INTRODUCTION
Only a few crustacean groups inhabit land, a way of life that requires morphological, physiological and behavioural adaptations. Among the terrestrial crustaceans, the order Isopoda presents a remarkable lineage that has widely invaded land from a marine ancestor: the Oniscidea (Warburg, 1993; Hornung, 2011; Broly et al., 2013). Today, the Oniscidea populate wide continental areas and represent a significant part of the soil macrofaunal (Warburg, 1993). The group currently conforms 3,710 species belonging to 37 families and 527 genera (Schmalfuss, 2003; Sfenthourakis and Taiti, 2015). The monophyly of this large suborder is now well accepted (reviewed in Schmidt, 2008). The Oniscidea is traditionally subdivided into five clades: Diplocheta (Ligiidae), Tylida (Tylidae), Microcheta (Mesoniscidae), Synocheta (e.g., Trichoniscidae, Styloniscidae) and Crinocheta (e.g., Armadillidae, Philosciidae, Porcellionidae) (Schmalfuss, 2003; Schmidt, 2008). The Diplocheta and the Tylida represent the most basal clades, mainly including semi-terrestrial species, whereas Synocheta and Crinocheta represent the more apical clades mainly including strictly terrestrial species (Schmidt, 2008).
The Crinocheta is the largest clade within Oniscidea, with about 80% of the species diversity of the Oniscidea. It includes the most terrestrial forms, some of them living in particularly dry habitats (Warburg, 1993).The Crinochetan species form a monophylum well supported by numerous characters, e.g., the pars molaris of mandibles replaced by a tuft of hairy setaeorthe male pleopod one endopodites with spermatic grooves (reviewed in Schmidt, 2008, but see Lins et al., 2017). However, the phylogenetic relations between the families of Crinocheta have not yet been fully resolved despite detailed consideration (Schmidt, 2002, 2003, 2008). Until now, the fossil record was unsufficient to reconstruct an evolutionary framework and solve taxonomic hypotheses. One major obstacle is that the fossil record of the Oniscidea is poor despite a likely Late Paleozoic origin (reviewed in Broly et al., 2013, 2015). One possible explanation is that isopods are difficult to fossilize in continental realms except under exceptional conditions (see Hyžný and Dávid, 2017). Yet numerous amber inclusions of Oniscidea have been recorded but, at the exception of some studies (e.g., Morris, 1979; Schmalfuss, 1980, 1984), they have only been superficially examined and figured. To date, the fossil record of the Crinocheta is presumably represented by members of the family Agnaridae, Armadillidiidae, Delatorreidae, Eubelidae, Oniscidae, “Philosciidae”, “Platyarthridae”, “Porcellionidae”, “Scleropactidae” and “Trachelipodidae” from the Cenozoic (see Broly et al., 2013). Recently, a Cretaceous fossil of a represent of the Armadillidae has been discovered in Burmese amber (Poinar, 2018).
This paper describes six crinochetan species new to science from the Early Miocene amber of Chiapas, Mexico. It includes the first fossil record of the family Detonidae, Olibrinidae, and “Stenoniscidae”. The aim of this paper is not to solve phylogenetic hypotheses in Oniscidea but to establish a first scanning of the palaeodiversity of the Crinocheta in these deposits. Several individuals also figured require future studies.
MATERIAL AND METHODS
The Chiapas amber is found mainly in three Oligocene-Miocene lithostratigraphic units, known as (from base to top) La Quinta Formation (Upper Oligocene-Lower Miocene), Mazantic Shale (Lower Miocene) and Balumtum Sandstone (Lower to Middle Miocene). The Campo La Granja mines are located within the uppermost member of the La QuintaFormation (Finca Carmitto Member) (see Serrano-Sánchez et al., 2015a, for location and geologic details). The Finca Carmitto Member (Lower Miocene, 23 Ma) includes coarse sandstone interbedded with gray shale and limestone lenses. Most amber pieces have clear layers of diverse thickness, from less than one to four millimeters, representing individual resin flows, separated by thin layers of sand. Stratified amber layers include quartz/rich sandstone similar to that of surrounding rock matrix of the Finca Carmitto Member. These amber pieces have distinct organic inclusions, different from other so-called Chiapas amber (from Mazantic Shale), and include a peculiar assemblage of estuarine meiofauna, including harpacticoid copepods (Huys et al., 2016), numerous ostracods (Matzke-Karasz et al., 2017), parasitic larvae of epicaridian isopods (Serrano-Sánchez et al., 2015b) and sesarmid crabs (Serrano-Sánchez et al., 2016). Amber samples from the overlying Mazantic Shale do not usually include sand and are more clean in organic matter contents, but their shape tends to be rounded and many show encrusting oysters and barnacles, as a result of exposure to estuarine environment, before definite burial. The estimated age for deposition of both types of amber is Early Miocene (23 Ma, see Vega et al., 2009; Perrilliat et al., 2010; Serrano-Sánchez et al., 2015a).
Amber samples were cut with a diamond saw and polished with Brasso. Specimens were investigated with a SZH Olympus stereo microscope and photographed with an Axio Zoom.V16 Zeiss microscope. Samples are deposited at Museo de Paleontología “Eliseo Palacios Aguilera” (Secretaría de Medio Ambiente e Historia Natural), Tuxtla Gutiérrez, Chiapas, Mexico, under the acronym IHNFG (Instituto de Historia Natural, Fósil Geográfico).
SYSTEMATIC PALAEONTOLOGY
Subphylum Crustacea Brunnich, 1772
Class Malacostraca Latreille, 1802
Order Isopoda Latreille, 1817
Suborder Oniscidea Latreille, 1802
Family Olibrinidae Budde-Lund, 1913
Genus Palaeolibrinus Broly, gen. nov.
Type species. Palaeolibrinus spinicornis Broly, gen. nov.sp. nov.
Diagnosis. Spiny appearance caused by the strong dorsal scale-setae. Second antennae with 7-jointed flagellum. Cephalothorax elongate, with linea frontalis and linea supraantennalis. Cephalic lines markedly convex. Uropods massive and long, exopodite longer than endopodite and sympodite. Uropod sympodites conical, slightly surpassing the pleotelson.
Derivation of name. The name of the new genus is derived from a combination of the Greek word palaios (meaning “ancient”) and Olibrinus, the type genus name of the Olibrinidae.
Comparison and systematic position. The autapomorphies of the Olibrinidae concern the mouthparts (Schmidt, 2008), poorly exploitable here because examinable only superficially in fossilspreserved in amber. However, the specimen is assigned to this family based on the general body shape, the massive conical uropods and on, the most remarkable character, the antennal flagellum with seven well distinct articles. Indeed, most Crinocheta have a 3- or 2-jointed flagella except for the Olibrinidae, the only family with species with more than four flagellar articles (up to 18 articles, although some species present only three or four flagellar articles; Schmidt, 2002).
The assignation of the Olibrinidae to the Crinocheta was a debated issue. Schmalfuss (1989) included the Olibrinidae in the Synocheta based on a number of symplesiomorphies. It is now well accepted that the Olibrinidae is one of the basalmost families within the Crinocheta (Schmidt, 2002, 2008).
Currently, the family Olibrinidae contains four small genera including OlibrinusBudde-Lund, 1913 (11 species, with probably numerous synonyms according to Schmalfuss, 2003); AdoniscusVandel, 1955 (three species); ParadoniscusTaiti and Ferrara, 2004 (two species) and NamiboniscusSchmidt, 2001 (one species).
The new fossil differs from Olibrinus in having uropods with exopodite longer than sympodites and uropod sympodites slightly surpassing the pleotelson. The new fossil differs from Namiboniscus in having a 7-jointed flagellum and long uropods with an exopodite longer than sympodite and endopodite. The shape of the cephalon of the new fossil, in having a linea frontalis and a linea supraantennalis, differs remarkably from the cephalons of Olibrinus, Adoniscus and Namiboniscus that present only one transverse line. By the same character, it resembles Paradoniscus, but the cephalon differs in its curved cephalic lines. In addition, the new fossil is easily distinguishable from Paradoniscus in having well developed eyes, well developed pleonal epimera with corners pointing backwards and 7-jointed flagellum.

Figure 1 Palaeolibrinus spinicornis gen. nov. sp. nov., ♂ adult holotype (IHNFG-4984); (a, b) General habitus, dorsaland ventral views; (c) Cephalon in dorsal view; (d) Antenna; (e) Pereiopod II; (f) Pereiopod VII; (g) Pleopod 2-5; (h) Pleotelson with uropods, dorsal view. holotype in dorsal view. Scale bars = 200 µm.
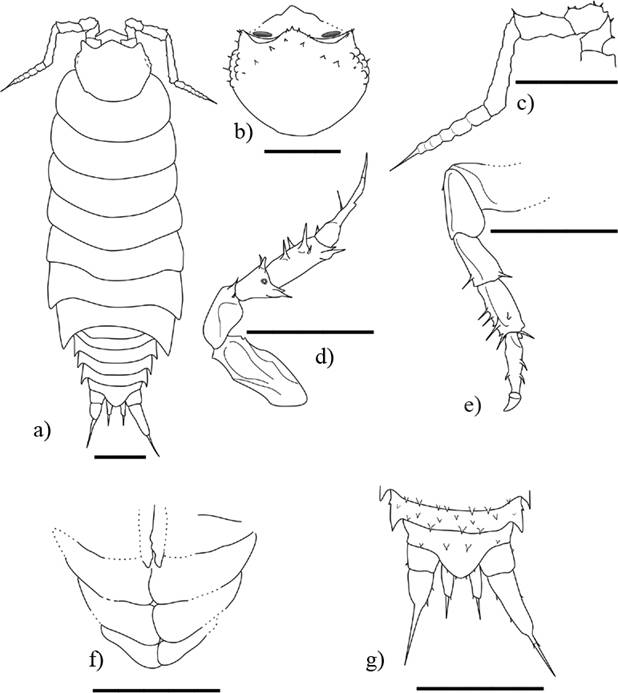
Figure 2 Palaeolibrinus spinicornis gen. nov. sp. nov., ♂ adult holotype (IHNFG-4984); (a) General habitus, lateral view; (b) Cephalon in dorsal view; (c) Antenna; (d) Pereiopod II; (e) Pereiopod VII; (f) Pleopod 2-5; (g) Pleotelson with uropods, dorsal view. Scale bars = 200 µm.
Diagnosis. As for the genus.
Derivation of name. The name of the new species refers to the spiny appearance of the dorsum surface of the fossil.
Holotype. IHNFG-4984, adult male preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Locality. Campo La Granja mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Finca Carmitto Member, La Quinta Formation, Early Miocene (Aquitanian, 23 Ma).
Description. ♂. Maximal dimensions: 1.8 × 0.6 mm. Brownish-grey. Body ovoidal, slightly laterally compressed at the 3rd thoracic segment (Figures 1a, 2a).
Cephalothorax elongate without lateral lobes, with linea frontalis and linea supraantennalis (Figures 1c, 2b). Frontal margin of vertex markedly convex, with several spine-like scale-setae. Compound eyes of around 10 ommatidia. Antennula indiscernible. Second antenna rather long, composed of five peduncular articles + flagellum. First four peduncular articles with sparse setae. Fifth peduncular article the longest, as long as flagellum. Flagellum conical of seven distinct articles gradually reducing in size; a seta as long as the two distal articles on the apical article (Figure 1d, 2c). Buccal pieces hardly discernible.
Pereion composed of seven pereionites (Figures 1a, 1b, 2a). Pereionite I slightly wider than the other pereionites. Anterior and posterior corners of the pereionite I rounded, posterior corners of pereionite II-IV right-angled, posterior corners of pereionites V to VII gradually acute and pointing backwards. Tergal surface covered by numerous spine-like scale-setae.
Seven pairs of pereiopods with long setae and only few tricorn-like setae (Figures 1e, 1f, 2d, 2e). Some of the pereiopods (e.g., pereiopod I) or parts of pereiopods (e.g., apical part of the left pereiopod VI) appear unusually slender.
Pleon composed of five pleonites distinctly narrower than pereion, with surface covered by numerous spine-like scale-setae (Figures 1a, 2a). Pleonites I-II framed by pereionite VII. Neopleurae of pleonites I and II reduced; well developed neopleurae pointing backwards in pleonites III-V. Pleopod I poorly visible; pleopod II-V as in Figures 1g and 2f. Pleopod II endopod with apex almost rounded. Telson relatively short, with concave sides and obtuse apex (Figures 1h, 2g). Uropod sympodites conical, slightly surpassing the pleotelson. Exopodites massive, conical, longer than sympodites. Exopodites around 2.5 times longer than endopodites, both apically ending in a long seta. Some sparse and short setae in outer and inner margin of exopodites and endopodites.
Family Detonidae Budde-Lund, 1906
Genus Armadilloniscus Uljanin, 1875
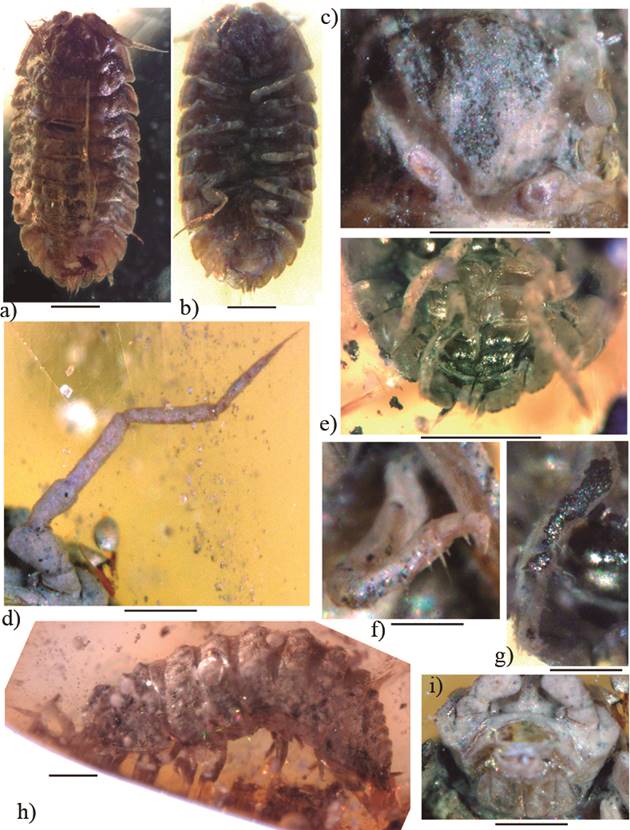
Figure 3 Armadilloniscus miocaenicus sp. nov., (a, b) juvenile paratype (IHNFG-4985) in dorsal and ventral views; (c) Cephalon in frontal view; (d) Second antenna; (e) Pleopod 2-5 and uropods in ventral view; (f) Pereiopod I; (g) Pereiopod VII; (h) ♀ adult holotype (IHNFG-4990) in lateral view; (i) Mouth. Scale bars = 200 µm.
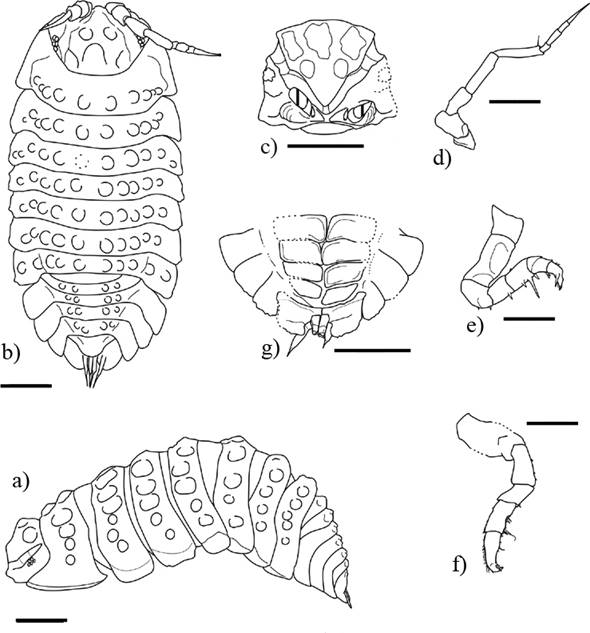
Figure 4 Armadilloniscus miocaenicus sp. nov., (a) ♀ adult holotype (IHNFG-4990) in lateral view; (b) juvenile paratype (IHNFG-4985) in dorsal view; (c) Cephalon in frontal view; (d) Second antenna; (e) Pereiopod I; (f) Pereiopod VII; (g) Pleopod 2-5 and uropods in ventral view. Scale bars = 200 µm.
Type species. ActoniscusellipticusHarger, 1878 by subsequent designation (see Taiti and Ferrara, 1989).
Diagnosis. Cephalothorax with developed lateral lobes with convex lateral margins. Vertex of the cephalon sculptured with five tubercles, three large posterior and two smaller anterior. Compound eyes of five ommatidia arranged in two rows. A large groove between the eyes and the cephalic sculptures. Body ornamentation composed of large and evenly rounded tubercles. Uropod exopodites and endopodites of similar length.
Derivation of name. The name of the new species is derived from “Miocene”, the geological age of the fossil.
Holotype. IHNFG-4990, adult female preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, TuxtlaGutiérrez, Chiapas, Mexico.
Paratype. IHNFG-4985, juvenile individual preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Locality. Campo La Granja mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Finca Carmitto Member, La Quinta Formation, Early Miocene (Aquitanian, 23 Ma).
Description. Maximum dimensions: 3.5 × 1.8 mm (holotype), 1.6 × 0.8 mm (paratype). Brownish. Body ovoidal, dorsoventrally compressed, dorsum surface with scaly appearance and markedly tuberculate (Figures 3a, 3h, 4a, 4b).
Cephalothorax with developed lateral lobes with convex lateral margins and median lobe acute-angled (Figures 3a, 3c, 4b, 4c). Lateral lobes delimited by the linea supraantennalis. Vertex of the cephalon sculptured with five tubercles, three large posterior and two smaller anterior (Figures 3a, 3c, 4b, 4c). Compound eyes of five ommatidia arranged in two rows. A large groove between the median and lateral lobes extending between the eyes and the cephalic sculptures, probably to accommodate the antennas when folded (Figures 3a, 3c, 4b, 4c). Antennula indiscernible. Second antenna robust, composed of five peduncular articles + flagellum. Peduncular article with sparse setae; fifth article with a particularly long seta on distal part (Figures 3d, 4d). Flagellum of four translucent articles; the apical article elongated, bearing a tuft of setae. Buccal pieces examinable only superficially, as in Figure 3o.
Pereion composed of seven pereionites with enlarged coxal plates (Figures 3a, 3h, 4a, 4b). Pereionite I about two times wider than the other pereionites. Coxal plates of pereionites I to VII pointing backward. Coxal plates of pereionite I widely pointing forward, framing the cephalon until its middle. Tergites with large, evenly rounded tubercles of various sizes.
Pereiopod I with a particularly long spine in the distal part of inner margin of the carpus (Figures 3f, 4e). Pereiopod II similar to pereiopod I, long spine of the carpus slightly shorter. Pereiopods seven hairy with numerous setae especially on the outer margin of the carpus and propodus, one long seta in inner margin of the propodus, one particularly long and two others smaller on the carpus (Figures 3g, 4f). Dactylus of all pereiopods with a smaller inner claw beside the large outer claw and a long featherlike dactylar organ.
Pleon composed of five pleonites with enlarged epimera pointing backwards, continuing the regular oval outline of the pereion. Pleonites and telson with two median tubercles.Telson short, trapezoidal (Figures 3a, 4b). Pleopods II-V rectangular to subrectangular as in Figures 3e, 4g. Uropod sympodite flattened, plate-like, in contact with the epimera of pleon segment V. Exopodites inserting on the inner margin of the sympodites. Exopodites and endopodites of similar size, slightly surpassing posterior margin of the sympodites.
Comparison and systematic position. The new specimen can be easily assigned to the extant genus ArmadilloniscusUljanin, 1875, based on the tuberculate body segments with enlarged coxal plates and epimera, on the cephalon with well developed median lobe and lateral lobes delimited by the linea supra-antennalis, and on the uropodal sympodite flattened with exopodite inserted on its medial margin (Taiti and Ferrara, 1989; Schmidt, 2002).
Currently, the genus Armadilloniscus contains about 30 species, but some are probably synonymous with others (e.g., A. bulgaricusFrankenberger, 1941 and A. letourneuxiSimon, 1885 are probably synonyms of A. ellipticusHarger, 1878; Schmalfuss, 2003). A. miocaenicus sp. nov. is easily distinguishable from A. binodulusLewis, 1992; A. conglobatorTaiti and Ferrara, 1989; A. holmesiArcangeli, 1933; A. indicusFerrara and Taiti, 1983; A. lamellatusTaiti and Ferrara, 1989; A. lanyuensisKwon and Wang, 1996; A. lindahliRichardson, 1905; A. malaccensisTaiti and Ferrara, 1989; A. mirabilisFerrara, 1974; A. nasatusBudde-Lund, 1908 and A. ornatocephalusLewis, 1992 that present faintly developed tergal ornamentation. By the presence of well developed eyes, A. miocaenicus sp. nov.is easily distinguishable from A. iliffeiTaiti and Ferrara, 1989 and A. hawaiianusTaiti and Ferrara, 1989. By the sculpture of the cephalon, A. miocaenicus sp. nov.differs from A. biltoniTaiti and Ferrara, 1989; A. coronocapitalisMenzies, 1950; A. hoonsooiKwon and Wang, 1996. A. miocaenicus sp. nov.differs from A. aegaeusSchmalfuss, 1981; A. candidusBudde-Lund, 1885; A. quadricornisVandel, 1971; A. steptusSchotte and Heard, 1991 in having uropod exopodites and endopodites of similar length. A. miocaenicus sp. nov.differs from the Japanese species A. albusNunomura, 1984; A. brevinaseusNunomura, 1984; A. japonicusNunomura, 1984; A. notojimensisNunomura, 1990 in having a trapezoidal telson.
The genus Armadilloniscus is currently distributed nearly worldwide in subtropical and tropical regions, and in the Mediterranean area (see map in Taiti and Ferrara, 1989). In the Caribbean region, only three species have been recorded: A. caraibicusPaoletti and Stinner, 1989; A. ellipticusHarger, 1878 and A. ninaeSchultz, 1984. A. miocaenicus sp. nov.differs from A. ellipticusHarger, 1878 in having uropod exopodites and endopodites of similar length and trapezoidal telson. A. miocaenicus sp. nov.differs from A. caraibicusPaoletti and Stinner, 1989 in having rounded tubercules (elongated ribs in A. caraibicus), cephalic grooves, and exopodites and endopodites of similar length. A. miocaenicus sp. nov.differs from A. ninaeSchultz, 1984 in having well developed tubercules and eyes of only five ommatidia.
Remarks. The ventral side of the adult specimen is marked by a deep gash at the level of the IV-V thoracic segments, removing the pereiopods III-V (Figure 3a). This scar could have been caused by a relatively large predator, such as a bird.
The antennae of the adult specimen have been unfortunately truncated during polishing process. The clear pictures taken before allowed, nonetheless, a description of the appendages.
Family “Scleropactidae” Verhoeff, 1938
Genus Palaeospherarmadillo Broly, gen. nov.
Type species. Palaeospherarmadillo mazanticus Broly, gen. nov.sp. nov.
Diagnosis. Cephalothorax with frontal shield pressed to the vertex. Eyes absent. Distal part of the fifth antennal article with a groove. Antennal flagellum three-jointed. First coxal plate with schisma. Noduli laterales indistinct. Pleotelson exceeded by uropods, not reaching the body outline. Uropod sympodite angular, flattened, broader than long in dorsal view. Uropod exopodite very small, inserted on the dorsal face near the median margin.
Derivation of name. The name of the new genus is derived from a combination of the Greek word palaios (meaning “ancient”) and of “Spherarmadillo”in reference to SpherarmadilloRichardson, 1907, a close extant genus.
Comparison and systematic position. Currently, the “Scleropactidae” are separated into two groups according to their geographical distribution: a Southeast Asian group (united in the subfamily Toradjinae, Ferrara et al., 1995) and a Neotropical group (Schmidt, 2007). Despite numerous phylogenetic investigations, a clear statement on the monophyly of the “Scleropactidae” cannot be made (Schmidt, 2003, 2007). Nonetheless, the new genus can be easily assigned to the “Scleropactidae” based on the shape of pleotelson and uropods. The new genus is very close to the extant genus SpherarmadilloRichardson, 1907 based on the coxal plate I with hind corner cleft, eyes totally absent, uropod sympodite flattened (wider than long and angulated in dorsal view) and uropod exopodite very small (not reaching the distal margin of the sympodite). However, SpherarmadilloRichardson, 1907 is remarkable in having distinct noduli laterales (Schmidt, 2007). Palaeospherarmadillo gen. nov. differs mainly from Spherarmadillo in lacking well developed noduli laterales.
Remarks. Given the position of the body of the new fossils, it is clear that Palaeospherarmadillo gen. nov. presented conglobational ability, as most of the species of “Scleropactidae”. However, it is difficult to decide wether the species corresponds to an exo or endoantennal conglobation.
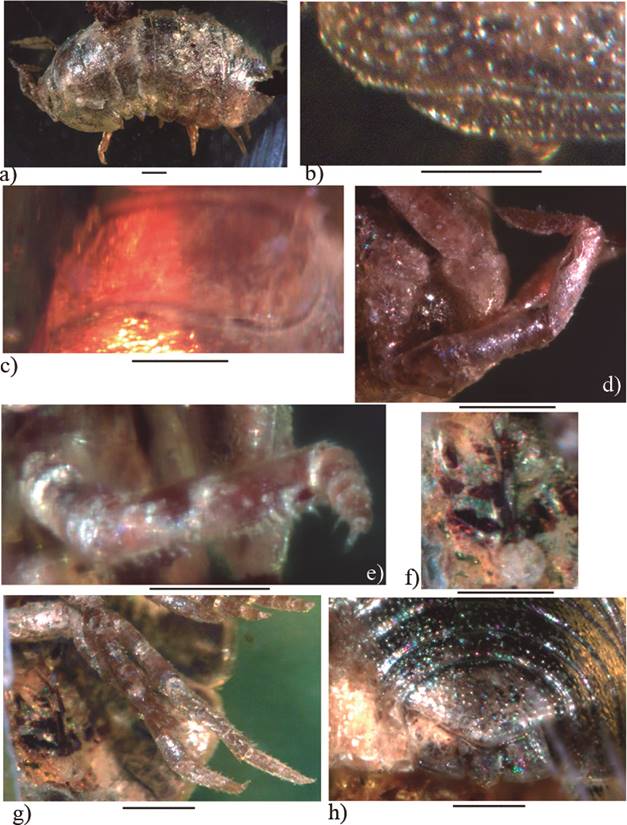
Figure 5 Palaeospherarmadillo mazanticus gen. nov. sp. nov., ♂ adult holotype (IHNFG-4997); (a) General habitus, lateral view; (b) Lateral margin of first coxal plate in ventral view; (c) Cephalon in dorsal view; (d) Antenna; (e) Pereiopod I; (f) Pleopod 3; (g) Pereiopod VII; (h) Pleotelson with uropods, dorsal view. Scale bars = 200 µm.
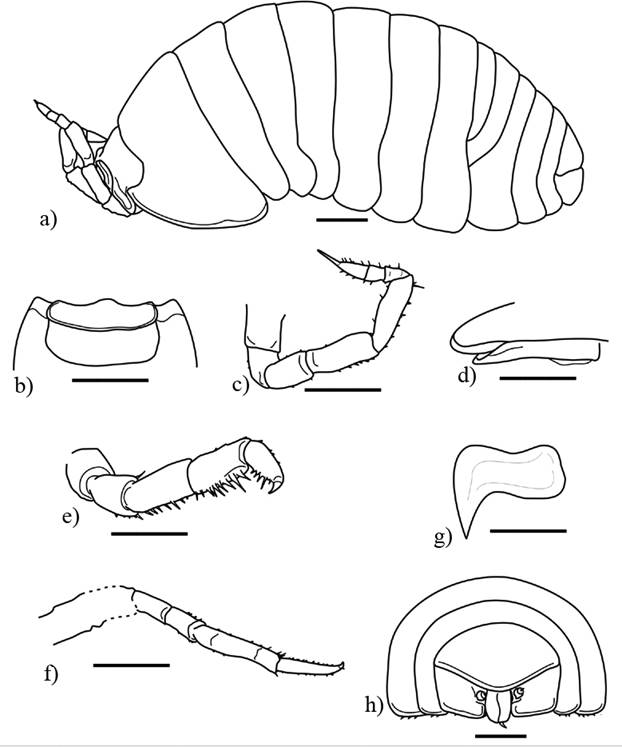
Figure 6 Palaeospherarmadillo mazanticus gen. nov. sp. nov., ♂ adult holotype (IHNFG-4997); (a) General habitus, lateral view; (b) Cephalon in dorsal view; (c) Antenna; (d) Lateral margin of first coxal plate in ventral view; (e) Pereiopod I; (f) Pereiopod VII; (g) Pleopod 3; (h) Pleotelson with uropods, dorsal view. Scale bars = 200 µm.

Figure 7 Palaeospherarmadillo rotundus gen. nov. sp. nov., adult holotype (IHNFG-5302); (a) General habitus, lateral view; (b) Antenna; (c) Cephalon in dorsal view; (d) Lateral margin of first coxal plate in ventral view; (e) Merus, carpus, propodus and dactylus of pereiopod VI; (f) Pleotelson with uropods in dorsal view. Scale bar = 200 µm.
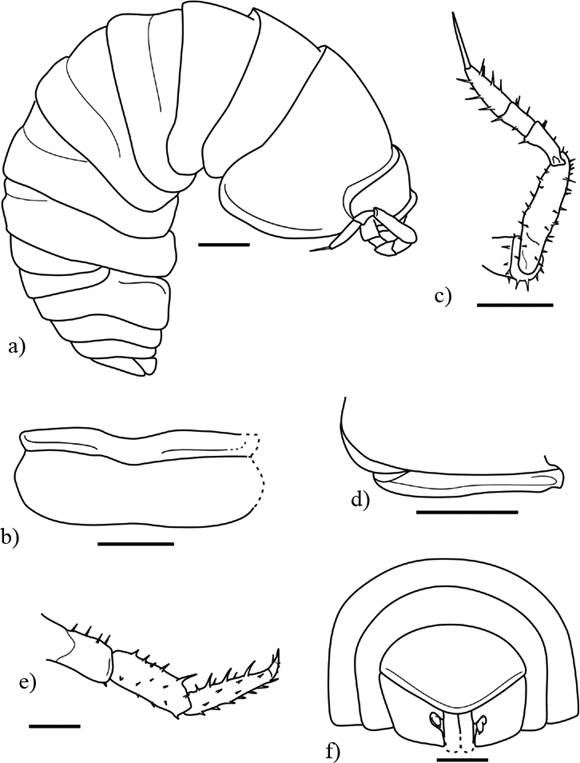
Figure 8 Palaeospherarmadillo rotundus gen. nov. sp. nov., adult holotype (IHNFG-5302); (a) General habitus, lateral view; (b) Cephalon in dorsal view; (c) Antenna; (d) Lateral margin of first coxal plate in ventral view; (e) Merus, carpus, propodus and dactylus of pereiopod VI; (f) Pleotelson with uropods in dorsal view. Scale bars = 200 µm.

Figure 9 Archeostenoniscus robustus gen. nov. sp. nov., ♀ adult holotype (IHNFG-4988/A); (a, b) General habitus, dorsal and ventral views; (c) Mouth; (d) Pereiopod I; (e) Pleon, telson and uropods in ventral view; (f) Second antenna; (g) Pleon, telson and uropods in dorsal view; (h) Pereiopod VII.Scale bars = 200 µm.

Figure 10 Archeostenoniscus robustus gen. nov. sp. nov., ♀ adult holotype (IHNFG-4988/A); (a) General habitus, dorsal view; (b) Second antenna; (c) Pereiopod I; (d) Pereiopod VII; (e) Pleon, telson and uropods in dorsal view. Scale bars = 200 µm.
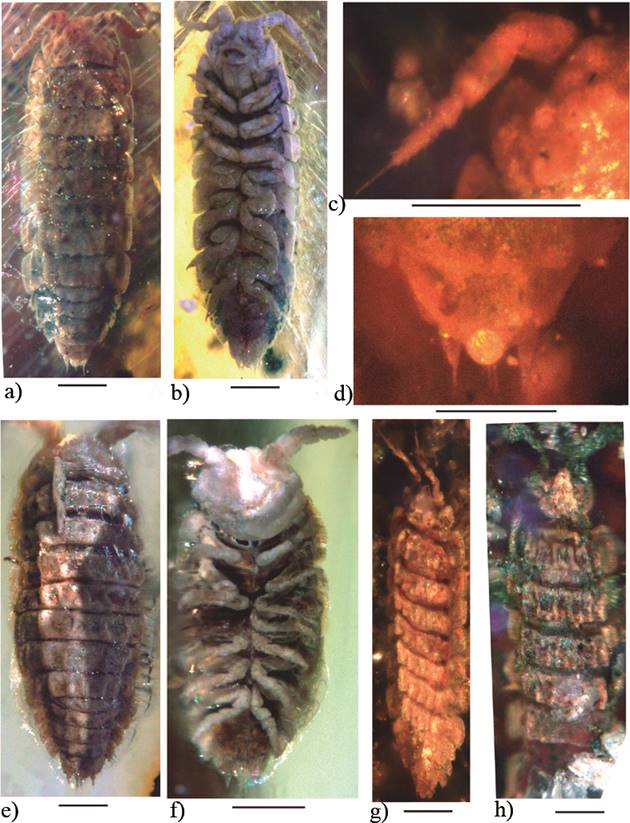
Figure 11 Archeostenoniscus mexicanus gen. nov. sp. nov., adult holotype (IHNFG-4996); (a, b) General habitus, dorsal and ventral views; (c) Two-jointed flagellum of the antenna; (d) Telson and uropods in dorsal view. Archeostenoniscus sp. 1, (IHNFG-5304); (e, f) General habitus, dorsal and ventral views. Archeostenoniscus sp. 2, (IHNFG-4989); (g) General habitus, dorsalview. (h) Incomplete specimen putatively assigned to Archeostenoniscus, (IHNFG-4988/B). Scale bars = 200 µm.

Figure 12 Archeostenoniscus mexicanus sp. nov., adult holotype (IHNFG-4996), general habitus, dorsal view; close-up of the antennal flagellum and of the telson and uropods are figured. Scale bar = 200 µm.

Figure 13 Palaeospherarmadillo mazanticus Broly, gen. nov.sp. nov., paratype (IHNFG-4998); (a-c) General habitus, lateral and ventral views. Undefined crinochetan specimens preserved in Chiapas amber. IHNFG-5305; (d, e) General habitus, dorsal and ventral views. IHNFG-5306; (f, g) General habitus, dorsal and ventral views. IHNFG-5307; (h, i) General habitus, dorsal and ventral views. IHNFG-5308; (j, k) General habitus, dorsal and ventral views. IHNFG-5309; (l, m) General habitus, dorsal and ventral views. IHNFG-2928; (n, o) General habitus, dorsal and ventral views. IHNFG-5310; (p) General habitus. Scale bars = 200 µm.
Derivation of name. The name of the new species is derived from the Lower Miocene Mazantic Shale, one of the amber-bearing lithostratigraphic units in Mexico.
Holotype. IHNFG-4997, adult male preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Paratype. IHNFG-4998, adult male preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Locality. Los Pocitos mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Mazantic Shale, Early Miocene (Aquitanian, 23 Ma).
Description. Maximum dimensions: 2.4 × 1.1 mm (holotype). Animal probably able to roll up into a ball. Dorsum surface dark slate-grey, smooth, covered with numerous small tricorn setae arranged in rows (Figures 5a, 5b, 6a).
Cephalothorax without antennal lobes, with frontal shield separated from vertex only by a suture. Its frontal margin is convex, medially slightly recurved in dorsal view (Figures 5c, 6b). Eyes absent. Antennula indiscernible. Second antenna composed of five peduncular articles + flagellum. Peduncular article with some short setae, particularly in the fourth and fifth articles. Distal part of the fifth article with a longer seta and a deep groove probably to host the flagellum in folded position (Figures 5d, 6c). Flagellum as long as the fifth peduncular article but much slender. It is composed of three short articles of similar length and covered with several setae and only a weak suture between the apical and second article. Apical cone slightly shorter than the distal flagellar article (Figures 5d, 6c). Buccal pieces indiscernible.
Pereion composed of seven pereionites with well developed coxal plates (Figures 5a, 5b, 6a). Pereionite I about three times wider than the other pereionites. Coxal plates of pereionite I pointing forward and widely backward, with hind corner cleft and posterior lobe more protruding (Figure 6d). Coxal plates of pereionites II to IV reduced in size, coxal plates of pereionites II to V mainly rounded, VI and VII subrectangular. Noduli laterales indiscernible, probably very small. Pereiopod I carpus enlarged with transverse brush composed of scales and numerous setae on ventral margin (Figures 5e, 6e). Following pereiopods long and slender with short setae. Pereiopods 7 similar to the others legs, with no apparent sexual dimorphic structures (Figures 5g, 6f).
Pleon composed of five pleonites. Epimera of the pleonites III-V well developed, continuing the regular oval outline of the body. Male pleopod 1 exopodite with a well developed distal lobe. Pleopod 2 endopodite exceeds the exopodite (Figures 5f, 6g). Telson triangular, with posterior margin rounded (Figures 5h, 6h). Pleotelson exceeded by uropod protopodites.Uropod protopodite flattened, wider than long and of trapezoidal shape in dorsal view (Figures 5h, 6h). Exopodite reduced, inserted on the dorsal face and on the inner margin of the protopodite (Figures 5h, 6h). Uropod endopodite short, slightly surpassing the posterior margin of the protopodite (Figures 5h, 6h).
Type species. Palaeospherarmadillo mazanticus Broly, sp. nov.
Derivation of name. The name of the new species comes from the latin word “rotundus”, in reference to the rounded posture taken by the holotype during death.
Holotype. IHNFG-5302, adult (gender indeterminable) preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Locality. Los Pocitos mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Mazantic Shale, Early Miocene (Aquitanian, 23 Ma).
Description. Maximal dimensions: 2.9 × 1.7mm (holotype). Animal capable to roll up into a ball. Dorsum surface dark slate-grey, smooth, covered with numerous small tricorn setae (Figure 7a, 7f). Cephalothorax without antennal lobes, with frontal shield separated from vertex only by a suture and medially slightly recurved in dorsal view (Figures 7c, 8b). Eyes absent. Antennula indiscernible. Second antenna composed of five peduncular articles + flagellum. Peduncular article with some short setae, more numerous in the fifth article. Distal part of the fifth article with a groove suitable to host the flagellum (Figures 7b, 8c). Flagellum as long as the fifth peduncular article, composed of three articles covered with numerous setae; first and second articles of similar length, third article slightly longer, only a weak suture between the third and second article. Apical cone slightly shorter than the distal flagellar article (Figures 7b, 8c). Buccal pieces indiscernible.
Pereion composed of seven pereionites with well developed coxal plates (Figures 7a, 8a). Pereionite I about three times wider than the other pereionites. Coxal plates of pereionite I pointing forward and backward, with hind corner cleftand posterior lobe slightly protruding (Figures 7d, 8d). Coxal plates of pereionites II to IV reduced in size, coxal plates of pereionites II to V mainly rounded, VI and VII subrectangular. Noduli laterales poorly discernible, one small discernible close to the posterior margins of the tergites 6 and 7. Pereiopods poorly discernible due to the rolling-up of the animal. Only apical part of some of them well discernible (Figures 7e, 8e). A particularly robust seta on the sub-apical part of the carpus. Inner face of the propodus with three smilar strong setae and numerous smaller. Inner claw of the dactylus shorter than outer claw.
Pleon composed of five pleonites. Epimera of the pleonites III-V well developed, continuing the regular oval outline of the body. Pleopods indiscernible. Telson triangular, with posterior margin rounded (Figures 7f, 8f). Pleotelson exceeded by uropod protopodites. Uropod protopodite flattened, wider than long and of trapezoidal shape in dorsal view (Figures 7f, 8f). Exopodite reduced, inserted on the dorsal face and on the inner margin of the protopodite (Figures 7f, 8f). Uropod endopodite short, slightly surpassing the posterior margin of the protopodite (Figures 7f, 8f).
Differential diagnosis and systematic position. The new fossil is assigned to the new genus Palaeospherarmadillo based on the coxal plate I with hind corner cleft, the poorly discernible noduli laterales, the exceedingly small uropod exopodite (not reaching the distal margin of the sympodite) and the uropod sympodite wider than long and angulated in dorsal view.
Palaeospherarmadillo rotundus sp. nov. differs from P. mazanticus in having a deep groove from the apical part of the 5th antennal segment to its middle (not reaching the top of the 5th article in P. mazanticus) and uropod protopodite slightly surpassing the posterior margin of the pleonite V (slightly shorter in P. mazanticus). The posterior corner of the coxal plates of pereionite I of Palaeospherarmadillo rotundus sp. nov. is rounder than in P. mazanticus. The other differences between Palaeospherarmadillo rotundus sp. nov. and P. mazanticus are mainly quantitative rather than qualitative. The antennal fifth article and flagellum of Palaeospherarmadillo rotundus sp. nov.present two times more and longer setae than in P. mazanticus. Similar findings for the apical part of pereiopod VI.
Family Stenoniscidae Verhoeff, 1908
Genus Archeostenoniscus Broly, gen. nov.
Type species. Archeostenoniscus robustus Broly, gen. nov.sp. nov.
Diagnosis. Body slender, sides parallel, 3-3.5 times as long as wide. First pleonite absent. Cephalon with numerous tubercles. Body ornamentation composed of six major rows of tergal ribs. Enlarged coxal plates, perpendicular to the body. Uropods short, apical half of the exopodites exceeds the telson in dorsal view.
Derivation of name. The name of the new genus is derived from a combination of the Greek word Arkhaios (meaning “ancient”) and Stenoniscus, the type genus name of the Stenoniscidae.
Comparison and systematic position. The new fossils, named as Archeostenoniscus robustus gen. nov sp. nov and Archeostenoniscus mexicanus gen. nov. sp. nov., are assigned to the Stenoniscidae Verhoeff, 1908 based on the elongate body sculptured by numerous rows of longitudinal ribs, the absence of a first pleonite, the coxal plates distinctly separated from tergites, and the short uropod largely covered by the telson in dorsal view.
Currently, the Stenoniscidae is a small family containing only two genera: StenoniscusAubert and Dollfus, 1890 (four species) and MetastenoniscusTaiti and Ferrara, 1982 (twospecies). See map of the current geographic distribution of the Stenoniscidae in Schmidt, 2003. In having coxal plates perpendicular to the body, uropodal propodites truncated and eyes developed, Archeostenoniscus gen. nov. is morphologically very close to Metastenoniscus (the coxal plates fall vertically, uropodal propodites are ovoidal and eyes are absent in Stenoniscus). However, the new genus differs remarkably from the two current genera in having uropods exceeding the pleotelson in dorsal view (pleotelson completely covering the uropods in Stenoniscus and Metastenoniscus; Schmidt, 2003, 2008).
By the present study, the new genus Archeostenoniscus includes at least two species: A. robustus sp. nov. (Figures 9, 10) and A. mexicanus sp. nov. (Figures 11a-11d, 12). Two other specimens (Figure 11e-11g) are morphologically very close (including tergal ribs, uropods reduced, two-jointed flagellum) and are assigned to the genus Archeostenoniscus. However, the quality of their preservation does not allow for an assignation to one of the two species described. One last specimen with tergal ribs (Figure 11h), poorly preserved, is putatively assigned to the genus Archeostenoniscus but its assignation must be taken with caution due to the pyritization of the head structures and the absence of the posterior part.
Diagnosis. As for the genus.
Derivation of name. The name of the new species refers to the robustness of the stocky antennal flagellum of the type specimen.
Holotype. IHNFG-4988/A, adult female preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, TuxtlaGutiérrez, Chiapas, Mexico.
Locality. Campo La Granja mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Finca Carmitto Member, La Quinta Formation, Early Miocene age (23 Ma).
Description. ♀. Maximum dimensions: 1.8 × 0.6 mm. Poorly pigmented. Body slender, sides parallel, 3.5 times as long as wide (Figures 9, 10).
Cephalon with small lateral lobes, sculptured with numerous tubercles (Figures 9a, 10a).Eyes present, composed of around four ommatidia. Antennula bi-articulate. Second antenna short, thick, composed of five peduncular articles + flagellum. Peduncular articles with some spare setae. Flagellum of two thickest articles; first article short, apical article two times longer, conical, ended by a robust apical cone (Figures 9f, 10b). Buccal pieces difficult to describe, only the maxilliped with rectangular base is superficially discernible.
Pereion composed of seven pereionites of similar size with enlarged coxal plates (Figures 9a, 10a). Anterior part of coxal plates of pereionites I pointing forward, framing the posterior part of the cephalon. Tergal surface sculpted with six major rows of ribs (not always completely delimited). The most lateral row of tergal ribs more salient on each side. Tergal ribs more flattened in pereionites I and II than in posterior ones. Posterior corner of the coxal plates rounded.
Seven pairs of pereiopods rather short, curled up on the ventral side of the body (Figures 9d, 9h, 10c, 10d). Basipodites enlarged, sculptured with a groove in which the ischium and merus fit. Carpus and propodus of pereiopod I with some sparse setae, one longer than others in the inner margin of the propodus.
Pleon composed of four pleonites (first pleonite absent). Pleonite III-V with epimera well developed in the continuity of the coxal plate of pereionites, with posterior part rounded, slightly pointing backward. Pleonite II with four ribs, flattening in the following pleonites. Pleopods III-IV subrectangular, Pleopods V subtriangular as in Figures 9e and 10e. Telson short, three-lobed, triangular with rounded apex (Figures 9e, 10e). Uropods with large sympodites and small conical exopodites ending by short setae. Endopodites shorter than sympodites and exopodites. The apical half of the exopodites exceeds the telson in dorsal view (see Figures 9a, 10a).
(Figures 11a-11d and 12)
Type species. Archeostenoniscus robustus Broly, sp. nov.
Diagnosis. Body slender, sides parallel, three times as long as wide. First pleonite absent. Cephalon with numerous tubercles. Antennal flagellum elongated with numerous setae. Body ornamentation composed of six major rows of tergal ribs. Enlarged coxal plates, perpendicular to the body. Uropods short, apical parts of the exopodites and endopodites exceed the telson in dorsal view.
Derivation of name. The name of the new species refers to Mexico, the country of origin of the holotype.
Holotype. IHNFG-4996, adult (gender indeterminable) preserved in Chiapas amber from Mexico, deposited in the Instituto de Historia Natural de Chiapas, Museo Eliseo Palacios Aguilera, Tuxtla Gutiérrez, Chiapas, Mexico.
Locality. Campo La Granja mine, near Simojovel, Chiapas, Mexico (Serrano-Sánchez et al., 2015a), Finca Carmitto Member, La Quinta Formation, Early Miocene age (23 Ma).
Description. Maximum dimensions: 1.6 × 0.4 mm. Poorly pigmented. Body slender, sides parallel, three times as long as wide (Figures 11a, 11b, 12).
Cephalon with small lateral lobes, sculptured with numerous tubercles (Figures 11a, 12). Eyes present, composed of around four ommatidia. Antennula bi-articulate. Second antenna composed of five peduncular articles + flagellum. Peduncular articles with sparse setae. Flagellum of two articles; first article short, apical article elongated, bearing numerous short setae and ended by a long seta (Figures 11c, 12). Buccal pieces discernible.
Pereion composed of seven pereionites of similar size with enlarged coxal plates (Figures 11a, 12). Anterior part of coxal plates of pereionites I pointing forward, framing the posterior part of the cephalon. Posterior corner of the coxal plates VI and more so VII, pointing backward. Tergal surface sculptured with six major rows of ribs. The most lateral row of tergal ribs more salient on each side. Tergal ribs more flattened in pereionites I and II than in posterior ones. Pereiopods discernible (Figure 11b).
Pleon composed of four pleonites (first pleonite absent). Pleonite III-V with epimera well developed in the continuity of the coxal plate of pereionites, with posterior part pointing backward. Pleonite II-V with four ribs, lateral ribs more flattened in the pleonites IV and V. Telson subtriangular with rounded apex and two flattened medial ribs (Figures 11d, 12). Uropods with conical exopodites. Endopodites as long as exopodites in dorsal view. The apical half of the exopodites and endopodites exceed the telson in dorsal view.
Remarks. The ventral appendages of the new fossil are indiscernible due to a layer of sandstone positioned just below the animal.
Comparison and systematic position. Despite the lack of information about the ventral face, Archeostenoniscus mexicanus is dorsally easily distinguishable from Archeostenoniscus robustus in: having a more slender and longer antennal flagellum; coxal plates with more acute corners; and in the shape of the pleotelson and uropodal endopodites exceeding the telson in dorsal view.
DISCUSSION
Palaeodiversity
In this paper, we describe six new crinochetan species from the Miocene amber of Chiapas, Mexico, including members of the family Olibrinidae (Palaeolibrinus spinicornis gen. nov. sp. nov.), Detonidae (Armadilloniscus miocaenicus sp. nov.), Stenoniscidae (Archeostenoniscus robustus gen. nov. sp. nov., Archeostenoniscus mexicanus sp. nov.) and “Scleropactidae” (Palaeospherarmadillo mazanticus gen. nov. sp. nov., Palaeospherarmadillo rotundus sp. nov.). This study represents the first fossil record of the family Detonidae, Olibrinidae and Stenoniscidae.
Previously, we recorded from the same deposits two gravid females (Aquitanoscia chiapasensis and A. maternus) assigned to the family “Philosciidae” (Broly et al., 2017). In the wider Caribbean region, several specimens assigned to the “Scleropactidae” (formerly Sphaeroniscidae; Protosphaeroniscus tertiarius) and to the Delatorreidae (formerly Pseudarmadillidae; Pseudarmadillo cristatus and P. tuberculatus) have been described from Dominican amber (respectively Schmalfuss, 1980, 1984). Numerous inclusions assigned to the “Philosciidae” and the “Platyarthridae” have been also listed in Dominican amber but not described and figured (Schmalfuss, 1984). Together, these studies reveal an early radiation of Crinocheta dating to the Miocene.
Implications for paleoenvironmental reconstruction
The distribution of oniscidean species is strongly driven by their degree of adaptation to terrestrial life. The environmental moisture conditions are preponderant and, to a lesser extent, the concentration of salts (sodium chloride and calcium carbonate) in the substrate, leads to a species gradient (e.g., Warburg et al., 1984). Therefore, study isopod communities provide precious insights to fossil paleoenvironments.
Most current species of the Olibrinidae are strictly littoral (intertidal zone), living in mangroves or sandy beach, burrowed in mud, sand or under rocks (Roman, 1977; Schmidt, 2001, 2002). Some species are also caverniculous and most of these species are probably amphibious (Taiti and Ferrara, 2004). The current species of Armadilloniscus are strictly littoral, living on rocky or sandy beaches, under stones or plant and seaweed debris, at close distance to the sea (Taiti and Ferrara, 1989). The current species of Stenoniscidae are strictly littoral (only one record from a cave near shore), living under algae and debris stranded on the beach (Paoletti and Stinner, 1989; Taiti and Ferrara, 1982; Schmidt, 2003). The current species of “Scleropactidae” are not found in littoral but rather in moist habitats, like forests or rainforests, or in caves (Schmidt, 2007). The “Philosciidae”, as previously described in Broly et al., 2017, are currently abundant in tropical biotopes and wetland habitats. It is generally accepted that Chiapas amber was deposited in ancient estuarine habitats resembling modern mangrove forests, but the predominant vegetation were plants Leguminosae related to the genus Hymenaea (Langenheim, 1966) (Calvillo-Canadell et al., 2010; Solórzano-Kraemer, 2010; Serrano-Sánchez et al., 2015a).
The typical estuarine fauna, including aquatic, semiaquatic and terrestrial crustaceans and insects, trapped in most pieces of Campo La Granja amber, suggests a tidal-flat environment, next to an estuary (Serrano-Sánchez et al., 2015a). The oniscidean assemblage presented here, including species presenting affinities with extant littoral groups and/or living in moist habitats, strongly supports a particularly wet paleoenvironment under a brackish water influence.
CONCLUSION AND PERSPECTIVES
The Oniscidea are considered to have emerged in the Late Paleozoic (Broly et al., 2013) and the first attested Oniscidea has been recently found in Cretaceous amber from Burma (Broly et al., 2015). Despite this long stratigraphic range, the fossil record of the Oniscidea is sparse and the group has received little interest from the scientific community. Several obstacles may have constrained the description of fossil species in the past. First, oniscidean species characterization requires access to the mouth parts or pleopods which are only superficially examinable or even totally inaccessible in fossils. Pereiopods can also be difficult to properly observe according to their position and may be absent due to mutilation. Second, calcite of the exocuticle may be affected by the acid components of the fresh resin, some times masking fine cuticular structures such as setae. So, the species characterization of isopods included in amber cannot be as complete as in modern taxa (Schmalfuss, 1984). However, in order to understand the palaeodiversity and its implications on the oniscidean evolutive history, we should try to incorporate data from the scarce fossil record of this group, without ignoring its limitations (Nel and Prokop, 2009; Allmon, 2013).
The diversity of terrestrial isopods found in the Chiapas amber is significative, and more studies are to be made in the near future, including some unidentified specimens shown here (Figure 13d-13p).











 nueva página del texto (beta)
nueva página del texto (beta)


