Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Chapingo. Serie horticultura
versión On-line ISSN 2007-4034versión impresa ISSN 1027-152X
Rev. Chapingo Ser.Hortic vol.14 no.3 Chapingo sep./dic. 2008
Arbuscular mycorrhizal fungi and Zn accumulation in the metallophytic plant Viola calaminaria (Gingins.) Lej.
Hongos micorrízico arbusculares en la acumulación de Zn en Viola calaminaria (Gingins.) Lej., una planta metalófita
O. Fernández–Fernández1, R. Carrillo–González2, J. Vangrosveld3 y M. C. González–Chávez2*
1 Universidad Autónoma Chapingo. Km. 38.5 Carretera México–Texcoco. Chapingo, Estado de México. 56230.
2 Colegio de Postgraduados, Campus Montecillo. Carretera México–Texcoco Km. 36.5 Montecillo, México 56230. Correo–e: carmeng@colpos.mx (*Autor responsable).
3 Environmental Biology Hasselt University, B–3590 Diepenbeek, Belgium.
Recibido: 19 de octubre, 2006
Aceptado: 7 de noviembre, 2008
Abstract
Arbuscular mycorrhizal fungi (AMF) play an important role in metal tolerance of their host plant, but their effects on metallophyte plants are not well known. The objectives of this research were to determine the level of mycotrophy in Viola calaminaria (Gingins.) Lej., a plant endemic to metal–enriched soils, and to investigate the effect of AMF on Zn accumulation. Two experiments were conducted. The first experiment (3 months long) was carried out in pots containing inoculated and non–inoculated plants grown in river sand as substrate under non Zn –polluted conditions. The second used plants from the first experiment; these were grown for 15 days under hydroponic conditions with different levels of Zn+2 (50, 200, 300 and 400 mg·liter–1). The AMF were Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe isolates BEG–132 and BEG–25 originating from soils polluted or non–polluted by metals, respectively. Experiment 1 indicated that V. calaminaria was colonized by both fungi but did not benefit from being colonized. Dry mass, root volume and P – Zn contents in inoculated plants were not different from that in non inoculated plants. Experiment 2 showed that total Zn content per plant increased when colonized by AMF and that accumulation was higher in plants colonized by the fungus from unpolluted soil (BEG–25). Fungal root colonization was not affected by Zn content. It remains to be determined whether AMF adapted to soils with high metal contents confer a growth and survival advantage on metallophyte plants in the field.
Key words: phytoremediation, heavy metals, mycorrhizal symbiosis, polluted soils.
Resumen
Los hongos micorrízico arbusculares (HMA) incrementan la tolerancia a metales en la mayoría de sus plantas hospederas, sin embargo, se conoce poco de su efecto en plantas metalófitas. Los objetivos de esta investigación fueron: 1) determinar la respuesta a la inoculación con dos HMA de Viola calaminaria (Gingins.) Lej., una planta endémica de suelos contaminados con metales pesados, y 2) investigar el efecto de la inoculación con HMA en la acumulación de zinc. Se establecieron dos experimentos: El primero duró tres meses y consistió en la inoculación de plantas de V. calaminaria con dos HMA utilizando arena de río como sustrato de crecimiento. El segundo experimento duró dos semanas y consideró el efecto de la inoculación de las plantas procedentes del primer experimento y que se transfirieron a condiciones de hidroponia con diferentes niveles de Zn+2 (50, 200, 300 y 400 mg·litro–1). Los HMA que se estudiaron fueron dos aislados de Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe: BEG–132– y BEG–25, los cuales se aislaron, respectivamente, de suelos contaminados o no con metales pesados. El experimento 1 mostró que las raíces de V. calaminaria se colonizaron por ambos aislados micorrízicos; sin embargo las plantas no presentaron beneficios en relación a crecimiento, desarrollo y nutrición. El segundo experimento mostró que la inoculación incrementó el contenido total de Zn por planta y que el aislado BEG 25 fue el que mayor influencia tuvo. La colonización micorrízica en las raíces de V. calaminaria no se afectó por las concentraciones de Zn. Resulta relevante continuar experimentos de este tipo para conocer si los HMA adaptados a altos contenidos de metales pesados confieren ventajas sobre crecimiento y supervivencia en campo en estas y otras plantas metalíferas.
Palabras clave: fito–remediación, metales pesados, simbiosis micorrízica, suelos contaminados.
INTRODUCTION
Several studies suggest that ornamental plants are able to deal with some contaminants such as heavy metals (Caselles et al., 2004; Sharma et al., 2004). For example, Caselles et al. (2004) showed that Petunia nyctaginiflora Juss plants grown in urban areas were able to control the contents of Mn and Zn when high concentrations of Al, Fe and Pb, and medium concentrations of Cu were deposited on its leaves. Hence, it is of interest to study other ornamentals with potential for use in remediation of metal–polluted soils.
Viola calaminaria is present on Pb– and Zn–polluted sites and abandoned mine sites (Hildebrandt et al., 1999; Colpaert and Vandenkoornhuyse, 2001; Tonin et al., 2001). Bizoux et al. (2004) mentioned that this plant is a threatened species, which has been found at sites in Belgium contaminated by atmospheric deposits from metal smelters, where there were elevated concentrations of Zn, Pb, and Cd. Tonin et al. (2001) noted that despite extremely high levels of metals in the soil, this yellow violet is an absolute metallophyte that accumulates metals only at very low levels. However, Baker et al. (2000) reported that V. calaminaria can accumulate up to 1% Zn in its leaves.
The removal of soil contaminants using plants (phytoremediation) is a relatively new approach to soil decontamination. Baker et al. (2000) suggest the use of hyperaccumulating metallophytes for this purpose.
Numerous reports show that soil microorganisms affect availability and uptake of metals by plants and also their tolerance to metals (Burd et al., 2000; González–Chávez et al., 2006; Turnau et al., 2006). Most metal–hyperaccumulating plant species tested so far accumulates Ni (145); others have been shown to accumulate Co (26), Cu (24), Zn (14), Pb (4) and Cr (2). Boyd and Martens (1998) speculated that some hyperaccumulators may form mycorrhizae and in some cases, the fungal endophyte may play a role in metal uptake. Now new information is available regarding the relationship between AMF and hyperaccumulator plants (Turnau and Mesjasz–Przybylowics, 2003; Regvar et al., 2003; Vogel–Mikûs et al., 2005;Leung et al., 2006).
Hildebrandt et al. (1999) reported that roots of V. calaminaria were consistently colonized by AMF. The presence of these microorganisms in the soil is likely to play a basic role in the tolerance to and accumulation of metals exhibited by the host plants. However, we are not aware of data on the relationship and interactions between V. calaminaria and AMF.
Tonin et al. (2001) studied the ability of AMF fungi isolated from V. calaminaria to colonize and reduce metal uptake by Trifolium subterraneum L. Use of these fungi resulted in lower metal accumulation than the use of a metal tolerant AM fungus isolated from a non–tolerant plant. Similar results were reported by Hildebrandt et al. (1999) who found that the Glomus isolate 'Brl' from V. calaminaria supported growth of maize (Zea mays L.) or alfalfa (Medicago sativa L.) more efficiently in heavy metal–enriched soils than a commonly used isolate of Glomus intraradices (Schenck & Smith).
Root colonization by AMF is important for plants in phytoremediation (Wang et al., 2005; González–Chávez et al., 2006; Turnau et al., 2006). Takács et al. (2005) observed a strong correlation between plant survival and the frequency of AMF colonization in seven clones of 1–year–old poplar established at an industrial site strongly polluted by heavy metals. These authors showed that two clones, which had not been colonized by AMF, had the lowest survival rates in contrast to those that were colonized. Hence, remediation and revegetation of heavy metal–contaminated sites might be facilitated by selection of tolerant plant species and isolation of tolerant AMF (González–Chávez et al., 2006; Turnau et al., 2006).
Since the role of AMF in roots of metallophyte V. calaminaria and their relation to plant physiology needs to be elucidated, the objectives of this study were to determine whether inoculation with AMF improves growth of Viola calaminaria, and to investigate the effect of two AMF on Zn accumulation under hydroponic conditions.
MATERIALS AND METHODS
Experiment 1. Response of V. calaminaria to colonization by two AMF
Isolates BEG–25 (from nonpolluted soil) and BEG–132 (from As– and Cu–polluted soil, Gonzalez–Chavez et al., 2002a) of Glomus mosseae were used to inoculate V. calaminaria seedlings. Sorghum (Sorghum bicolor (L.) Moench) cultures, inoculated with the isolates BEG–25 or BEG–132, were used as inocula. Sorghum roots (90% colonization) were cut into 1 cm long segments and mixed with the original trap–culture sand which contained at least 50 spores per 10 g. The inoculum was placed in the planting hole before transplanting one Viola seedling per pot. V. calaminaria seeds were collected on the site of a former Pb and Zn mine in Plombières (Belgium) that was highly contaminated with heavy metals, particularly Zn, Pb and Cd. Seeds were sterilized with 10% sodium hypochlorite and germinated in sand. The experimental units were plastic pots containing 1 kg of coarse sand and planted with one 15–day–old V. calaminaria seedling. The sand was acid–washed and autoclaved. Plants were grown for 3 months in a growth chamber arranged at random. They were subjected to a 16 h photoperiod (400 W Na–vapor lamps) and day/night temperatures of 24 / 12 °C. Pots were gravimetrically adjusted with distilled water every 2 days to maintain soil moisture at 80% water–holding capacity. Plants were watered every week with 100 ml of Long–Ashton nutrient solution (Hewitt, 1966).
The experiment was arranged in a completely random design with three AMF treatments (control, BEG–25 and BEG–132), each with 16 replicates, for a total of 48 experimental units. Differences between treatments were tested by ANOVA followed by the Tukey test (P<0.05), using the software SAS v6.12 (Statistical Analysis System, 1998).
Dry weights of aerial part and roots, and root volume (liquid displacement method) were determined. Phosphorous and Zn concentrations were determined in shoots and roots after acid digestion with concentrated nitric acid and analyzed by ICP–ES (Varian inductively coupled plasma emission spectrometer). A sub–sample (0.5 g) was taken from each root system to evaluate the percentage of root colonization by AMF. Roots were cleared and stained with acid glycerol Trypan Blue (Koske and Gemma, 1989). One hundred stained root segments were mounted on slides in 50% aqueous glycerol (v/v) and examined under a compound microscope (Nikon Alphaphot 2 YS2) at 100x magnification. The frequency of AMF structures was estimated by rating the presence of fungal structures (arbuscules, vesicles and hyphae) expressed as a percentage. There were three replicates for each root sub–sample (total of 300 root segments per plant root sample).
Experiment 2. Effect of fungal inoculation on Zn accumulation V. calaminaria under hydroponic system
After 12 weeks of growth, plants from the first experiment were separated from the sand by gently washing off all sand particles adhering to the roots. They were then placed in 1–liter pots containing a buffered (0.01 M HEPES) Long Asthon nutrient solution with four different zinc levels (50, 200, 300 and 400 mg Zn·liter–1). The 16 replicates of each of the three AMF treatments from Experiment 1 were divided into four parts by assigning four plants to each of the four new Zn treatments. Thus, Experiment 2 had a 3 x 4 factorial design with AMF (3 levels) and Zn (4 levels) as factors, and the 12 new treatments were replicated four times. Plants were maintained hydroponically for 2 weeks in a growth chamber under the conditions of Exp 1. After these 2 weeks at the four Zn concentrations, plants were harvested. Aerial part and root dry weights, root volume, P and Zn concentrations were determined in aerial part and roots as described for Experiment 1. Differences between treatments were tested by ANOVA followed by the Tukey test (P<0.05), using the software SAS v6.12 (Statistical Analysis System, 1998).
A quality control program was implemented including reagent blanks, duplicate samples, in–house reference material. Detection limit was 0.01 for Zn.
RESULTS AND DISCUSSION
Experiment 1. Response of V. calaminaria to inoculation with two AMF
Colonization of V. calaminaria roots was 19% by BEG–132 and 47% by BEG–25. Besides this difference in fungal colonization, after three months of fungal inoculation, no changes in plant growth were observed between AMF treatments. Neither shoot and root dry masses nor P and Zn concentrations in shoots and root parts were significantly (P<0.05) different between the control and the two AMF treatments (Table 1). This suggested that Viola calaminaria did not behave as a mycotrophic plant in these experimental conditions. However, it would be interesting to know whether, under field conditions, AMF might be useful to V. calaminaria and other metallophytes growing under conditions of restricted, low essential nutrient concentrations or stress. These fungi may be important in multiple responses of their host in addition to plant growth alone, which Whitfield et al. (2004) mentioned.
Experiment 2. Effect of fungal inoculation on Zn accumulation V. calaminaria under Zn stress
Colonization by two fungal isolates of Glomus mosseae was little affected by Zn application (Table 2). Root colonization by G. mosseae BEG–132 was lower (19 a 26%) than by BEG–25 (44 a 60%). Liu et al. (2005) obtained similar results in fungal colonization when exposing plants to different levels of As (0 to 75 mg·kg–1 soil).
Bizoux et al. (2004) mentioned that many of metalliferous species are actually dependent on high concentrations of metals in soils and grow less well in uncontaminated soils. In our study, Zn content in shoots and roots of V. calaminaria increased with increasing Zn concentration in the growth medium (Figure 1a, b). However, this concentration was not high enough to consider V. calaminaria a hyperaccumulator plant according to the definition by Baker (1981) who reported that, to consider a plant species a Zn hyperacumulator, the threshold is 10,000 μg·g–1 dry matter in leaves. Our results are in agreement with the findings of Tonin et al. (2001), who found that V. calaminaria accumulated only low levels of metals although it was growing in highly polluted soil.
In this experiment, compared with the control Zn level (50 mg·liter–1 of Zn), shoots and roots of inoculated and non–inoculated plants showed significantly higher Zn concentrations when exposed to higher Zn levels. These increases were at least 7 and 29 times higher between 200 and 400 mg·liter–1 Zn, respectively, relative to Zn concentrations in the control. Inoculation with BEG–132 significantly increased (P<0.05) Zn concentration in shoots at 50 to 300 mg·liter–1, but at 400 mg·liter–1 all treatments behaved similarly (Figure 1a). Significant differences in root Zn concentrations were only observed by inoculation with BEG–132 at 50 mg·liter–1 and by two fungi at 200 mg·liter–1. At higher exposures inoculated and non inoculated plants did not show statistical differences (Figure 1b). Whitfield et al. (2004) reported that mycorrhizal colonization increased tissue Zn concentrations. V. calaminaria seeds used in this research were collected from soil polluted with Pb, Cd and Zn. Studies considering the species' association with AMF and the effects not only of Zn but also of Pb and Cd, should be conducted.
Zinc concentrations in roots were much higher than in shoots (Figure 1b). This result confirms other research, where roots are the main plant organ retaining metals such as Cd or Pb (Salt et al., 1997; Vogel–Mikûs et al., 2006). The present results suggest that the isolate BEG–25 contributed significantly more to Zn accumulation in the roots than BEG–132. Higher root colonization by BEG–25 than by BEG–132 (approximately two times more) may be a reason for this effect, especially if the endophyte itself should be an accumulator of Zn, and this remains to be shown.
Inoculation with the isolate BEG–25 significantly (P<0.05) increased (58 to 114%) shoot dry weight and total dry weight (51 to 99%) at all Zn concentrations (except 300 mg·liter–1). A similar trend was observed for root dry weight at 200 and 400 mg·liter–1 of Zn (Table 2). Controls and BEG–132 plants did not differ significantly (P>0.05) in their shoot or root dry masses.
The total Zn content was significantly higher in plants inoculated with each fungus at 200 mg·liter–1. However, no difference between inoculation treatments was found at 300 mg·liter–1, while at 400 mg·liter–1 BEG–25 plants showed a higher Zn content compared to BEG–132 inoculated plants and controls (Figure 2). BEG–25 plants tended to accumulate more Zn than BEG–132 plants. These results suggest that the two fungi mediate, in different ways, Zn toxicity and concomitant Zn accumulation to their host plant. The isolate BEG–132 originated from a soil polluted by mainly As and Cu, but also containing increased Zn levels (297 mg·kg–1 DTPA–available), while BEG–25 was isolated from an uncontaminated agricultural soil.
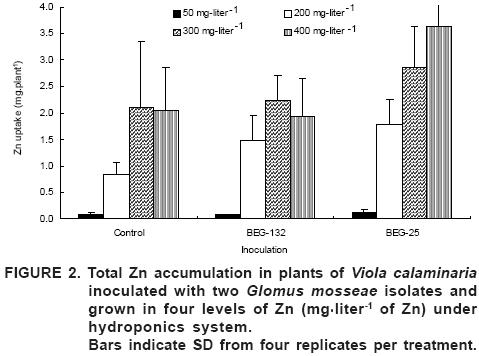
Gonzalez–Chavez et al. (2002b) reported that G. mosseae isolates BEG–132 and BEG–25 both suppressed high affinity arsenate/phosphate transport into the roots of both resistant and non–resistant Holcus lanatus. Toler et al. (2005) showed reduced Cu acquisition under elevated Cu conditions by fungal isolates from heavy–metal polluted soils in sorghum exposed to elevated Cu relative to fungal isolates from non polluted soil. In the present experiment only the BEG–25 isolate, a fungus from non–polluted soil, significantly increased Zn accumulation in Viola plants. The mechanism involved is not known. BEG–132, an isolate from As– and Cu–polluted soil, did not significantly affect Zn accumulation. Perhaps tolerance to one metal does not confer tolerance to another; furthermore this isolate has been propagated in pollutant–free substrates for more than two years. Tolerance may have been lost as reported previously by Gonzalez–Chavez et al. (2004) and Malcová et al. (2003).
There is increasing evidence on the role of AMF in phytoextraction in metal hyperaccumulating and non–hyperaccumulating plants. Wang et al. (2005) studied the effect of inoculating Elsholtzia splendens with a fungal consortium MII (Gigaspora margarita ZJ37, Gigaspora decipens ZJ38, Scutellospora gilmori ZJ39, Acaulospora spp. and Glomus spp). This mycorrhizal fungal consortium increased phytoextraction of several heavy metals. Turnau and Mesjasz–Przybylowics (2003) found Ni–hyperaccumulating species of the Asteraceae family consistently colonized by AMF, and Turnau et al. (2006) mentioned that preliminary results in the Ni–hyperaccumulating Berkheya coddii plants more than doubled Ni content and shoot biomass after inoculation with native AMF (Gigaspora spp. and Glomus tenue). Leung et al. (2006) and Liu et al. (2005) also reported increased As–accumulation in As hyperaccumulating Pteris vittata L. fern after inoculation with AMF. Citerio et al. (2005) also observed that inoculation increased accumulation of Ni, Cd and Cr in Cannabis sativa L. plants.
Janouskova et al. (2005) found that G. intraradices decreased the Cd–phytoextraction efficiency of transgenic tobacco plants bearing yeast metallothionein CUP1 combined with a polyhistidine cluster, while it increased that of non–transgenic plants. However, since these authors did not mention whether their fungus was isolated from polluted or nonpolluted soils, comparison is difficult.
Regvar et al. (2003) and Vogel–Mikûs et al. (2005) examined natural colonization by AMF in three Thlaspi species, metallophytes growing on sites similar to those of V. calaminaria. Thlaspi especies (Brassicaceae) have been regarded as non mycorrhizal plants. Their results showed sparse but distinct AMF colonization. Recently, Vogel–Mikûs et al. (2006) reported that under a greenhouse experiment AMF colonized Thlaspi praecox, a Zn, Cd and Pb hyperaccumulator plant, only during the plant reproductive period were changes in metal tolerance strategy observed. Cd and Zn uptake was reduced at higher soil metal contents. The results presented in our paper show effective symbiosis during the entire period of study with V. calaminaria, with increased Zn accumulation in shoot and root.
More work should be done with other plants and metals in order to improve the chances of successful phytoremediation. The increment of metal accumulation in shoots and higher production of shoot biomass by BEG 25 are two relevant factors for phytoextraction, which give potential to its application.
CONCLUSIONS
Symbiotic fungi can be used to enhance growth and metal accumulation in Viola calaminaria plants. The results presented in this paper point to more detailed study of the role of AMF in Viola and other metallophyte plants.
ACKNOWLEDGEMENTS
This research was funded through project SEMARNAT–CONACyT C0–01–2002–739.
LITERATURE CITED
BAKER, A. J. M. 1981. Accumulators and excluders –strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3: 643–654. [ Links ]
BAKER, A. J. M.; MCGRATH, S. P.; REEVES, R. D.; SMITH, J. A. C. 2000. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediaton of metal–polluted soils, pp. 85– 107. In: Phytoremediation of Contaminated Soil and Water. TERRY, N.; BAÑUELOS. G. S. (eds.). CRC Press Inc. Boca Raton, Florida, USA. [ Links ]
BIZOUX, J. P.; BREVERS, F.; MEERTS, P.; GRAITSON, E.; MAHY, G. 2004. Ecology and conservation of Belgian populations of Viola calaminaria, a metallophyte with a restricted geographic. Belgian Journal of Botany 137: 91–104. [ Links ]
BOYD, R. S.; MARTENS, S. N. 1998. The significance of metal hypera–ccumulation for biotic interactions. Chemoecology 8: 1–7. [ Links ]
BURD, G. I.; DIXON, D. G.; GLICK, B. R. 2000. Plant growth –promoting bacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology 4: 237–245. [ Links ]
CASELLES, J.; COLLIGA, C.; ZORNOZA, P. 2004. Evaluation of trace element pollution from vehicle emissions in petunia plants. Water, Air and Soil Pollution 136: 1–9. [ Links ]
CITTERIO, S.; PRATO, N.; FUMAGALLI, P.; AINA, R.; MASSA, N.; SANTAGOSTINO, A.; SGORBATI, S.; BERTA, G . 2005. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 59: 21–29 [ Links ]
COLPAERT, J. V.; VANDENKOORNHUYSE, P. 2001. Mycorrhizal fungi, pp. 37–58. In: Metals in the Environment. PRASAD, M.N.V. (ed.). Marcel Dekker Inc. New York, USA. [ Links ]
GONZÁLEZ–CHÁVEZ, M. C.; D'HAEN, J.; VANGRONSVELD, J. J.; DODD, J. C. 2002a. Copper sorption and accumulation by the extraradical mycelium of different Glomus spp. (arbuscular mycorrhizal fungi) isolated from the same polluted soil. Plant Soil 240: 287–297. [ Links ]
GONZÁLEZ CHÁVEZ, C.; HARRIS, P.J.; DODD, J.; MEHARG, A.A. 2002b. Arbuscular mycorrhizal fungi confer enhanced arsenate resistance on Holcus lanatus. New Phytologist 155: 163–171. [ Links ]
GONZÁLEZ–CHÁVEZ, M. C.; CARRILLO–GONZÁLEZ, R.; WRIGHT, S. F.; NICHOLS, K. 2004. The role of glomalin, protein produced by arbuscular mycorrhizal fungi in sequestering potentially toxic elements. Environmental Pollution 130: 317–323. [ Links ]
GONZÁLEZ–CHÁVEZ, M. C.; J. VANGRONSVELD, J. COLPAERT, C. LEYVAL. 2006. Arbuscular mycorrhizal fungi and heavy metals: Tolerance mechanisms and potential use in bioremediation, pp. 211–234. In: PRASAD, M.N.V.; SAJWAN, K. S.; NAIDU, R. (eds.). Trace Elements in the Environment. Biogeochemistry, Biotechnology, and Bioremediation. CRC Press. Boca Raton, Florida, USA. [ Links ]
HEWITT, E. 1966. Sand and water culture methods used in the study of plant nutrition. Commonwealth Agricultural Bureau Technical Communication 22, Farnham Royal, UK. [ Links ]
HILDEBRANDT, U.; KALDORF, M.; BOTHE, H. 1999. The zinc violet and its colonization by arbuscular mycorrhizal fungi. Journal of Plant Physiology 154: 709–717. [ Links ]
JANOUSKOVA, M.; PAVLIKOVA, D.; MACEK, T.; VOSATKÁ, M: 2005. Arbuscular mycorrhiza decreases cadmium phytoextraction by transgenic tobacco with inserted metallothionein. Plant Soil 272: 29–40. [ Links ]
KOSKE, R. E.; GEMMA, J. N. 1989. A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92: 486–505. [ Links ]
LEUNG, H. M.; YE, Z. H.; WONG, M. H. 2006. Interactions of mycorrhizal fungi with Pteris vittata (As hyperaccumulator) in As–contaminated soils. Environment Pollution 139: 1–8. [ Links ]
LIU, Y. ; ZHU, Y. G.; CHEN, B. D.; CHRISTIE, P.; LI, X. L. 2005. Influence of the arbuscular mycorrhizal fungus Glomus mosseae on uptake of arsenate by the As hyperaccumulator fern Pteris vittata L. Mycorrhiza 15: 187–192. [ Links ]
MALCOVÁ, R.; FYDOVÁ, J.; VOSATKÁ, M. 2003. Metal–free cultivation of Glomus sp., BEG 140 isolated from Mn–contaminated soil reduces tolerance to Mn. Mycorrhiza 13: 151–157. [ Links ]
REGVAR, M.; VOGEL, K.; IRGEL, N.; WRABER, T.; HILDEBRANDT, U.; WILDE, P.; BOTHE, H. 2003. Colonization of pennycresses (Thlaspi spp.) of the Brassicaceae by arbuscular my–corrhizal fungi. Journal of Plant Physiology 160: 615–626. [ Links ]
SALT, D. E.; PICKERING, I. J.; PRINCE, R. C.; GLEBA, D.; DUSHENKOV, S.; SMITH, R. D.; RASKIN, I. 1997. Metal accumulation by aquacultured seedlings of indian mustard. Environmental Science and Technology 31: 1636–1644. [ Links ]
SHARMA, K.; SHARMA, K. P.; GROVER, R. 2004. In vitro studies to analyze the response of some ornamental plant species to heavy metals at germination and other stages. Nature, Environmental Pollution and Technology 3: 369–376. [ Links ]
TAKÁCS, D.; RADIMSZKY, L.; NEMETH, T. 2005. The arbuscular mycor–rhizal status of poplar clones selected for phytoremediation of soils contaminated with heavy metals. Zeitschrift fur Naturforschung C–A J. Biosciences 60: 357–361. [ Links ]
TOLER, H. D.; MORTON, J. B.; CUMMING, J. R. 2005. Growth and metal accumulation of mycorrhizal sorghum exposed to elevated copper and zinc. Water, Air and Soil Pollution164: 155–172. [ Links ]
TONIN, C.; VANDENKOORNHUYSE, P.; JONER, E.J.; STRACZEK, J.; LEYVAL, C. 2001. Assessment of arbuscular mycorrhizal fungi diversity in the rhizosphere of Viola calaminaria and effect of these fungi on heavy metal uptake by clover. Mycorrhiza 10: 161–168. [ Links ]
TURNAU, K.; MESJASZ–PRZYBYLOWICS, J. 2003. Arbuscular mycorrhizal of Berkheya coddii and other Ni–hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 13: 185–190. [ Links ]
TURNAU, K.; JURKIEWICZ, A.; LINGUA, G.; BAREA, J. M.; GIANINAZZI–PEARSON, V. 2006. Role of arbuscular mycorrhiza and associated microorganisms in phytoremediation of heavy metal polluted sites, pp. 235–252. In: PRASAD, M.N.V.; SAJWAN, K. S.; NAIDU, R. (eds.). Trace Elements in the Environment. Biogeochemistry, Biotechnology, and Bioremediation. CRC Press. Boca Raton, Florida, USA. [ Links ]
VOGEL–MIKÛS, K.; DROBNE, D.; REGVAR, M. 2005. Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environmental Pollution 133: 233–242. [ Links ]
VOGEL–MIKÛS, K.; PONGRAC, P.; KUMP, P.; NECEMER, M.; REGVAR, M. 2006. Colonization of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular my–corrhizal fungal mixtures induces changes in heavy metal and nutrient uptake. Environmental Pollution 139: 362–371. [ Links ]
WANG, F. Y. ; LIN, S. G.; YIN, R. 2005. Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant Soil 269: 225–232. [ Links ]
WHITFIELD, L.; RICHARDS, A. J.; RIMMER, D. L. 2004. Effects of mycorrhizal colonization on Thymus polytrichus from heavy–metal–contaminated sites in northern England. Mycorrhiza 14: 47–54. [ Links ]














