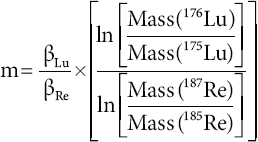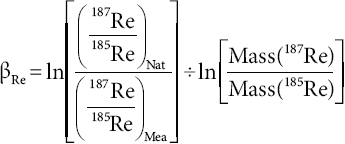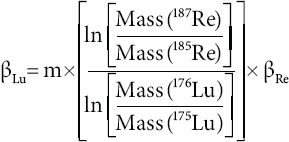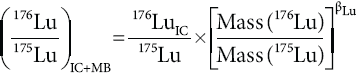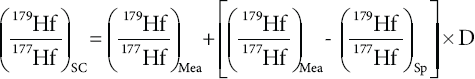INTRODUCTION
Besides being a powerful geochronometer for garnet-bearing meta-morphic rocks, especially eclogites (e.g., Skora et al., 2006; Smit et al., 2010; Estrada- Carmona et al., 2015) and phosphates (e.g., Barfod et al., 2003; Larsson and Söderlund et al., 2005), the geological application of the Lu-Hf isotope system (β-decay of 176Lu to 176Hf, t1/2 = 37.2 G a; Scherer et al., 2001; Söderlund et al., 2004) is mainly focused on the chemical differentiation of the silicate Earth with respect to the formation of crust and mantle (e.g., Vervoort et al., 2000; Blichert-Toft and Puchtel, 2010; Guitreau et al., 2013), crustal contamination of mantle sources (e.g., Cai et al., 2014; Guo et al., 2016), and sedimentary environments (e.g., Bayon et al., 2009; Vervoort et al., 2011). In addition, when taken in combination with another isotopie system like Sm-Nd, mixing models can be calculated and deviations from the terrestrial trend can be identified (e.g., Schmitz et al., 2004; Vervoort et al., 2011).
The first approaches to measure Lu-Hf isotope ratios in geological materials were made with Thermal Ionization Mass spectrometry (TIMS, e.g., Patchett and Tatsumoto, 1980). After the development of Multi-Collector Inductively Coupled Plasma Mass-Spectrometry (MC-ICP-MS) instruments in the early nineties, having a much greater ionization efficiency, as well as improvements in the chemical separation that diminished the is ob arie interferences and matrix effects of other major and trace elements (Blichert-Toft et al., 1997; Blichert-Toft, 2001; Le Fèvre and Pin, 2001, 2005; Münker et al., 2001; Bizzarro et al., 2003; Ulfbeck et al., 2003; Lapen et al., 2004; Connelly et al., 2006; Lu et al., 2007; Sprung et al., 2010; Yang et αl., 2010; Bast et al., 2015), the Lu-Hf isotope system became more popular both for applications in geochronology and in isotope geochemistry. In this context, the Lu-Hf separation method for whole-rock samples developed by Münker et al. (2001) is a benchmark procedure that is now emulated by many other laboratories (e.g., Bast et al., 2015). Recently, Sprung et al. (2010) improved this separation method performing additional Lu and Hf clean-up steps. This separation procedure facilitates additional separation of elements for other isotope systems like Rb-Sr and Sm-Nd from the same sample aliquot.
However, the contrasting chemical behavior of Lu and Hf, the content of refractory minerals (like garnet, zircon or rutile) in some igneous and metamorphic rocks, and the behavior of Hf from the spike, hampers the complete sample digestion and sample-spike equilibration. These complications may lead to systematic errors of up to ten εHf-units, especially in zircon-bearing rocks and even more when recalculated to an initial value in relatively old (pre-Mesozoic) rocks (Mahlen et al., 2008). Problems with incomplete sample digestion by standard bench-top digestion methods were identified by various authors (Blichert-Toft et al., 1997; Münker etal., 2001; Ulfbeck et al., 2003; Blichert-Toft et al., 2004; Lapen et al., 2004; Mahlen et al., 2008; Vervoort et al., 2011) and were dealt by employing acid pressure digestion vessels (Parr® bombs) that consist of a steel jacket with a Teflon® liner. To ensure complete digestion in Parr® pressure vessels, the samples have to be digested typically for 5 days at ~190°c. However, a separate bomb is required either for every individual sample or two samples in Savillex® beakers can be put together in a larger Parr® bomb.
With the Picotrace® pressure digestion system (DAS®) 16 (or 32) samples can be digested simultaneously. The system consists of one (or two) Teflon-coated pressure block(s) made of metal alloy that hosts 16 Teflon pressure vessels, a Teflon-coated hot plate, and a hot plate controller. Besides that, the system is equipped with an evaporation device, with which strong acids such as perchloric and hydrofluoric acid are evaporated and neutralized in a closed system with no need of a perchloric acid fume hood. However, since the digestion block is heated on a hot plate and not in an oven, the active internal temperature is limited to ~165°C, due to loss of heat between the Teflon-coated hot plate, which is limited to a maximum temperature of 240°C and operated generally at 215°C, and the Teflon-coated digestion block that is located in a laminar flow clean-bench.
In this contribution we report (1) the chemical separation method established in the cleanlab facilities at the Geology Department, Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE), México; (2) the analytical protocol employed for the Thermo Neptune MC-ICP-MS installed at Centro de Geociencias, UNAM, Junquilla, Querétaro, México; (3) the equations used to correct the measured lutetium and hafnium mass intensities for isobaric interfer-enees, instrumental mass-bias, and spike, which form the basis for the data reduction software written and developed in R language (R Core Team, 2016) and presented here (IsotopeHf®). External reproducibility and accuracy of the methods is validated by analyses of several internation al reference standards and replicate analyses of unknown samples. In addition, to evaluate the digestion efficiency of the Picotrace DAS® pressure digestion system some samples were digested in both the Parr® Acid Digestion Vessel and the Picotrace DAS®.
ANALYTICAL METHODS
Sample descriptions
Eight different international reference rock powders recommended by the United States Geological Survey (USGS, n=6) and the Geological Survey of Japan (GS J, n=2) were analyzed, as well as six replicate digestions of unknown whole-rock samples. The unknown samples are from the Chiapas Massif, Southern México, and they were selected on the basis of their mineralogical composition and age: Two felsic orthogneiss samples (age ~1.0 G a) and one garnet-bearing sillimanite schist (age ~0.45 Ga) were chosen by containing significant amounts of resistant phases such as zircon or garnet, and three zircon-poor mafic amphi-bolite samples (age ~0.45 Ga) were analyzed to further examine the accuracy and precision of our method. Additionally, five single zircon grains and four fractions of amphibole concentrates were analyzed. A brief description of the samples is provided in Table 1.
Table 1 Descriptions of selected analyzed samples.
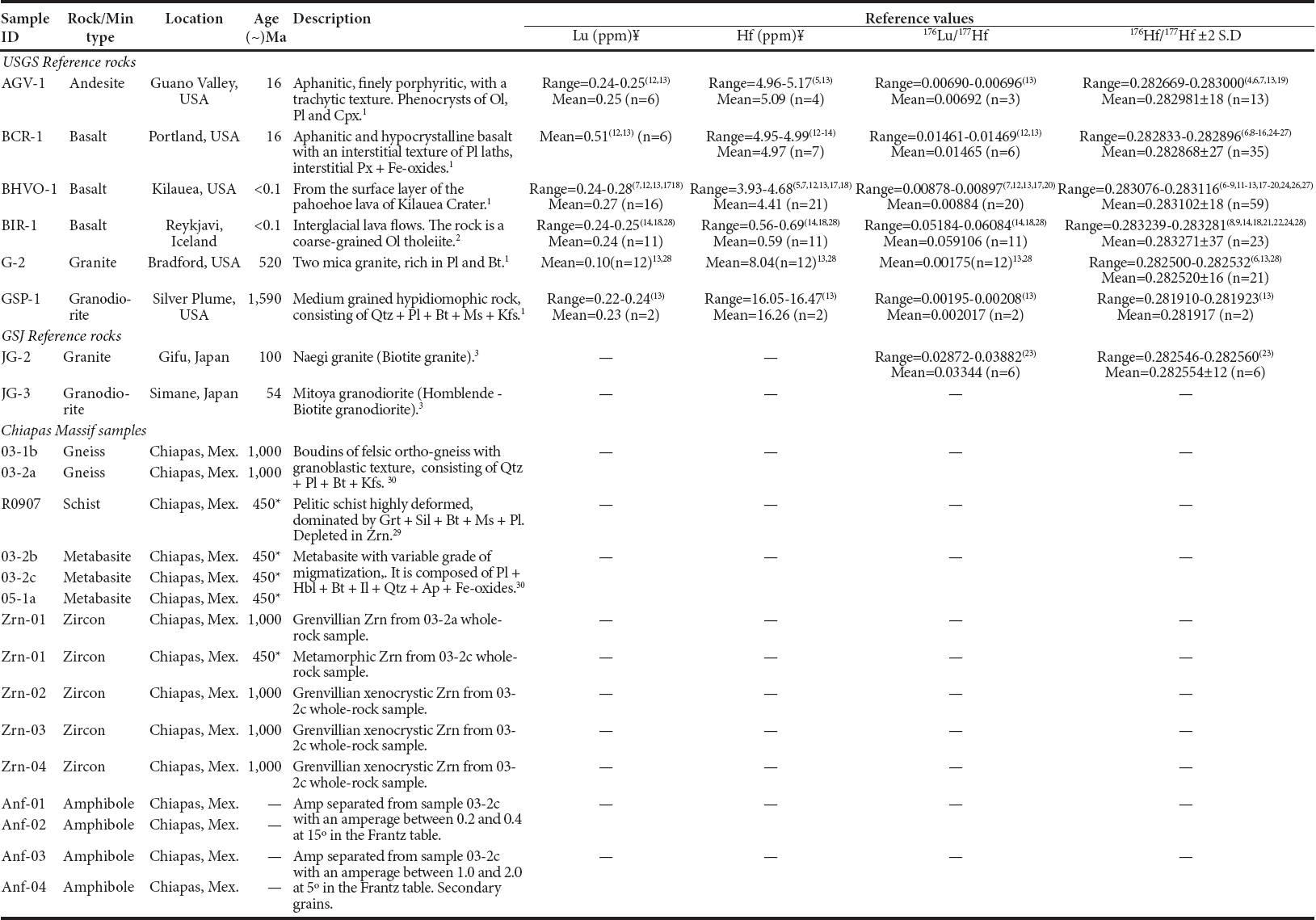
Abbreviations: Amp, amphibole; Ap, apatite; Bt, biotite; Cpx, clinopiroxene; Grt, garnet; Hbl, hornblende; Il, ilmenite; Kfs, alkaline feldspar; Ms, muscovite; Ol, olivine; Pl, plagioclase; Px, pyroxene; Qtz, quartz; Sil, sillimanite; Zrn, zircon.¥ Reported from ID analyses. * Metamorphic age. References: 1Flanagan (1967); 2Flanagan (1984); 3Ando and Shibata (1988); 4Chauvel et al. (2010); 5Lu et al. (2007); 6Weis et al. (2007); 7Pourmand and Dauphas (2010); 8Bizarro et al. (2003); 9Blichert-Toft (2001); 10Chu et al. (2002); 11Jochum et al. (2006); 12Lapen et al. (2004); 13Mahlen et al. (2008); 14Münker et al. (2001); 15Ulfbeck et al. (2003); 16van de Flierdt et al. (2007); 17Connelly et al. (2006); 18Le Fèvre and Pin (2005); 19Lu et al. (2007); 20Vervoort et al. (2004); 21Chu et al. (2011); 22Hanyu et al. (2005); 23Nebel et al. (2010); 24Bizimis et al. (2005); 25Ellam (2006); 26Li et al. (2006); 27Salters et al. (2011); 28Bast et al. (2015); 29Weber et al. (2008); 30González-Guzmán et al. (2014).
Chemical procedures
Chemical preparation and element separation were performed in PicoTrace® clean benches within class 1000 cleanlab facilities at the Geology Department, CI CESE. All acids used were double distilled in sub b oiling Teflon® systems, and all Teflon® beakers were previously cleaned in aqua regia, HNO3, HF, and Milli-Q® water. Between 50-150 mg of powdered whole rock aliquot or amphibole concentrate was weighted (to 0.01 mg precision) into a digestion vessel and spiked with amixed 180Hf-176Lu tracer (MS-WR-1,180Hf=98.266% and 176Lu=71.610%) provided by the Institut für Mineralogie, Universität Münster, Germany. When the Picotrace DAS® was used, a mixture of concentrated HF, HNO3, and HClO4 (3-4 mL,~1 mL and 3-4 drops, respectively) was added to each sample and placed on a hot plate at 215ºC for one week. When the Parr® Acid Digestion Vessel was used for digestion, a mixture of HF-HNO3 (5:1) was added to the samples previously weighted into 7 ml Savillex® beakers. Two Savillex® beakers were placed into the Teflon liner of a 125 ml Parr® Acid Digestion Vessel together with concentrated HF as a pressure medium and heated at 190 ºC for five days in an oven.
Evaporation of acids at subboiling conditions (including НСІО4) was performed with the Picotrace DAS® (for both DAS® and Parr® digested samples), changing the system to evaporation assembly and using hotplate temperatures starting at 120ºC (~75 。c evaporation temperature for HF and HNO3) and a stepwise heating program that goes up to 160 ºC (~120 ºC evaporation temperature) to dry down the perchloric acid. Strong acid fumes are extracted from the DAS ® with a clean air current in a closed system and neutralized by passing them through wash-bottles filled with 5% NaOH solution. The resulting Perchlorates were converted into chlorides by adding ~5 mL of 6M HCl. Sample-spike equilibration was achieved by leaving this solution in the closed vessels overnight at 80 ºC before drying down again.
Elemental separation
Lu-Hf extraction was achieved by two cation-exchange resin methods, based on Münker et al. (2001) and Sprung et al. (2010) with some modifications. In order to avoid matrix effects and the Lu tail in the Hf cut, the three-stage separation scheme after Sprung et al. (2010) was applied for most samples. This procedure is listed in Table 2. Most of the matrix elements including Light Rare Earth Elements (LREE) were eluted sequentially with 2-3 M and 3M HCL The solution was collected in 50 mL Teflon® PFA beakers and used for further element separation for other isotope systems like Sm-Nd or Rb-Sr. The H REE fraction was then eluted with 6 M HCl and evaporated to dryness. After H REE elution, the column was rinsed with 6M HCL Subsequently, Ti was eluted using a mixture of 0.45 M HN03, 0.09 M citric acid (HCit), and 1 vol % of H202. Prior to Hf collection, Zr was extracted from the column with a mixture of 6 M HCl and 0.06 M HR Finally, Hf was collected with 12 mL of 2 M HF in 30 mL Teflon® p FA beakers and gently evaporated to dryness.
Table 2 Three-stage HREE-Hf separation scheme based on Sprung et al. (2010) using ion-exchange chromatography of 1-1.2 mL of Eichrom Ln-spec resin with 50-100 mesh; resin bed length: 3.8-4.0 cm.
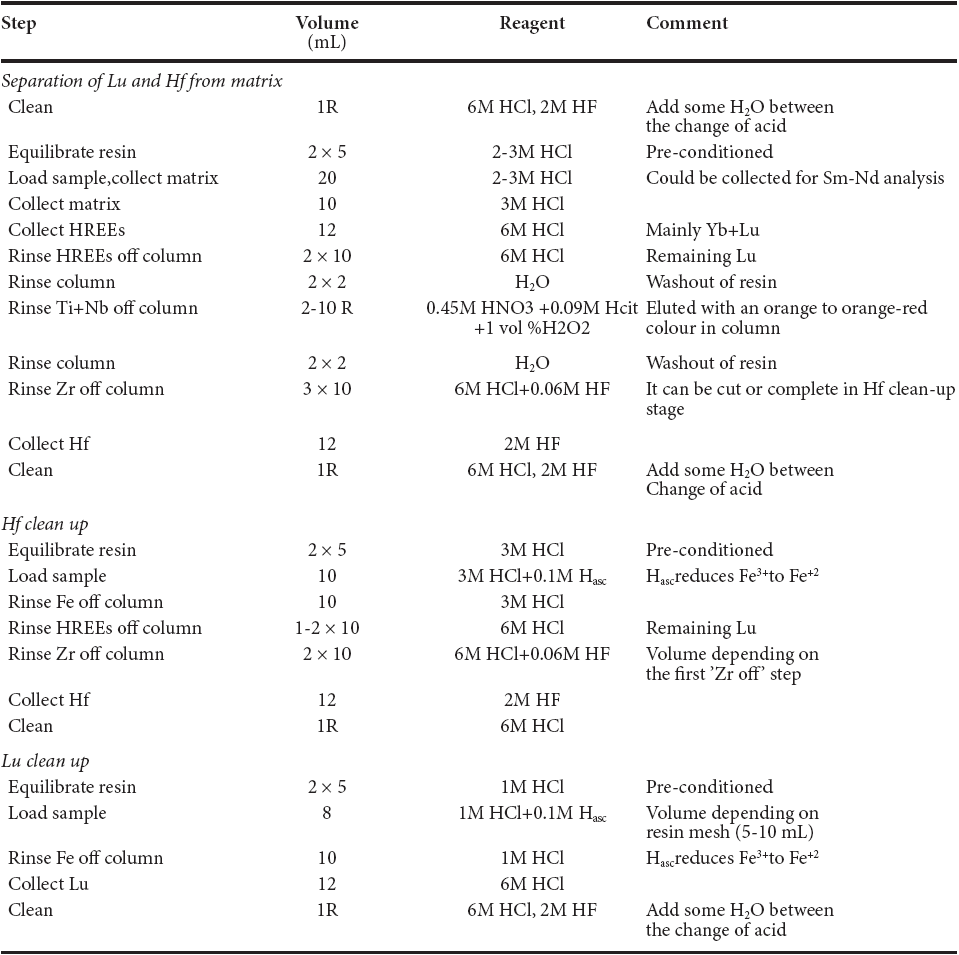
R= Reservoir; here refers to column volume. The organic acid and H2O2 reagent should be used fresh. The Ti+Nb separation step uses the acid volume necessary to achieve a colourless eluate and then another 5-10 additional mL.
Since Fe3+ was eluted together with Hf4+ in the first separation step, the Hf cut was loaded again to the previously cleaned column in a mixture of HCl and 0.1M ascorbic acid. Ascorbic acid reduces Fe3+ to Fe2+, which was then rapidly eluted in the subsequent step. (To avoid the ascorbic acid in the LREE cut for further Sm-Nd or Rb-Sr separation, the sample was not loaded with ascorbic acid in the first separation step). The Hf clean-up was completed essentially in the same way as the first separation procedure except for the Ti elution that was omitted. This procedure reduced significantly Lu+Yb interferences on the 176Hf signal. Finally the Lu cut was loaded with ascorbic acid to the precleaned columns to further reduce the Yb/Lu. It is important to note that this chemical separation method is time-consuming and the oxidation procedure (Ti elution step) can result in strong reactions that may lead to loss or cross-contamination of samples by ejecting material.
Mass spectrometry
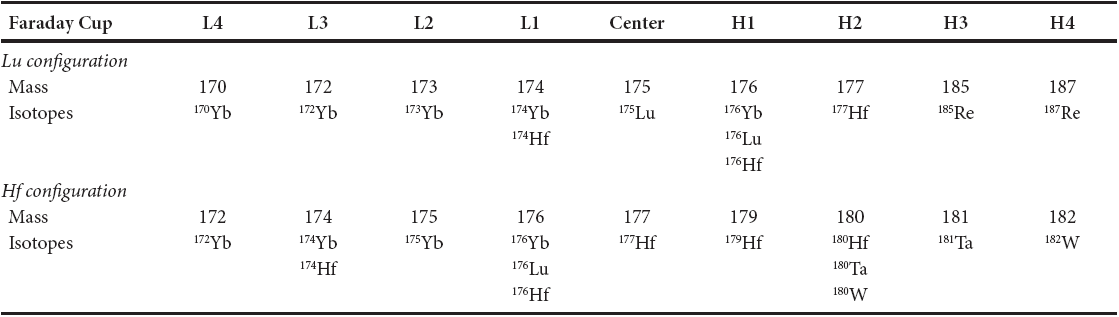
The determination of Lu and Hf isotope ratios was carried out on a Thermo Neptune Plus® MC-ICP-MS installed at the Centro de Geociencias, Universidad Nacional Autonoma de México, in Juriquilla, Querétaro. All masses were measured on Faraday cups in static mode. The cup configuration is summarized in Table 3 and the typical signals are depicted in Figure 1. The sample solutions were introduced to the plasma via an Aridus® desolvating sample introduction system using an Ar carrier gas and a blended Ar + N2 sweep gas. The beam intensities were then optimized by adjusting the torch position, gas flows, ion focusing, and magnet field settings. The Hf fraction was taken up with 1 mL of 0.56 M HNO3-0.24 M HF solution and the Lu fraction from 0.6 mL of 0.1 M HN03 solution.
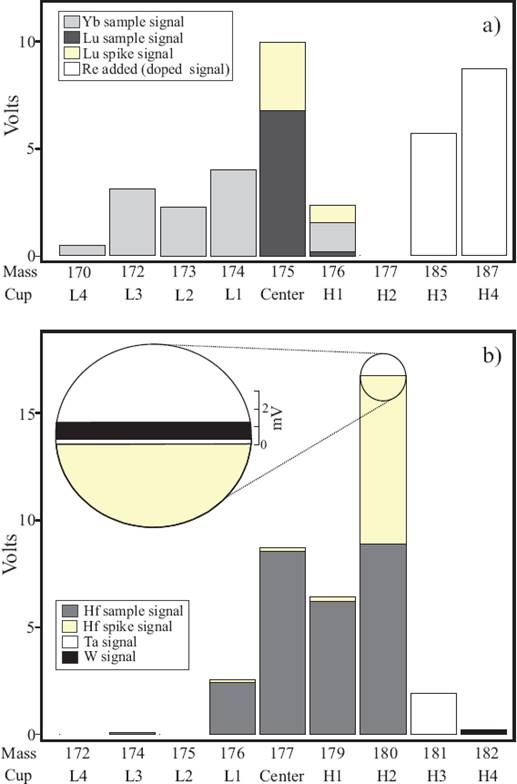
Figure 1 Bar chart illustrating the relative contributions of spike and sample components for typical MC-ICP-MS Lu-Hf analyses, (a) Typical signals for the Lu analysis and (b) typical signals for the Hf analysis. Notice the minimal 180Ta and 180W contribution on 180Hf.
The isotope concentrations of Hf and Lu in the sample solutions were estimated by measuring peak sizes of diluted sample fractions prior to isotope data acquisition to make sure that signals are not too high to saturate the Faraday cups. Samples were then adjusted to ~50 ppb for Hf and ~10 ppb for Lu isotope analyses. Washouts were pertormed with MQ Water and 0.45 M HNO3-HF trace for 120-240 seconds or until all signal intensities were below 5 X 10-4 V. Baselines were acquired measuring backgrounds at peak positions (on-peak-zeroes, OPZ) at least twice during each analytical session, using the same acid solution used for the washouts. The OPZ intensities were subtracted from the corresponding peaks when the baseline intensities were higher than 5 X 10-4 V.
Lu isotope analysis
For Lu isotope data acquisition one block of 40 cycles with 4 seconds integration time each was performed. The 177Hf intensity was measured to monitor for isobaric interference of 176Hf on the 176Lu signal. For the mass bias correction, each sample was doped with ~10 ppb of Re and the masses 185 and 187 were measured simultaneously on cups H3 and H4 (Table 3). Lu-Re (10 ppb Lu + 15 ppb Re) and/ or Yb-Lu-Re (10 ppb Yb + 10 ppb Lu +15 ppb Re) standard solutions were measured after every 3-5 unknowns to account for differences in instrumental mass bias between the elements.
Hf isotope analysis
For Hf isotope data acquisition 8 blocks with 10 cycles per block and an integration time of 4 seconds per cycle were measured. Isobaric interferences of 176Yb and 176Lu on the 176Hf signal were monitored by measuring 172Yb and 175Lu. Moreover, 181Ta and 182w were measured to monitor for isobaric interferences of 180Ta and 180w on the spiked isotope 180Hf (Table 3). The interference correction was calculated from the known natural ratio of the isotopes from the interfering element.
To examine the accuracy of Hf isotope measurement, a 50 ppb JMC 475 Hf standard solution was measured after every 4-5 unknowns. The average 176Hf/177Hf ratio of JMC 475 measured over the last three years during a total of six analytical sessions is 0.282149 ± 0.000025 (n = 41), which is slightly below but within errors of cited values (e.g., Blichert-Toft et al., 1997: 0.282163 ± 0.000009; Münker et al., 2001: 00.282151 ± 0.000013; Lapen et al., 2004: 0.282165 ± 0.000013; Vervoort et al., 2004: 0.282144 ± 0.000014, all errors are 2S.D). Therefore, all measured 176Hf/177Hf ratios were normalized to the well-accepted JMC 475 value of 0.282160. The results of the JMC 475 standard measurements are shown in Figure 2.
DATA REDUCTION
For offline reduction of the raw data extracted from the mass spectrometer, an R-based software package (CRAN) was written. The Hf isotope data reduction Toolkit for R (in short: IsotopeHf®) can be requested from the corresponding author. This contribution aims to introduce this package, with integrated functions for data corrections, calculation, and graphical output, focused on determining elemental concentrations and isotopie ratios for Lu and Hf, as well as the corresponding uncertainties. It is important to note that IsotopeHf® is a cross-platform program that can be run on any operating system with R environment installed (version 3.1.1 or later). For a proper functionality of IsotopeHf® other packages (CRANs) must be installed; namely "ggplot2" "plyr", and "dplyr" (Wickham, 2009; Wickham, 2011; Wickham and Francois, 2015). IsotopeHf® runs via the command-line interface. The user's guide is included in the Appendix of this paper (Electronic Supplementary File) and it can be displayed within the program. Advantages, disadvantages and known bugs of IsotopeHf® are listed in Table 4. In the following sections, the main equations used in IsotopeHf® to correct the raw data from the Lu and Hf data acquisition by MC-ICP-MS are explained.
Table 4 Avantages, disadvantages and known bugs of IsotopeHf®.
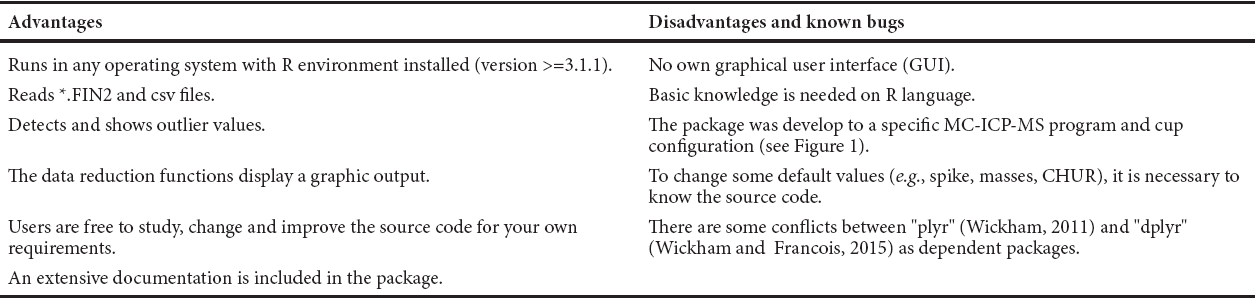
*output file from Thermo Neptune® MC-ICP-MS.
Lu isobaric interference correction (and Yb mass bias correction)
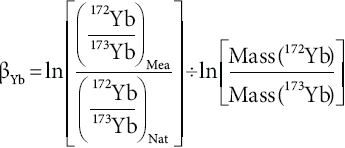
Isobaric interferences for 176Lu occur with 176Yb and 176Hf. After the three-stage elemental separation procedure employed in this study that includes a Lu clean-up procedure, the 176Hf interference is insignifìcant. However, Yb and Lu show similar adsorption behavior on Ln-Spec® resin and are difficult to separate from each other. Therefore, the contribution of 176Yb on the 176 mass peak has to be corrected, considering also the mass bias for Yb isotope ratios. In a first step for isobaric interference correction on 176Lu, the Yb mass bias factor βYb is calculated applying the exponential law (Russell et al., 1978) as follows:
Then, the interference corrected 176LuIC signal is calculated by subtracting the mass bias corrected 176Yb fraction from the measured 176 mass signal using the equation (Vervoort et al., 2004):
where the subscripts IС, Mea, and Nat are interference corrected, measured, and natural, respectively. To correct for mass bias of Yb and to calculate its isobaric contribution on 176Lu, natural Yb isotope compositions reported by Segal et al. (2003) were used (172Yb/173Yb = 1.35428 and 176Yb/173Yb = 0.79381). It is noteworthy that Vervoort et al. (2004) noted an over-correction of the 176Lu/175Lu values by subtracting the 176Yb interference from 176Lu using this protocol. To solve this, an empirical correction factor (typically 0.9996, Vervoort et al., 2004) can be used to adjust (173Yb/176Yb)Nat in Equation 2. Our mixed Lu-Yb-Re standard solution (with Yb/Lu = 1) yielded 176Lu/175Lu ranging from 0.02651 to 0.02655 (mean = 0.02653 ± 0.00002 2S.D., n=14). These values correspond within analytical uncertainties to the natural isotopie composition of Lu reported by several authors (Blichert-Toft et al., 1997; Chu et al., 2002; Kleinhanns et al., 2002; Vervoort et al., 2004, Figure За). Considering that the chemical separation method after Sprung et al (2010) decreases the Yb/Lu value to about 1 in the Lu cut and that 176Lu/175Lu remained constant on the Neptune® MC-ICP-MS over the whole period of analysis (three years), an additional correction factor as proposed by Vervoort et al (2004) was not necessary, at least in this study.
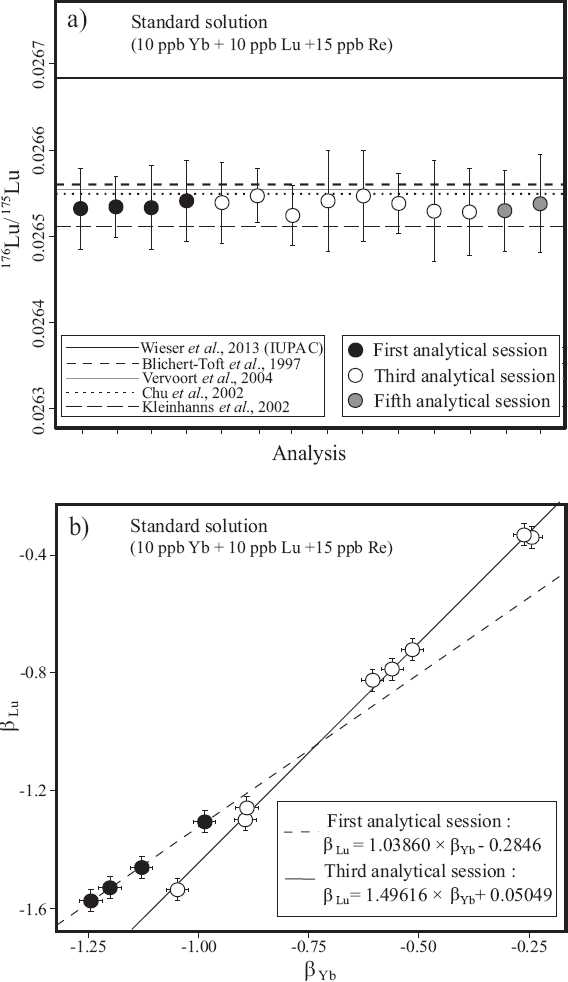
Figure 3 (a) Relationship between 176Lu/175Lu corrected for mass bias + Yb interference on mixed Yb+Lu+Re solution in some analytical sessions and true 176Lu/175Lu reported by Blichert-Toft et al. (1997); Chu et al. (2002); Kleinhanns et al. (2002); Vervoort et al. (2004) and Wieser et al. (2013). (b) Variations of mass bias between Yb and Lu during two analytical sessions. The mass-bias factors were characterized using Yb + Lu + Re solution. βYb was calculated by Equation 1. βLu was calculated by Re-Lu doping method.
Lu mass bias correction
Instrumental mass bias behavior of Lu cannot be determined by using the natural constant isotope ratio, because Lu has only two naturally occurring isotopes (175Lu and 176Lu), of which the 176Lu signal is altered by the spike isotope. The mass bias can be corrected by normalizing to an external standard of known isotopie composition that is simultaneously measured with the multi-collector system. For this purpose the samples were doped with admixed Re (see Figure 1a). The doping method has been successfully employed for mass-bias correction in many isotope systems, such as Cu isotopes with admixed Zn (Maréchal et al., 1999), Rb isotopes with admixed Zr (Nebel et al., 2005), and Lu isotopes with admixed W (Wimpenny et al. 2013).
Rhenium is ideal as doping agent for Lu measurements, since its isotope mass range is close to that of Lu and it contains no isobaric interferences. Rhenium doping was first intro duced by Scherer et al. (1999) and applied in several Lu-Hf studies (e.g., Münker et al., 2001; Scherer et al., 2001; Kleinhanns et al., 2002; Weber et al., 2010; Wimpenny et al., 2013). The method is based on the assumption that instrumental mass bias of Re and Lu in MC-ICP-MS does not vary independently in the same solution at the same time. Hence, this behavior can be used to cross-calibrate the mass bias factors (β). The relation between mass bias factors obtained with the exponential law can be calculated from the slope (m) of the linear array plotted in ln(187Re/185Re) vs. ln(176Lu/175Lu) (Figure 4):
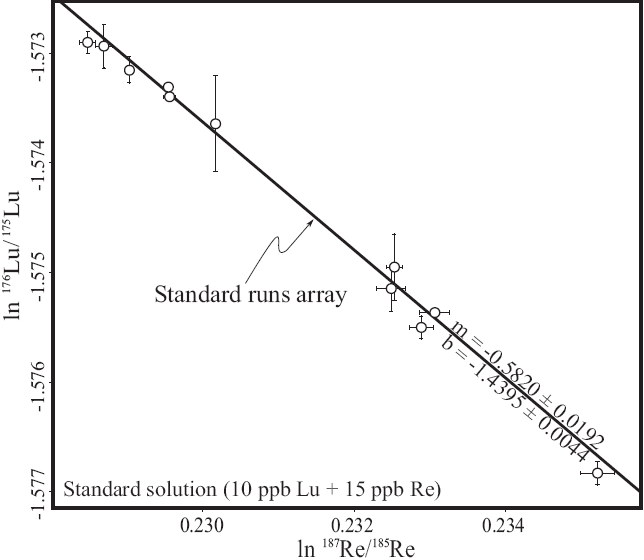
Figure 4 Re vs. Lu log-log plot showing the slope (m) obtained from the external isotopie standard (Lu + Re solution) during a typical Lu working session. This slope is used to correct for Lu sample + Re standard mixtures. The two mass bias factors (βRe and βLu) are proportional throughout the working session. The errors bars represent 2S.D.
The regression line with the slope (m) is calculated from standard solutions containing both Re and natural Lu, which were measured during a working session after every 3-5 unknowns. The property oí the linear array still holds in the unknown sample solutions, inasmuch as their two mass bias factors (βRe and βLu) are proportional throughout the working session. We first calculated βRe in the sample:
Then, βLu is calcdated from the βRe [Equation 4, (187Re/185Re)Nat =1.67398 after Gramlich et al., 1973] and from the slope (m) of the linear array of the log-log plot.
Finally, the mass bias corrected 176Lu/175Lu is calculated by using the interference corrected 176Lu and βLu.
where the subscripts MB and IС are mass bias corrected and interference corrected, respectively.
The Re doping method is an approach that deals with the problem that Lu and Yb do not fractionate equally. Besides that the differences between the mass bias factors may vary from one instrument to another, from one analytical session to another, and also during a long analytical session (>16h). Variations in the Yb and Lu mass bias factors (β) during two different analytical sessions are depicted in Figure 3b.
Hf mass bias correction and correction for spike
Correction of 176Hf/177Hf for mass bias in un spike d samples is usually achieved by normalizing to the accepted natural 179Hf/177Hf of 0.7325. Due to alteration of the natural isotope ratios by the admixed isotope tracer that is never 100% pure spike isotope, correction with natural 179Hf/177Hf is inaccurate. Thus the "True" 179Hf/177Hf for the mixed solution needs to be calculated. Besides that, for the interference correction of 176Lu on 176Hfby using the 175Lu monitor, the measured and corrected 175Lu/176Lu has to be used, since this isotope ratio is also altered by the spike. This can introduce an additional systematic error on the resulting 176Hf/177Hf if a significant Lu-tail is present in the Hf cut.
Several approaches have been suggested to correct the 176Hf/177Hf in spiked samples. Lapen et al. (2004) used closed-form equations, modified from the double-spike approach that simultaneously provides a solution for the spike and mass-bias corrections on 176Hf/177Hf. However, Lapen et al. (2004) used a spike enriched in 178Hf, and they used the 180Hf/177Hf to test the reliability of the correction method. Such an approach will need long-term comparison to external reproducibility of both un spiked and spiked samples. On the other hand, Lu et al. (2007) reported an iterative solution for a simultaneous determination of the Hf concentration and 176Hf/177Hf ratio using a spike enriched in 179Hf, yielding identical analytical results in both spiked and unspiked samples of geochemical reference standards. The Hf concentration is derived from the spike-to-sample molar ratio, Sprung et al. (2010) preferred a 179Hf/177Hf-normalizedprocedure derived from considerations of Maréchal et al. (1999), by using the linear trend of the measured Hf standard solutions to cross-calibrate the mass bias factors (β) of the samples.
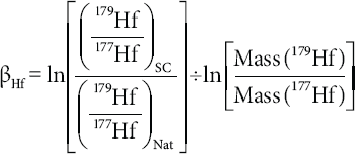
For the IsotopeHf® software an empirical method based on the equations of Boelrijk (1968), Qiao (1988), and Gopalan (2002), and developed by Chu et al. (2011) was applied, first obtaining the spike contribution on 179Hf/177Hf, then using the spike-corrected 179Hf/177Hf to calculate the mass bias factor (βHf), and finally correcting the effects of both spike and mass bias on the 176Hf/177Hf.
In a first step, the interference corrections of 180Ta and 180w on the 180Hf signal (close-up in Figure 1b) are calculated:
Since the mass bias cannot be corrected without considering the spike, the contribution of the spike in the sample (D = 177NSp/177NNat) is calculated:
where 177Ν is the number of moles of 177Hf, and the subscripts Nat, sp, 1С, and MB are for natural, spike, interference corrected, and mass bias corrected, respectively. Although the spike used in this study is artificially enriched in the 180Hf isotope to more than 98%, the spike contribution on the 179Hf/177Hf and 176Hf/177Hf is significant and needs to be corrected using the following equations:
where the subscripts SC and Mea are spike corrected and measured, respectively. Herewith, the mass bias factor (βHf) is obtained from the 179Hf/177Hf without the contribution of the spike after
to finally correct the 176Hf/177Hf and 180Hf/177Hf for mass bias.
A test for the correct spike subtraction is obtained by comparing the mass bias and spike corrected 179Hf/177Hf relative to the accepted natural value (0.7325).
RESULTS AND DISCUSSION
The corrected Lu-Hf isotope ratios and elemental compositions for international reference rock standards, unknown whole-rock, and mineral samples are listed in Table 5. Deviations of the obtained data from the recommended values for reference standard (from Table 1) as well as differences between the Parr® and the DAS® digestion methods and replicate analyses for both reference standard and unknown rock samples are listed.
Table 5 Lu-Hf isotope and concentration data of analyzed samples. Comparisons between replicate analysis and between this study and reference values
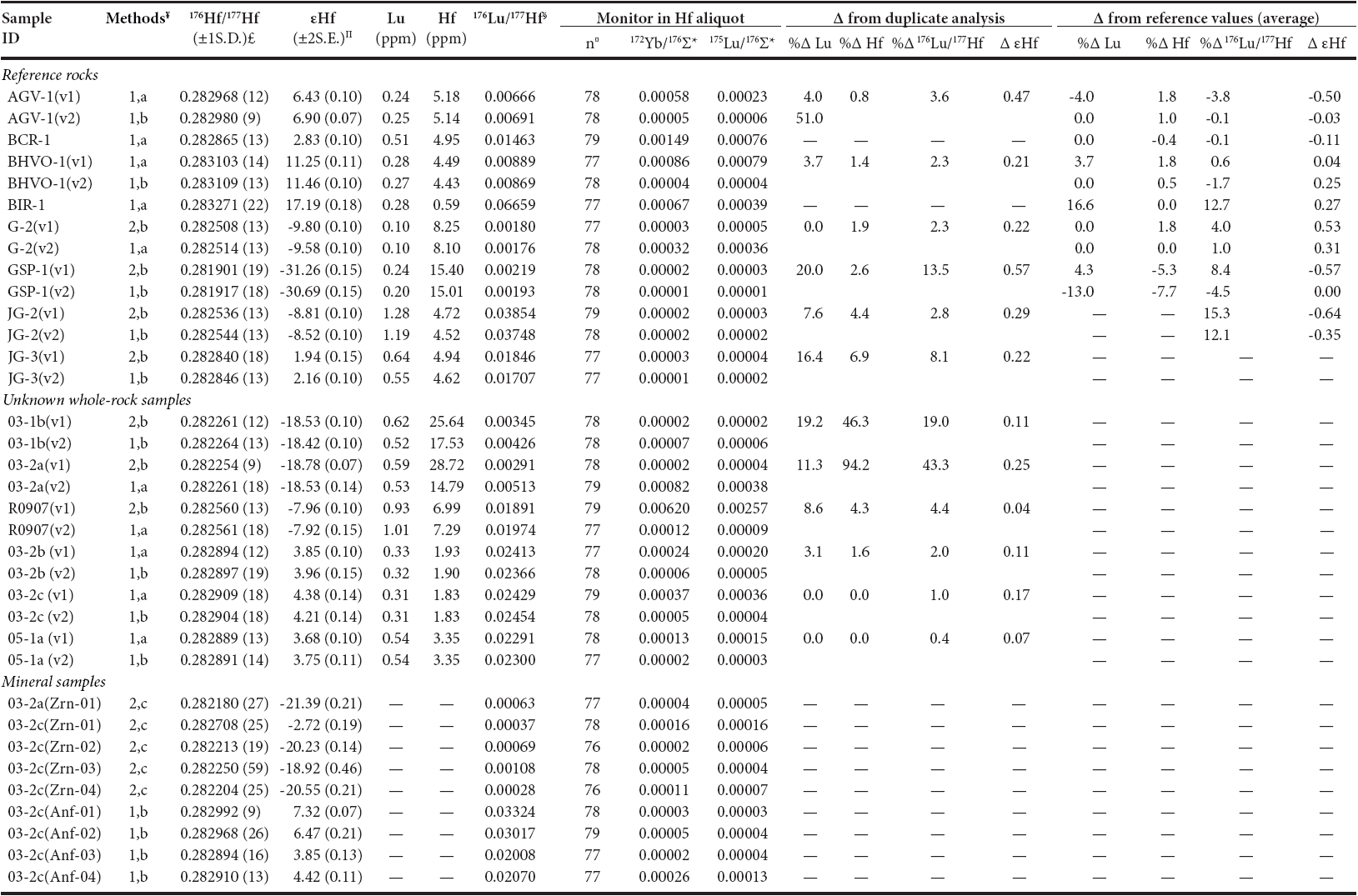
¥Digestion method: 1=DAS® system, 2=Parr® bomb. Separation method: a = Münker et al. (2001), b = Sprung et al. (2010), c = Nebel-Jacobsen et al. (2005).
£Errors refer to the last digit(s) and are one standard deviations. Π Errors refer to the last digits and are 2S.E. = 2σ/√n. §The 176Lu/177Hf errors are typically < 0.2% (2σ).
¤Number of measured isotope ratios after outlier rejection. *Isotope ratios measured in Hf aliquot.176Σ= 176Yb + 176Lu + 176Hf
The εHf values were calculated following CHUR 176Hf/177Hf value = 0.282785 (Bouvier et al., 2008).
The precision of the analytical procedure and reduction algorithms used by IsotopeHf® for mafic rocks (n=5, unknowns and standards), is illustrated in Figure 5 comparing the results from duplicate analysis using DAS® pressure digestion system. The duplicates show good agreement, yielding correlation coefficients (R2) of 0.9970 for 176Hf/177Hf (Figure 5a) and 0.9992 for Lu/Hf, respectively (Figure 5b).
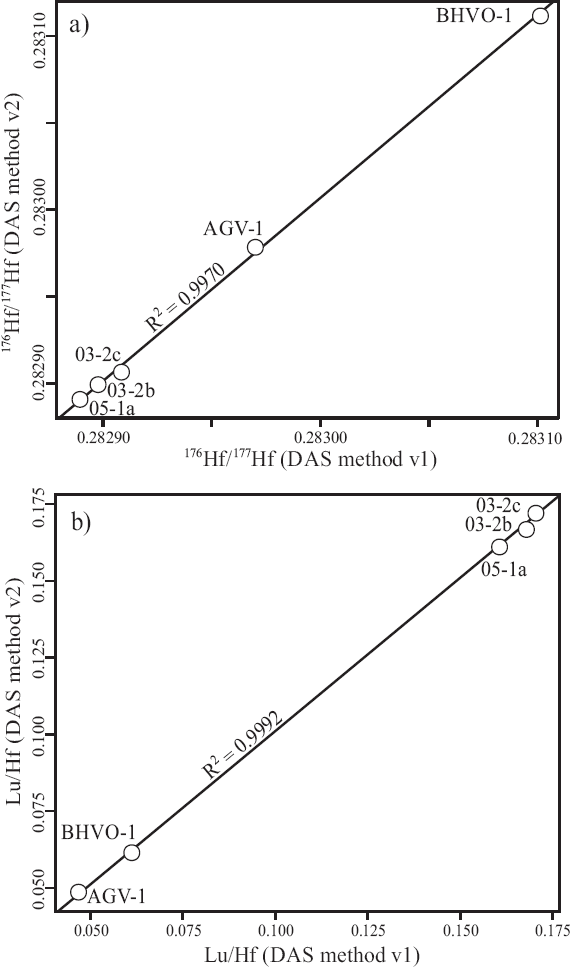
Figure 5 Comparison between results of (a) 176Hf/177Hf and (b) Lu/Hf on duplicate analyses of mafic rocks using DAS® system.
Validation of the separation technique
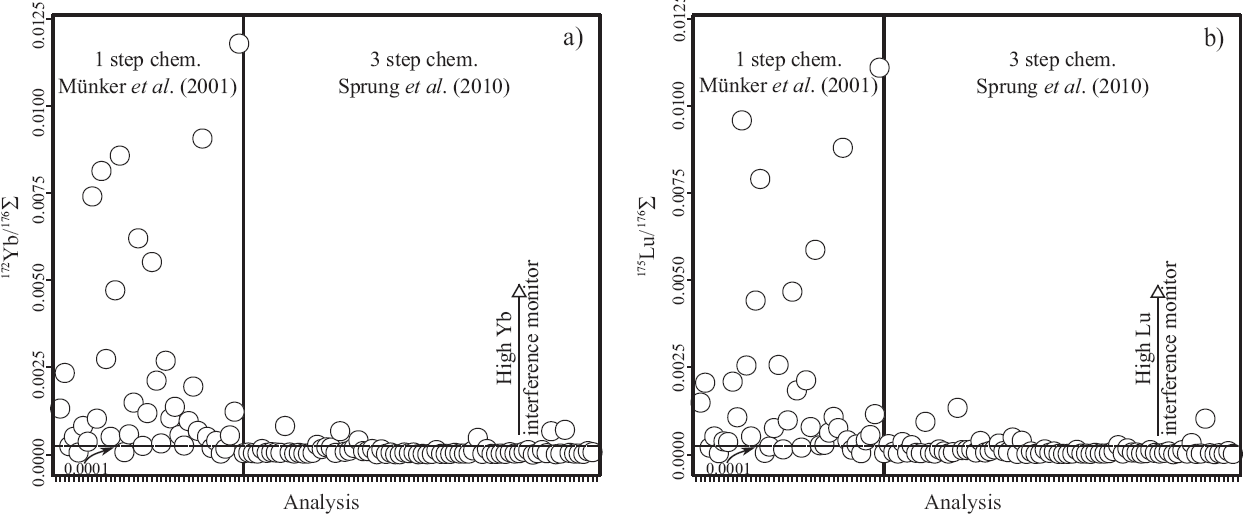
The quality of Hf separation from Yb and Lu is indicated by 172Yb/176Σ and 175Lu/176Σ values below 0.0001 (Bast et al., 2015), which was the case for all whole-rock samples separated with the three-stage separation scheme that included an additional Hf clean-up (after Sprung et al., 2010, Figure 6). Samples separated with the single column procedure (Münker et al., 2001) occasionally show significant Yb and Lu-tails in the Hf cuts (172Yb/176Σ up to 0.0118 and 175Lu/176Σ up to 0.0113, Figure 6). The necessary correction for 176Lu increases significantly the error on 176Hf/177Hf, especially because the measured 175Lu/176Lu for spiked Lu and its corresponding error has to be considered for the correction. However, after running data reduction with IsotopeHf® there is no significant difference on the resulting εHf values, neither for the reference standard with the highest 175Lu/176Σ of 0.00079 (BHVO-l(v1)) ΔεΗf = 0.04, Table 5), indicating that interference corree-tion including the spike works properly with IsotopeHf®.
Accuracy and reproducibility of Hf-isotope ratios
The corrected 176Hf/177Hf values of international reference rock standards calculated with IsotopeHf® are accurate within analytical uncertainties with most published data (Figure 7). All standard basalts analyzed in this work (Figure 7a, 7b and 7d) yield 176Hf/177Hf values that deviate from the average reported values by less than 0.00001, corresponding to less that 0.3 εHf-units (BCR-1 = -0.11, BHVO-1 = from 0.04 to 0.25, BIR-1 = 0.27 εHf-units). One analysis of the standard andésite AGV-1 yielded a 176Hf/177Hf that is slightly lower (by -0.50 εΗΓ-units) than the average but still within analytical errors of most reported values (Figure 7c).
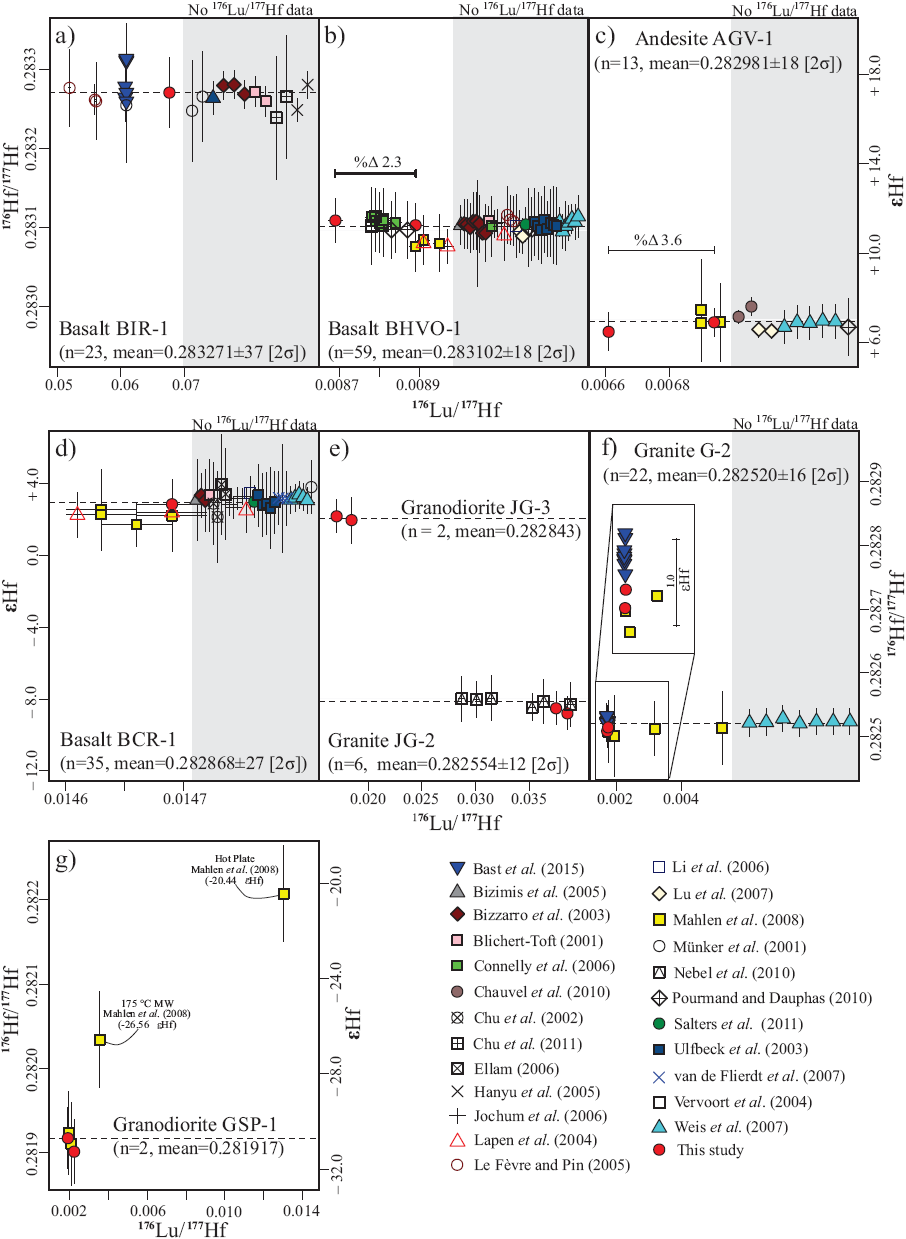
Figure 7 176Lu/177Hf and 176Hf/177Hfdata of (a) BIR-1} (b) BHVO-1, (c) AGV-1, (d) BCR-1, (e) JG-2 and JG-3, (f) G-2, and (g) GSP-1 international reference rock standards obtained in this study together with available data from literature (Table 1). Dashed lines represent the mean for compiled 176Hf/177Hf data, except for Granodiorite JG-3. The error bars are 2σ S.D.
Granitic reference rock standards are much more problematic, because 176Hf/177Hf values of the G-2, GSP-1, and JG-2 standards are poorly reported in literature and because the reported data are highly disperse (Mahlen et al., 2008; Bast et al., 2015, Figure 7e-7g). However, the corrected 176Hf/177Hf values of standard granitoids G-2, GSP-1, and JG-2 analyzed here do not differ from the reported values by more than 0.64 εHf-units. It is noteworthy that we report the first Lu-Hf isotope data for granodiorite standard JG-3.
The external reproducibility for 176Hf/177Hf of all analyzed samples ranges between 0.00 and 0.57 εHf-units but only two out of twelve duplicate analyses differ by more than 0.3 εHf-units: (1) Granodiorite standard GSP-1 (Δ = -0.57 εHf-units), which was digested once in Parr® bomb and once in DAS®, suggests inhomogeneity or somewhat incomplete digestion of an inherited zircon component (with lower εΗΓ) in DAS® yielding a slightly higher 176Hf/177Hf by 0.000012 and (2) Andésite standard AGV-1 (Δ = 0.47 εHf-units), which was separated once by the three-stage separation scheme and once by single column separation.
Accuracy and reproducibility of 176Lu/177Hf by ID
Accuracy and reproducibility of 176Lu/176Hf by isotope dilution analysis (ID) in whole rock samples depends on (1) the precise knowledge of isotope compositions and concentrations of spike isotopes in the mixed spike, (2) equilibration between sample and spike, (3) adequate amount of spike for given Lu and Hf concentrations to avoid error amplification, and (4) in complete dissolution of all phases, in particular Hf-rich phases like zircon. Since all of these sources of error may worsen the overall accuracy and reproducibility and because not all laboratories do ID analyses, there are only few 176Lu/177Hf data published from the studied international reference rock standards to compare with. The published data are mostly from reference basalts (BCR, BHVO and BIR) and andésite AGV-1 (Münker et al., 2001; Vervoort et al., 2004; Lapen et al., 2004; Le Fèvre and Pin, 2005; Connelly étal, 2006; Mahlen et al., 2008; Pourmand and Dauphas, 2010; Bast et al., 2015). Only three publications present data from granitoid standards (Mahlen et al., 2008; Nebel et al., 2010; Bast et al., 2015).
The best accuracy with respect to the reported 176Lu/177Hf values from mafic standards was obtained from basalt BCR-1 and from one analyses of andesite standard AGV-1, yielding deviations from the reported average values of 0.1% (Figure 7c and 7d). A second analysis of AGV-1 yielded a 3.8% lower 176Lu/177Hf, mainly due to a 4% lower Lu-content, compared to the reference values (Figure 7c).
The 176Lu/177Hf from two analysis of BHVO-1 differ by 2.3% but the values are between -1.7 and 0.6% compared with the average of 20 reported values from literature (Table 5, Figure 7b). Basalt standard BIR-1 yielded a 176Lu/177Hf of 0.06659, which IS 16.6% higher compared with the reported values (from 0.05184 to 0.06085, n = 11: Figure 7a). However, the Hf concentration calculated from our analysis is in agreement with the average value reported in literature (Table 5), suggesting that incomplete digestion was not an issue. Therefore, the difference is either due to poor sample-spike equilibration or inhomogeneity of BIR-1 with respect to Lu/Hf.
The 176Lu/177Hf values of granitic standard rocks differ from the most reliable reported values from 1.0 to 4.0% (G-2), -4.5 to 8.4% (GSP-1) and 12.1 to 15.3% (JG-2), respectively (Figure 7e-7g). Nevertheless, the large differences in the granite JG-2 and granodiorite GSP-1 may be due to the high dispersion of JG-2 data reported by Nebel et al. (2010) and thepoorly reported GSP-1 data in literature (Figure 7e, 7g). The differences between duplicate analyses in granitoids standards vary from 2.3 (G-2) to 13.5% (GSP-1). It is noteworthy that for granitic whole-rock samples incomplete dissolution of refractory phases like zircon is the most problematic issue for 176Lu/177Hf reproducibility. Similar results were obtained for our replicate analyses from selected unknown samples. Whereas 176Lu/177Hf values from metabasite samples vary by acceptable 0.4 to 2.0%, Grenvillian orthogneisses differs by up to 43%, depending on the digestion method used (Table 5).
Although most 176Hf/177Hf and εΗf values of the international refer-enee rocks analyzed in this work agree, within analytical errors, with the data reported from literature, discrepancies in the 176Lu/177Hf values affect the recalculated initial 176Hf/177Hfi and εΗfi values of ancient rock samples more severely. The effect on the initial values depends not only on the random and systematic errors introduced by instrumental counting statistics, mass-bias, and spike-stripping corrections, but also on the absolute 176Lu/177Hf and the error in age (which is not considered here). For example, a ±19.0% difference (between two replicate digestions) in the 176Lu/177Hf value of a Grenvillian orthogneiss (03-lb, 176Lu/177Hf = 0.00345 and 0.00426, Table 5) produces only ±0.43 εΗf units difference in the initial value by recalculating to 1.0 Ga (Figure 8a). By assuming the same initial age and difference in 176Lu/177Hf for a metabasite (03-2c(v2), 176Lu/177Hf = 0.02454, Table 5) wodd change the εHf1Ga by ±3.00 (Figure 8b). Hence, it is rather the absolute error of the 176Lu/177Hf values that counts for the time-integrated error in εΗfi and at a minor extent the percent error.
Comparison of sample digestion techniques
Mahlen et al (2008) tested the effects of four different digestion methods (tabletop hotplate, low-temperature microwave (175°C), high-temperature microwave (200°C), and Parr® bomb) for Lu-Hf isotope analysis of whole-rock samples. The authors concluded that any of the digestion methods might produce reliable 176Hf/177Hf values in mafic rocks. However, for felsic rocks incomplete digestion occurs with the tabletop hotplate and the low-temperature microwave methods that might affect not only 176Lu/177Hf but also the 176Hf/177Hf values.
In this work, the Picotrace DAS® digestion system is compared with the Parr® bomb digestion vessels. As mentioned above, there are only minor or insignificant differences in 176Hf/177Hf data between the two digestion system methods, but there are important differences in the 176Lu/177Hf values, particularly in some felsic rocks. Whereas lower 176Lu/177Hf values with the Picotrace DAS® digestion system compared with Parr® bomb digestion cannot be explained by incomplete dissolution but rather by poor sample-spike equilibration (mainly GSP-1 and JG-3), 19.0% and 43.3% higher 176Lu/177Hf values in orthogneiss samples performed with the Picotrace DAS® digestion system (03-lb and03-2a) are most likely the result of incomplete dissolution of zircon. In such cases, assuming that complete sample-spike equilibration was achieved, the variations in 176Lu/177Hf and 176Hf/177Hf should correlate, and the data should plot along a regression line whose slope corresponds to the time of crystallization of the sample (Blichert-Toft et al., 2004; Lapen et al., 2004; Mahlen et al., 2008). If this is true - and the age of the rock is well-known - then incomplete dissolution of the whole-rock sample does not have any effect on the calculated initial 176Hf/177Hf. On the other hand, if poor sample-spike equilibration or sample in-homogeneity is the case, then the data plot off the supposed reference isochron in a 176Lu/177Hf vs. 176Hf/177Hf plot. The different effects can be illustrated in 176Lu/177Hf vs. 176Hf/177Hf plots for samples with known age by including the corresponding reference isochrones (Figure 9).
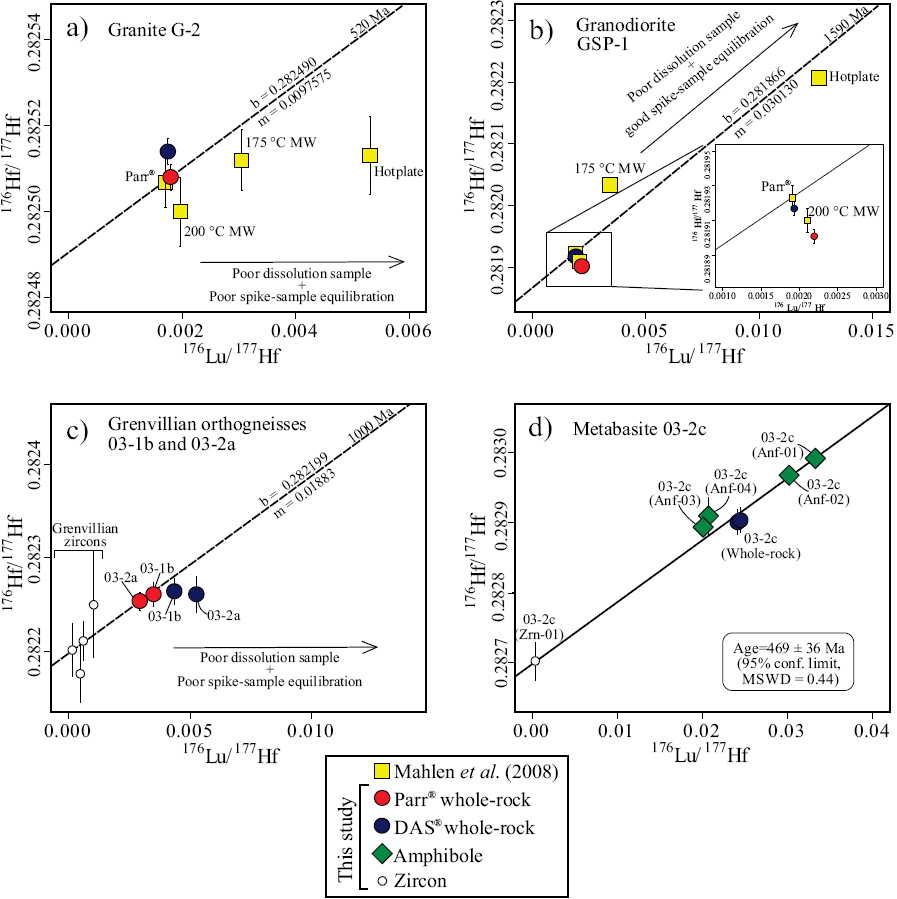
Figure 9 176Lu/177Hf vs. 176Hf/177Hf isochron diagrams for (a,b) zircon-bearing international reference rocks standards (G-2 and GSP-1), (c) orthogneiss samples (03-lb and 03-2a), and (d) metabasite sample (03-2c). Reference isochrons of the standard granitoids correspond to their reported ages, which were plotted through the average from data points of Parr® bomb digestion. The slope of the reference isochron for orthogneiss samples corresponds to their 1.0 Ga U-Pb zircon age reported by González-Guzmán et al. (2014)) and is plotted through the zircon data point. Discrepancies introduced by incomplete sample dissolution or incomplete spike-sample equilibration are illustrated by the deviations from the calculated reference isochrones. Note that the largest deviations are associated with samples processed using tabletop hotplate dissolution (~120°C, Mahlen et al., 2008). The five-point isochron for the amphibolite 03-2c (d) is calculated from two whole rock aliquots, two magnetic hornblende (Anf-01 and Anf-02) separates, and one zircon. Note that secondary low-magnetic amphiboles (Anf-03 and Anf-04) plot off the isochrones, indicating later-stage alteration (error bars are 1σ S.D.)
The results from granitoids standards G-2 and GSP-1 (Figure 9a, 9b) are compared with the data published by Mahlen et al. (2008). The results from Parr® and DAS® digestion (this work) are similar to Parr® digestion by Mahlen et al. (2008). Slightly differing results, however, do not plot on the reference isochrones, indicating sample inhomogeneity and/or poor equilibration with spike instead of incomplete dissolution, as observed in the hot plate digestion and the low-temperature microwave methods (Figure 9a, 9b; Mahlen et al., 2008). On the other hand, replicate analyses of garnet-bearing sample R0907 show a slight but significant difference in the 176Lu/177Hf value (4.4 %). However, this discrepancy is most likely the result of sample inhomogeneity and not incomplete sample digestions, since higher Lu and Hf concentrations were obtained by DAS® (Table 5).
Inasmuch as zircon has high Hf and low Lu concentrations typically of about 1% Hf and Lu in the 100 ppm-range, measured Hf isotope ratios of zircon are close to their initial values and therefore insensitive to uncertainties in 176Lu/177Hf. On a 176Lu/177Hf vs.176Hf/177Hfplot (Figure 9c) the results of whole-rock analysis from Grenvillian orthogneisses (samples 03-lb and 03-2a) digested with Parr® together with the results from four Parr® digested zircon grains, plot within errors on a 1.0 Ga reference isochron that corresponds to the approximate U-Pb zircon age of the rocks (Manjarrez-Juárez, 2013; González-Guzmán et al., 2014). The data from the same whole-rock samples digested with DAS® do not plot on this reference isochron (Figure 9c). The offset of DAS® digested runs suggests incomplete sample dissolution. The observed differences between DAS® and Parr® bomb digestion is due to large differences in the Hf concentrations determined by ID between DAS® digestion (17.5 and 14.8 ppm Hf) and Parr® bomb digestion that yielded 25.6 and 28.7 ppm Hf, for sample 03- lb and sample 03-2a, respectively. Hence, samples with relatively high Hf contents that implies also high zircon content are most vulnerable to incomplete digestion if temperatures are below 190°C typically used in Parr® bombs.
For the relatively zircon-poor metabasite samples, incomplete sample digestion is not observed and whole-rock analyses are reproducible by using DAS® with differences in 176Lu/177Hf of 2.0% or less and ΔεHft between replicate analysis at 450 Ma (regional high-grade event, González-Guzmán et al., 2014) of 0.11 (sample 03-2b), 0.17 (sample 03-2c) and 0.07 (sample 05-la) εHf-units. From the sample 03-2c, in addition to duplicate analysis of the whole-rock powder, a single zircon grain (Parr® bomb digestion) and four amphibole concentrates (two primагу an d two secondary) were performed with DAS® digestion (Table 5). The primary amphiboles (Anf-01 and Anf-02), yielding 176Lu/177Hi greater than the whole-rock, plot on a regression line together with the whole-rock and zircon in a 176Lu/177Hf vs. 176Hf/177Hf diagram (Figure 9d). The slope of this regression line corresponds to an age of 469 ± 36 Ma (95% conf. limit, MSWD = 0.44), which is within errors identical to the time of high-grade metamorphism and anatexis in the area (González-Guzmán et al., 2014). The secondary amphiboles (Anf-03 and Anf-04) with 176Lu/177Hf lower than whole-rock, plot above the isochron confirming their secondary origin. The results demonstrate that besides complete sample dissolution with DAS® digestion, both spike-sample equilibration and accurate spike-subtraction was attained.
CONCLUSIONS AND RECOMMENDATIONS
The three-stage chemical separation technique of Lu and Hf from whole-rock samples applied in this study (modified from Sprung et al., 2010) yielded significantly better results compared to the singlestage separation technique after Münker et al. (2001), with virtually no isobaric interferences of 176Lu and 176Yb on the 176Hf signal. The 176Hf/177Hf values calculated with IsotopeHf® data reduction that in-eludes spike and mass bias corrections yields reliable results. Accuracy and reproducibility of 176Lu/177Hf by ID depend on the digestion method, sample-spike equilibrium, and homogeneity of the sample aliquot. Sample digestion with the Picotrace DAS® pressure digestion system at 165°c is reproducible and comparable with digestion in Parr® bombs at 190°C for mafic rocks and felsic rocks with moderate Hf content (<15 ppm). For Hf-rich rocks that incorporate most of their Hf in zircon complete sample dissolution cannot be achieved with the DAS® at 165°C, producing large errors on 176Lu/177Hf values and consequently erroneous initial Hf isotope ratios for ancient rock samples. For zircon-rich whole-rock samples the Parr® bomb digestion method at 190°C for five days is recommended or the DAS® pressure digestion system should be improved by reducing temperature loss to achieve significantly higher internal temperature.











 nueva página del texto (beta)
nueva página del texto (beta)