating more knowledge in this area will allow standardizing the different results obtained and improving the implementation of effective treatments for controlling postharvest diseases without harming man and the environment.
ACKNOWLEDGEMENTS
This work was funded by the Spanish government through project AGL2011-30519-C03-03 and by Mexico's National Science and Technology Council through funds FOMIX-QRO-2010-C01-145514.
REFERENCES
Agrios, G. (2007). Plant pathology (5a ed). San Diego, USA: Academic Press. [ Links ]
Alkan, N., Davydov, O., Sagi, M., Fluhr, R., & Prusky, D. (2009). Ammonium secretion by Colletotrichum coccodes activates host NADPH oxidase activity enhancing host cell death and fungal virulence in tomato fruits. The American Phytopathological Society, 22(12), 1484-1491. doi: 10.1094/MPMI-22-12-1484 [ Links ]
Anand, T., Bhaskaran, R., Raguchander, T., Samiyappan, R., Prakasam, V., & Gopalakrishnan, C. (2009). Defence responses of chilli fruits to Colletotrichum capsici and Alternaria alternata. Biologia Plantarum, 53(3), 553-559. doi: 10.1007/s10535-009-0100-5 [ Links ]
Ballester, A. R., Izquierdo, A., Lafuente, M. T., & González-Candelas, L. (2010). Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest Biology and Technology, 56, 31-38. doi: 10.1016/j.postharvbio.2009.10.002. [ Links ]
Ballester, A. R., Lafuente, M. T., Forment, J., Gadea, J., De Vos, R. C. H., Bovy, A. G., & González-Candelas, L. (2011). Transcriptomic profiling of citrus fruit peel tissues reveals fundamental effects of phenylpropanoids and ethylene on induced resistance. Molecular Plant Pathology, 12(9), 879-897. doi: 10.1111/J.1364-3703.2011.00721.X. [ Links ]
Ballester, A. R., Lafuente, M. T., & González-Candelas, L. (2013). Citrus phenylpropanoids and defence against pathogens. Part II: Gene expression and metabolite accumulation in the response of fruits to Penicillium digitatum infection. Food Chemistry, 136, 285-291. doi:10.1016/j.foodchem.2012.08.006. [ Links ]
Ben-Yehoshua, S., & Mercier, J. (2005). UV irradiation, biological agents, and natural compounds for controlling postharvest decay in fresh fruits and vegetables. In S. Ben-Yehoshua (Ed.), Environmentally friendly tecnologies for agricultural produce quality (pp. 265-299). Boca Ratón, Florida: CRC Press. [ Links ]
Beno-Moualem, D., & Prusky, D. (2000). Early events during quiescent infection development by Colletotrichum gloeosporioides in unripe avocado fruits. Phytopathology, 90, 553-559. doi: 10.1094/PHYTO.2000.90.5.553. [ Links ]
Berrocal-Lobo, M., Molina, A., & Solano, R. (2002). Constitutive expression of ethylene-response-factor1 in Arabidopsis confers resistance to several necrotrophic fungi. The Plant Journal, 29(1), 23-32. doi: 10.1046/j.1365-313x.2002.01191.x. [ Links ]
Boller, T., & Yang He, S. (2009). Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324(5928), 742-744. doi: 10.1126/science.1171647. [ Links ]
Cantu, D., Vicente, A. R., Greve, L. C., Dewey, F. M., Benett, A. B., Labavitch, J. M., & Powell, A. L. T. (2008). The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proceedings of the National Academy of Sciences, 105(3), 859-864. doi:10.1073/pnas.0709813105. [ Links ]
Casado, D. A., Encinas-Villarejo, S., De Los Santos, B., Schiliro, E., Yubero-Serrano, E. M., Amil-Ruíz, F.....Caballero, J. L. (2006). Analysis of strawberry genes differentially expressed in response to Colletotrichum infection. Physiologia Plantarum, 128, 633-650. doi: 10.1111/j.1399-3054.2006.00798.x. [ Links ]
Chiu, C. M., You, B. J., Chou, C. M., Yu, P. L., Yu, F. Y., Pan, S. M.,...Lee, M. H. (2012). Redox status-mediated regulation of gene expression and virulence in the brown rot pathogen Monilinia fructicola. Plant Pathology, 62(4), 809-819. doi: 10.1111/ppa.12006. [ Links ]
Dou, D. L., Kale, S. D., Wang, X. L., Chen, Y. B., Wang, Q., Wang, X.....Brett, M. T. (2008). Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell, 20(4), 1118-1133. doi: 10.1105/tpc.107.057067. [ Links ]
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 45, 185-209. doi: 10.1146/annurev.phyto.42.040803.140421. [ Links ]
El-Kereamy, A., El-Sharkawy, I., Ramamoorthy, R., Taheri, A., Errampalli, D., Kumar, P., & Jayasankar, S. (2011). Prunus domestica pathogenesis-related protein-5 activates the defense response pathway and enhances the resistance to fungal infection. Plos One, 6(3), e17973. doi: 10.1371/journal.pone.0017973. [ Links ]
Elvira, M. I., Molina, G. M., Gilardi, P., García-Luque, I., & Serra, M. T. (2008). Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. Journal of Experimental Botany, 59(6), 1253-1265.doi: 10.1093/jxb/ern032. [ Links ]
Ferreira, R. B., Monteiro, S., Freitas, R., Santos, C. N., Chen, Z., Batista, L. M.....Teixera, A. R. (2006). Fungal pathogens: The battle for plant infection. Critical Reviews in Plant Sciences, 25, 505-524. doi: 10.1080/07352680601054610. [ Links ]
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology, 43, 205-227. doi:10.1146/annurev.phyto.43.040204.135923. [ Links ]
González-Aguilar, G. A., Tiznado-Hernández, M. E., Zavaleta-Gatica, R., & Martínez-Tellez, M. A. (2004). Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochemical and Biophysical Research Communications, 313, 694-701. doi: 10.1016/j.bbrc.2003.11.165. [ Links ]
González-Candelas, L., Alamar, S., Sanchez-Torres, P., Zacarias, L., & Marcos, J. (2010). A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biology, 10(1), 194-211. doi: 10.1186/1471-2229-10-194. [ Links ]
Grant, J. J., & Loake, G. J. (2000). Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiology, 124, 21-29. doi: 10.1104/pp.124.1.21. [ Links ]
Guan, Y., Chang, R., Liu, G., Wang, Y., Wu, T., Han, Z., & Zhang, X. (2015). Role of lenticels and microcracks on susceptibility of apple fruit to Botryosphaeria dothidea. European Journal of Plant Pathology. doi: 10.1007/s10658-015-0682-z. [ Links ]
Hassan, M. K., Dann, E. K., Irving, D. E., & Coates, L. M. (2007). Concentrations of constitutive alk(en)ylresorcinols in peel of commercial mango varieties and resistance to postharvest anthracnose. Physiological and Molecular Plant Pathology, 71, 158-165. doi: 10.1016/j.pmpp.2007.12.005. [ Links ]
Janisiewicz, W. J., & Korsten, L. (2002). Biological control of postharvest diseases of fruits. Annual Review of Phytopathology, 40, 411-441. doi: 10.1146/annurev. phyto.40.120401.130158. [ Links ]
Janisiewicz, W. J., Saftner, R. A., Conway, W. S., & Forsline, P. L. (2008). Preliminary evaluation of apple germplasm from Kazakhstan for resistance to postharvest blue mold in fruit caused by Penicillium expansum. HortSciencie, 43(2), 420-426. Obtenido de http://ucanr.edu/datastoreFiles/234-1665.pdf. [ Links ]
Jones, J. D. G., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323-329. doi:10.1038/nature05286. [ Links ]
Jurick II, W. M., Janisiewicz, W. J., Safner, R. A., Vco, I., Gaskins, V. L., Park, E.....Conway, W. S. (2011). Identification of wild apple germplasm (Malus spp.) accessions with resistance to the postharvest decay pathogens Penicillium expansum and Colletotrichum acutatum. Plant Breeding, 130, 481-486. doi: 10.1111/j.1439-0523.2011.01849.x. [ Links ]
Kelley, B. S., Lee, S. J., Damasceno, C. M. B., Chakravarthy, S., Kim, B. D., Martin, G. B., & Rose, J. K. (2010). A secreted effector protein (SNE1) from Phytophthora infestans is a broadly acting suppressor of programmed cell death. The Plant Journal, 62(3), 357-366. doi: 10.1111/j.1365-313X.2010.04160.x. [ Links ]
Khadivi-khub, A. (2014). Physiological and genetic factors influencing fruit cracking. Acta Physiologiae Plantarum, 37, 1718. doi: 10.1007/s11738-014-1718-2. [ Links ]
Lamb, C., & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Molecular Biology, 48, 251-275. doi: 10.1146/annurev.arplant.48.1.251. [ Links ]
Lanza, B., & Di Serio M. G. (2015). SEM characterization of olive (Olea europea L.) fruit epicular waxes and epicarp. Scientia Horticulturae, 191, 49-56. doi: 10.1016/j.scientia.2015.04.033. [ Links ]
Lee, M. H., & Bostock, R. M. (2007). Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox?. Phytopathology, 97(3), 269-277. doi: 10.1094/PHYTO-97-3-0269. [ Links ]
Liu, J. J., & Ekramoddoullah, A. K. (2006). The family 10 of plant pathogenesis-related proteins: their structure, regulation, and function in response to biotic and abiotic stresses. Physiological and Molecular Plant Pathology, 68(1), 3-13. doi: 10.1016/j.pmpp.2006.06.004. [ Links ]
Macarisin, D., Cohen, L., Eick, A., Rafael, G., Belausov, E., Wisniewski, M., & Droby, S. (2007). Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Postharvest Pathology and Micotoxins, 97, 1491-1500. doi: 10.1094/PHYTO-97-11-1491. [ Links ]
Marcos, J. F., González-Candelas, L., & Zacarías, L. (2005). Involvement of ethylene biosynthesis and perception in the susceptibility of citrus fruit to Penicillium digitatum infection and the accumulation of defense-related mRNAs. Journal of Experimental Botany, 56(148), 2183-2193. doi: 10.1093/jxb/eri218. [ Links ]
Mcdowell, J. M., & Dangl, J. L. (2000). Signal transduction in the plant immune response. Trends in Biochemical Sciences, 25(2), 79-82. doi: 10.1016/S0968-0004(99)01532-7. [ Links ]
Mengiste, T. (2012). Plant immunity to necrotrophs. Annual Review of Phytopathology, 50, 267-294. doi: 10.1146/annurev-phyto-081211-172955. [ Links ]
Mittler, R., Vanderauwera, S., Gollery, M., & Breusegem, F. V. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490-498. doi: 10.1016/j.tplants.2004.08.009. [ Links ]
Mysore, K. S., & Ryu, C. M. (2004). Nonhost resistance: how much do we know?. Trends in Plant Sciences, 9(2), 97-104. doi: 10.1016/j.tplants.2003.12.005. [ Links ]
Nüberger, T., & Lipka, V. (2005). Non-host resistance in plants: New insights into an old phenomenon. Molecular Plant Pathology, 6, 335-345. doi: 10.1111/J.1364-3703.2004.00279.X. [ Links ]
O'Brien, J. A., Daudi, A., Butt, V. S., & Bolwell, G. P. (2012). Reactive oxygen species and their role in plant defense and cell wall metabolism. Planta, 236, 765-779. doi: 10.1007/s00425-012-1696-9. [ Links ]
Prusky, D., Mcevoy, J. L., Saftner, R., Conway, W. S., & Jones, R. (2004). Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology, 94, 44-51. doi: 10.1094/PHYTO.2004.94.1.44. [ Links ]
Torres, M. A. (2010). ROS in biotic interactions. Physilogia Plantarum, 138, 414-429. doi: 10.1111/j.1399-3054.2009.01326.x. [ Links ]
Torres, M. A., Jones, J. D. G., & Dangl, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiology, 141, 373-378. doi: 10.1104/pp.106.079467. [ Links ]
Torres, R., Valentines, M. C., Usall, J., Viñas, I., & Larrigaudiere, C. (2003). Possible involvement of hydrogen peroxide in the development of resistance mechanisms in 'Golden Delicious' apple fruit. Postharvest Biology and Technology, 27, 235-342. doi: 10.1016/S0925-5214(02)00110-2. [ Links ]
Torres, R., Teixidó, N., Usall, J., Abadias, M., Mir, M., Larrigaudiere, C., & Viñas, I. (2011). Anti-oxidant activity of oranges after infection with the pathogen Penicillium digitatum or treatment with the biocontrol agent Pantoea agglomerans CPA-2. Biological Control, 57, 103-109. doi: 10.1016/j.biocontrol.2011.01.006. [ Links ]
Treutter, D. (2006). Significance of flavonoids in plant resistance: a review. Environmental Chemistry Letters, 4, 147-157. doi: 10.1007/s10311-006-0068-8. [ Links ]
Valentines, M. C., Vilaplana, R., Torres, R., Usall, J., & Larrigaudiere, C. (2005). Specific roles of enzymatic browning and lignifications in apple disease resistance. Postharvest Biology and Technology, 36, 227-234. doi: 10.1016/j.postharvbio.2005.01.002. [ Links ]
Van-Loon, L. C., Geraats, B. P., & Linthorst, H. J. (2006). Ethylene as a modulator of disease resistance in plants. Trends in plant science, 11(4), 184-191. doi:10.1016/j.tplants.2006.02.005. [ Links ]
Vázquez, B. M. E., Martínez, P. R. Á., & Fernández, E. E. (2001). Development of toxigenic Aspergillus flavus and A. parasiticus on kernels of native pecan [Carya illinoensis (Wangenh) K. Koch] genotypes under different water activities. Scientia Horticulturae, 89(2), 155-169. doi:10.1016/S0304-4238(00)00225-9. [ Links ]
Vilanova, L., Teixidó, N., Torres, R., Usall, J., & Viñas, I. (2012). The infection capacity of P. expansum and P. digitatum on apples and histochemical analysis of host response. International Journal of Food Microbiology, 157, 360-367. doi: 10.1016/j.ijfoodmicro.2012.06.005. [ Links ]
Vilanova, L., Viñas, I., Torres, R., Usall, J., Jauset, A. M., & Teixidó, N. (2012). Infection capacities in the orange-pathogen relationship: Compatible (Penicillium digitatum) and incompatible (Penicillium expansum) interactions. Food Microbiology, 29, 56-66. doi: 10.1016/j.fm.2011.08.016. [ Links ]
Vilanova, L., Torres, R., Viñas, I., González-Candelas, L., Usall, J., Fiori, S.....Teixidó, N. (2013). Wound response in orange as a resistance mechanism against Penicillium digitatum (pathogen) and P. expansum (non-host pathogen). Postharvest Biology and Technology, 78, 113-122. doi: 10.1016/j.postharvbio.2012.12.013. [ Links ]
Viñas, I., Abadias, M., Teixidó, N., Usall, J., & Torres, R. (2013). Aspectos Básicos de la Patología de la Poscosecha. In I. Viñas, I. Recasens, J. Usall, J. Graell (Eds.), Poscosecha de manzana, pera y melocotón (pp 203-242). Madrid España: Mundi Prensa. [ Links ]
Wood, R. K. S. (2012). Active defense mechanisms in plants. NATO Advanced study institutes series. Series A, Life Sciencies. Cape, Greece. [ Links ]
Yu, O., & Jez, J. M. (2008). Nature's assembly line: biosynthesis of simple phenylpropanoids and polyketides. The Plant Journal, 54, 750-762. doi: 10.1111/j.1365-313X.2008.03436.x. [ Links ]
Zeng, K., Cao, J., & Jiang, W. (2006). Enhancing disease resistance in harvested mango (Mangifera indica L. cv. 'Matisu') fruit by salicylic acid. Journal of the Science of Food and Agriculture, 86, 694-698. doi: 10.1002/jsfa.2397. [ Links ]
Zhao, J., Davis, L. C., & Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances, 23(4), 283-333. doi:10.1016/j.biotechadv.2005.01.003. [ Links ]














