INTRODUCTION
Absolutely dated vertebrate fossils sites that provide late Pleistocene paleoenvironmental information for northern Mexico are rare, and their distribution is highly fragmentary (Metcalfe et al., 2000; Arroyo-Cabrales et al., 2010; Ferrusquía-Villafranca et al., 2010). The majority of such sites are located in the central and southern parts of the country and the resulting paleoenvironmental information displays a similar geographic bias (Ferrusquía-Villafranca et al., 2010). Late Pleistocene fossil sites from Sonora only comprise about 2% of all known Mexican localities (Arroyo-Cabrales et al., 2002; Ferrusquía-Villafranca et al., 2010). The Térapa fossil vertebrate site, located in northcentral Sonora, Mexico, near the towns of San Clemente de Térapa and El Llano (hereafter Térapa; Figure 1), contains an exceptional and extensive array of Late Pleistocene aquatic and terrestrial faunal remains (Mead et al., 2006, 2007; Hodnett et al., 2009; Steadman and Mead, 2010; Oswald and Steadman, 2011; Czaplewski et al., 2015) preserved in an 11 m thick deposit of marsh and fluvial sediments that has been absolutely dated to 40-43 ka, or to mid Oxygen Isotope Stage (OIS) 3 (Bright et al, 2010). Notably, the Térapa vertebrate fauna is a non-analogue fauna; several of the fossil vertebrates are from more temperate animals (e.g., mammoth, bison) while others are extinct or have tropical affinities and are no longer found in the area today (e.g., crocodile, capybara, glyptodont; Mead et al., 2006; Ceballos et al., 2010).
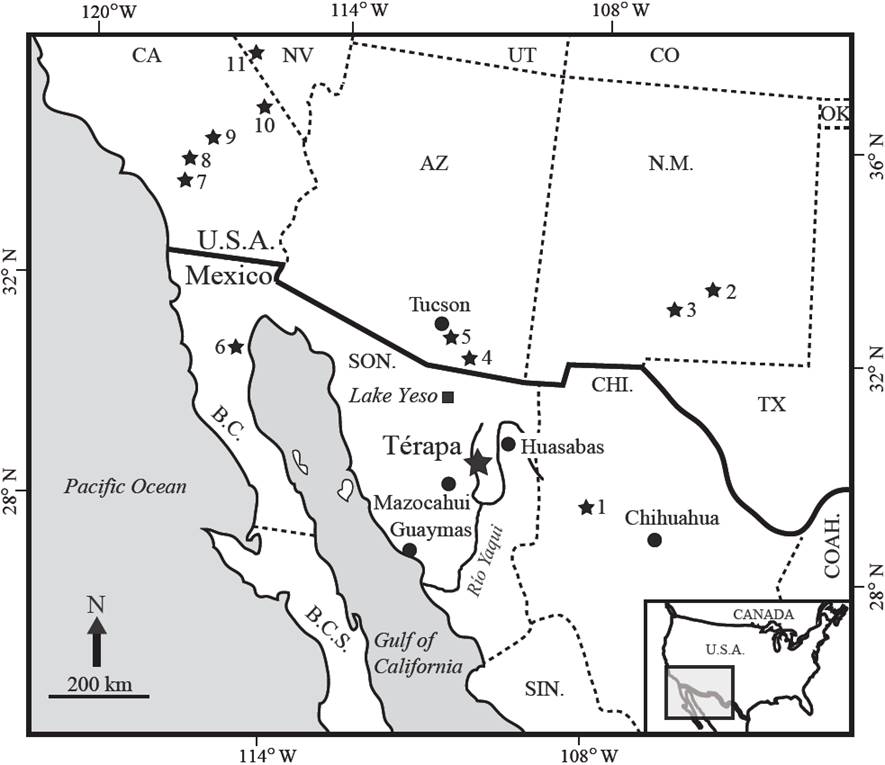
Figure 1 Illustration showing the location of lerapa, Sonora, Mexico (large black star) in relation to Lake Yeso (black square), to the southwestern U.S., and to northwestern Mexico. Small black stars are locations of climate records that extend to OIS 3. 1- Laguna Babicora; 2- Fort Stanton; 3- Lake Otero; 4- Murray springs; 5- Cave of the Bells; 6- San Felipe; 7- Diamond Valley; 8- Baldwin Lake; 9- Harper Lake and Lake Manix, 10- Valley Wells; 11- Devils Hole. Figure modified from Bright et al. (2010).
A preliminary ostracode faunal assemblage for Térapa was reported in Mead et al. (2006). This study expands on those results by reporting several additional ostracode species, and reconstructs in greater detail the paleoenvironment at Térapa through the use of the ostracode assemblage and stable isotope (δ18O and δ13С) analysis of ostracode valve calcite. The ostracode δ18O values provide insight into the seasonality of peak surface discharge, assuming that the water temperature at the time of calcification can be reasonably estimated. The ostracode δ13C values can provide insight into the vegetation community growing around the site because respired C02 from plant roots contributed to the dissolved inorganic pool of the streams and wetland that deposited the Térapa sediments. In 2010, Nunez et al. published a paleoenvironmental interpretation for Térapa that was based on δ18O and δ13C values from herbivore teeth recovered from throughout the same deposit. The enamel δ18O values were very high and the enamel δ13C values suggested that a strong c4 plant comm unity was present. Our ostracode δ18O and δ13C data provide an excellent opportunity to compare paleoenvironmental interpretations for a single deposit that are derived from two very different and comm only used archives - aquatic ostracode calcite and terrestrial tooth enamel. To our knowledge, this is the first time such a comparison has been attempted. We demonstrate that seasonality of precipitation and ecologie variables must be considered when reconstructing paleohydrology from different stable isotopie archives, especially in environments that experience marked differences in the seasonality of precipitation and vegetation cover. Our results provide an important, albeit brief, absolutely dated record of paleoenvironmental conditions in northeastern Sonora at a time when biotic communities and their associated environmental gradients were considerably different than today.
SITE DESCRIPTION AND BACKGROUND
The town of Térapa (29°41’ N; 109°39’ W; Figure 1) is located in the Moctezuma River valley, in the northwestern portion of the Sierra Madre Occidental, at an elevation of 605 meters above sea level (m a.s.l..). The vegetation community at Térapa is classified as foothills thorn-scrub (Van Devender et al., 1997; Martin et al., 1998). Oak and oak-pine woodlands dominate at higher elevations (> 1,400 m a.s.l.) and the vegetation below about 600 m a.s.l.. is classified as Sonoran Desert (Búrquez et al., 1992; Orvis, 1998).
The Moctezuma Valley is located between the op о sura Mountains (max. elevation∼1,800) to the west and the Sierra La Madera (max. elevation∼2,300 m a.s.l.) and another lower (max. elevation∼1,800 m a.s.l.) unnamed range to the east (Figure 2). The local geology consists of Lower Cretaceous fossilifero us limestone, granites, Eocene to Mio cene-aged ignimbrites, Miocene and Pliocene-aged basin fill, and Quaternary-aged basalts of the Moctezuma Volcanic Field (Paz-Moreno et al., 2003).
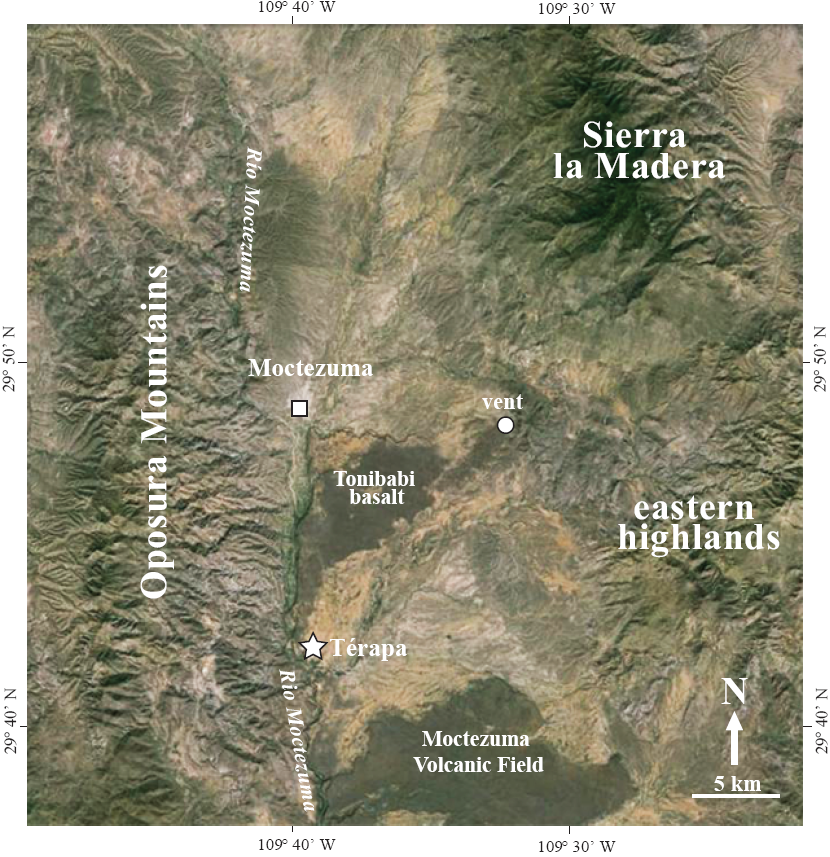
Figure 2 Satellite image of Térapa, Sonora, Mexico (white star) and its location in relation to the town of Moctezuma (white square), the Río Moctezuma, the Tonibabi lava flow and its vent (white circle), the Moctezuma Volcanic Field, and the surrounding highlands. Image modified from Google Earth.
The mean annual temperature (MAT) and mean annual precipitation (MAP) at Térapa are approximately 22°c and 58 cm-yr1, respectively (Figure 3). Average minimum monthly temperatures do not fall below 4°c and average maximum monthly temperatures appro ach 40°C in the summer (Figure 3). Monsoon rainfall during just two months (July-August) accounts for nearly 50% of the total annual precipitation (Figure 3). Winter precipitation is derived from Pacific storms that pass over the area as the polar jet stream and the associated storm track shift southward during the winter (Magaña et al, 2003; Nicholas and Battisti, 2008).
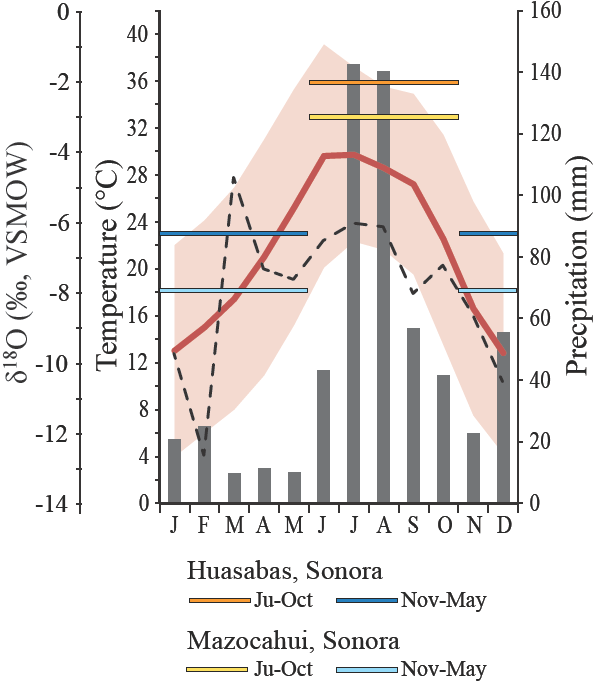
Figure 3 Climate data for Térapa, Sonora, Mexico. Average monthly precipitation totals in mm (gray columns); average monthly air temperatures (°C) (red line); average minimum and average maximum monthly air temperatures (°C) (red shading); and δ18O values of summer (June-October, warm colored horizontal lines) and winter (November-May, cool colored horizontal lines) precipitation collected at the towns of Huasabas and Mazocahui (see Figure 1) from 2002-2004. Dashed black line shows model-pre dieted δ18O values in Térapa precipitation from http://www.waterisotopes.org. Temperature and precipitation data are from http://smn.conagua.gob.mx/climatologia/normales/ estacion/son/NORMAL26251 at the time of writing. Period of record, 1971-2000.
The δ18O value (VSMOW) of precipitation at Térapa is not monitored, but mo deled values range from about -12‰ in the winter to -6‰ in the summer, with an average annual value of -7 ± 2‰ (Bowen, 2014; Figure 3). In contrast to modeled values, limited seasonal precipitation collections (2003-2004) at two stations within 50 km of Térapa (Figure 1) produced average δ18O values for winter (November-May) and summer (June-October) precipitation that were -7 ± 1‰ and -3 ± 2‰, respectively (n = 4; Figure 3; C. Eastoe, unpublished data). The winter precipitation δ18O value falls near the modeled value, but the summer precipitation δ18O value is much higher. The δ18O value of summer and winter precipitation in the southwestern U.S. varies by several per mille from year to year (Wright et al., 2001; Eastoe and Dettman, 2016). This variability likely explains the discrepancy between the modeled δ18O value for summer precipitation and the limited seasonal measurements near Térapa (Figure 3).
The fossiliferous sedimentary sequence at Térapa was deposited in a roughly 1 km x 2 km-wide basin along the eastern edge of the Tonibabilava flow (Figure 2). The basin was created when the Tonibabi lava flow spilled southward from its vent and down the Río Moctezuma Valley (Figure 2). The Tonibabi lava flow blocked and altered the course of the Río Moctezuma and many small tributaries. The blocking of these tributaries caused water and sediments to be impounded along the edges of the flow (Mead et al., 2006).
The source of the water and sediments that inundated the Térapa basin has not been conclusively identified (Mead et al., 2006). Given the overall fine grain size of the sediments and the orientation of the modern stream network, the water and sediments were most likely derived from several small washes that are sourced from small catchments in the eastern highlands (Figure 2; Mead et al., 2006). The basin at Térapa was two orders of magnitude smaller than its upstream source area, making it sensitive to changes in effective moisture in the eastern mountains.
The sedimentólogy and age of the Térapa fossil deposit is covered in detail in Mead et al. (2006) and Bright et al. (2010). Briefly, the entire sediment package is about 11 m thick (Figure 4). The basal 0.5 m of sediment (units As1 and As2) is comprised of coarse-grained sands that are deposited directly on the Tonibabi lava. Unit As2 is overlain by about 1.5 m of silty clay (Unit Ac). A well-sorte d wedge of sand (Unit As3) occurs within Unit Ac. The basal A-series sediments are overlain by roughly 1.5-2 m of blocky fossiliferous clays (Unit Bpl), which are in turn overlain by about 4 m of faintly bedded sandy silt (Unit Bc). A fairly prominent oxidized sorted sand occurs near the top of Unit Bc, which here is denoted Bc-s ("s" for sand). Unit Bc is overlain by another blocky fossiliferous unit that is about 1.5 m thick (Unit Bp2). Coarse pebble-armored stream channel deposits are formed into Unit Bp2 (Figure 4). Abruptly on top of both Unit Bp2 and the Tonibabi basalt are 2 to 4 m of coarse cobbles and sands of Unit C. Unit C likely represents coarse alluvial valley fill that spread acro ss the valley flo or after the Térapa basin was completely filled in with sediment. The Térapa locality has been dated, using multiple geochronological techniques, to about 40-43 ka, or to mid-marine Oxygen Isotope Stage (OIS) 3 (Bright et al., 2010).
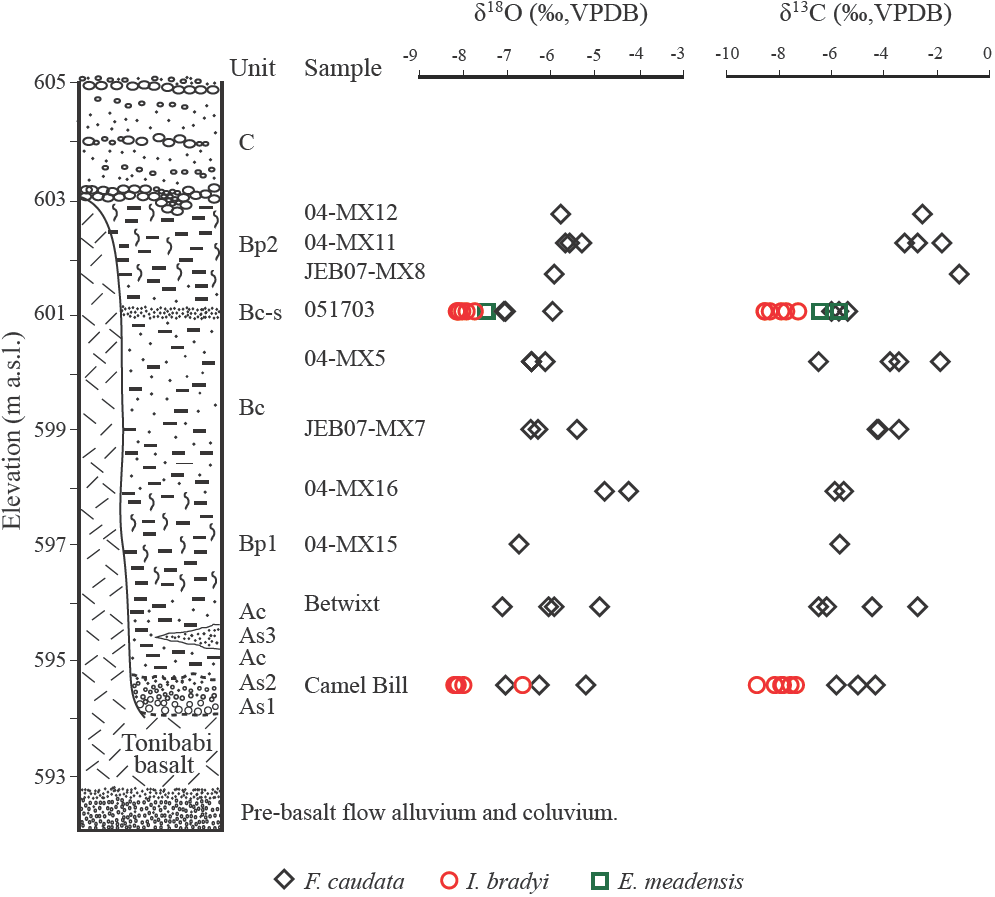
Figure 4 Stylized composite stratigraphie column for the Térapa deposit (modified from Mead et al. (2006)). Uncorrected ostracode calcite δ18O and δ13C values (‰,VPDB) are displayed in stratigraphie context. Black diamonds ־ Fabaeformiscandona caudata; red circles - Ilyocypris bradyi; green squares - Eucypris meadensis. Unit designations and sample names are the same as in Table 2 and Figure 6.
METHODS
Ostracode faunal analysis
Thirty four sediment samples from Térapa were analyzed for their ostracode faunas. Each sediment sample was placed in relative position within the master stratigraphie column of Mead et al. (2006). The majority of the sediment samples (n = 26) were processed at Northern Arizona University's Amino Acid Geochronology Laboratory using a modified version of Forester (1988). Sediments were disaggregated in a weak (3 gm-L-1) solution of sodium hexametaphosphate and sodium bicarbonate instead of Calgon®. The sediment solutions were washed over 150 μm sieves using hot deionized water. All residues were rinsed with deionized water and then air-dried. Northern Arizona University's Laboratory of Paleontology provided eight samples of hand-picked ostracode valves that were collected during their screen washing process (> 300 μm). The larger screen size means these samples were biased towards larger ostracode valves and species. Ostracode species were tabulated as presence-absence data, rather than individual valve counts. While individual valve counts and percent data are preferred for paleoenvironmental reconstructions, presence-absence data have been shown to be equally reliable (Muller et al., 2002) and will suffice for the purpose of this paper. Ostracode species were identified to genus, and where possible, species level based on drawings and images in Gutentag and Benson (1962), Meisch (2000), Delorme (1970a, b, c, d; 1971), Smith and Delorme (2010), and Ferguson (1967). The genus Stenocypris was identified by R. Forester (USGS, deceased).
δ18O and δ13C analysis of ostracode valve calcite
Whole, adult valves from fossil Fabaeformiscandona caudata, Ilyocypris bradyi, and Eucypris meadensis were manually picked from residues where they were most abundant and best preserved. These three species were selected because of their affinity for flowing water (e.g., Curry, 1999; Quade et al., 2003), which minimizes the effect of evaporation. The valves were cleaned by manual brushing with a fine paintbrush followed by gentle sonication and thorough rinsing using distilled water. The F. caudata and I. bradyi valves were sub-sampled into aliquots containing up to 4 valves (n = 37). The E. meadensis valves were large enough to be analyzed individually (n = 2). The δ18O and δ13 С values of the ostracode valves were measured using an automated KIEL-III carbonate preparation device coupled to a Finnigan MAT 252 gas-ratio mass spectrometer. The samples were reacted with dehydrated phosphoric acid under vacuum at 70°C. The isotope ratio measurement is calibrated based on repeated measurements of NBS-18 and NBS-19 standards. The stable isotope results are reported in standard delta (δ) notation where: δ‰ = [(Rsampie/Rstd)-1] x 103; and R = ratio of 18O/16О or 13C/12C. Rstd refers to the standard Vienna Peedee belemnite (VPDB). Precision for δ18O and δ13C measurements are ±0.1‰ and ±0.08‰, respectively.
RESULTS
Ostracode fauna
The fossil ostracode fauna at Térapa currently consists of 13 species from 12 genera (Table 1). The nearctic ostracodes Fabaeformiscandona caudata, Physocypria pustulosa, Cypridopsis vidua, and the cosmopolitan ostracode Darwinula Stevensoni (Figure 5) were encountered most frequently (Table 1). Pelocypris cf. tuberculatum, Cypridopsis okeechobei, Chlamydotheca arcuata, Limno су there paraornata, and Heterocypris incongruens (Figure 5) are species not previously reported in Mead et al (2006). The ostracode fauna is generally similar and consistent throughout the deposit (Table 1).
Table 1 Fossil ostracode species presence (P) or absence (-) listed by sample and stratum, Térapa, Sonora, Mexico.

Fc - Fabaeformiscandona caudata; Pp - Physocypria pustulosa; Cyp - Cypridopsis vidua; Ds - Darwinula stevensoni; Ib - Ilyocypris bradyi;. Pot - Potamocypris sp.; Em - Eucypris meadensis; Stn - Stenocypris sp.; Cha - Chlamydotheca arcuata; Hi - Heterocypris incongruens; Lp - Limnocythere paraornata; Pt - Pelocypris cf. P. tuberculatum. a Samples processed by N.A.U. Laboratory of Paleoecology and screen washed at 300 μm. b Species used for stable isotope analysis. c Includes several valves of Cypridopsis okeechobei.

Figure 5 Digital images of the fossil ostracode fauna at Térapa, Sonora, Mexico. Group a) Fabaeformiscandona caudata ; i- male right valve, ii- male left valve, iii- female right valve, IV - female left valve. Group b) Cypridopsis vidua; i- right valve, ii- left valve. Group c) Cypridopsis okeechoebi; i- right valve, ii- left valve. Group d) Darwinula stevensoni; i- right valve, ii- left valve. Group e) Limnocythere paraornata; i- male right valve, ii- male left valve, in- female right valve, iv- female left valve, f) Potamocypris sp., left valve. Group g) Stenocypris sp.; i- right valve, ii- left valve. Group h) Ilyocypris bradyi, right valve; Group i) Physocypria pustulosa; i- right valve, ii-left valve, j) Heterocypris incongruens; right valve, k) Eucypris meadensis; right valve. 1) Chlamydotheca arcuata; right valve, m) Pelocypris cf. R tuburculatum; left valve. All images are external lateral views taken under transmitted light using an Olympus SXZ-12 binocular microscope fitted with an Olympus DP71 digital camera. Images were modified in Photoshop CS3 for contrast and clarity.
δ180 and δ13с values from ostracode valve calcite
The δ 18OOST and δ 13COST values (VPDB) range from about -4‰ to -8‰ and from about -1‰ to -9‰, respectively (Figures 4 and 6; Table 2). With the exception of 2 outliers, Ilyocypris bradyi valves have the lowest δ18O and δ13C values (Figures 4 and 6; Table 2). The average δ18O and δ13C values from I. bradyi valves are -8 ± 0‰ and -8 ± 1‰, respectively (n = 12). The average δ18O and δ13C values from F. сaudata valves are -6 ± 1‰ and -4 ± 2‰, respectively (n = 25). The two E. meadensis valves produced δ18O and δ13C values of about -8‰ and -6‰, respectively (Figures 4 and 6; Table 2). The δ18OOST and δ13COST values are positively correlated (r = 0.74), although the low δ18O and δ13C values from I. bradyi control much of the correlation (Figure 6a). Excluding the I. bradyi values reduces the correlation considerably (r = 0.37).

Figure 6 Uncorrectea δ18O and δ13C values (‰, VPDB) from ostracode calcite preserved at Térapa, Sonora, Mexico, a) Cross-plot of indmdual δ18O and δ13C values are grouped by ostracode taxon and sedimentary unit. Sedimentary unit and sample names are as in Figure 4. b) Box-and-whisker plots of uncorrected δ18O values (blue boxes) and δ13C values (green boxes) from Fabaeformiscandona caudata and Ilyocypris bradyi valves. Thick black line - median value, colored box - 1st to 3rd quartile values, dashed lines and whiskers - maximum and minimum as 1.5 x interquartile range, open circles - outliers. Indmdual δ18O and δ13C values from two Eucypris meadensis valves are plotted as small black boxes.
DISCUSSION
Paleohydrology reconstruction
Ostracodes indicative of flowing-water settings (I. bradyú E. meadensis) are sometimes found intermixed with ostracodes indicative of vegetated, more wetland-like conditions (H. incongruens, C. vidua, C. arcuata). The mixing of flowing- and stagnant-water ostracodes suggests environmental heterogeneity or possibly post-mortem reworking of the valves. Infrequent occurrences of C. okeechobei and E. meadensis may be indicative of spring discharge. The presence of C. arcuata and an unidentified Stenocypris species (Figure 5) is notable. Both Chlamydotheca and Stenocypris have circumtropical distributions (Tressler, 1949). At Térapa, these two tropical ostracode species are frequently found in association with F. caudata, an ostracode with a range that is currently restricted to the continental United States and Canada (Forester et al., 2005).
The fossil ostracode fauna at Térapa is indicative of a persistent fresh-water setting. The majority of the fossil ostracode species have upper total dissolved solids (TDS) limits generally < 1,000 mg∙L-1, and both F. caudata and L. paraornata commonly inhabit water with TDS values between about 200 and 600 mg∙L-1 (Forester et al., 2005). To date, no ostracode species indicative of chemically evolved water, such as Limnocythere staplini or Limnocythere sappaensis, has been recovered at Térapa even though they were common in Late Pleistocene-aged closed-basin lakes from the southwestern U.S. and northern Mexico (e.g., Palacios-Fest et al., 2002; Wells et al., 2003; Allen et al., 2009; Chávez-Lara et al., 2012). The persistent low-TDS ostracode fauna suggests that the Térapa impoundment was through-flowing. Over-spilling or leakage through the Tonibabi basalt are also reasonable mechanisms that would have suppressed evaporative chemical enrichment and maintained a freshwater environment.
The presence of P. pustulosa, C. arcuata, and the unidentified Stenocypris species are key warm-water indicators at Térapa. Physocypria pustulosa requires at least 2 consecutive months with water temperatures above 20°C to complete its lifecycle (Forester et al., 1987). Chlamydotheca and stenocypris are both considered circumtropical genera (Tressler, 1949). Chlamydotheca and stenocypris have both been reported in the continental United States, but reports are typically from southern and Gulf-coast states or from warm (geothermal) spring settings (Furtos, 1933; Hoff, 1944; Ferguson, 1962,1964,1966; Bowen, 1976; Davis, 1980; Forester, 1991, 1999; Ourso and Höring, 2000; Curry and Baker, 2000). Being tropical genera, Chlamydotheca and Stenocypris are probably unable to survive cold winter temperatures (e.g., Addo-Bediako et al., 2000; Sunday et al., 2011). Uncontrolled experiments have shown that water temperatures below about 20°c are lethal to c. arcuata (Forester, 1991). Both of the tropical ostraco des at Térapa undoubtedly required optimal water temperatures that were much warmer than 20°C. For example, Bowen (1976) reported that Stenocypris sp. would only appear in a pond in South Carolina when the minimum water temperature exceeded 28°C, and Forester (1991) recovered Chlamydotheca from several springs in the western U.S. and northern Mexico where water temperatures were between 25°C and 32°C.
The water temperature requirements of the tropical ostracode species at Térapa provide insight into summer temperatures in northeastern Mexico at 40-43 ka. A modern shallow lake in north-central Sonora (Lake El Yeso; Figure 1) has mean monthly lake water temperatures that are, on average, about 3°C cooler than the mean monthly air temperatures (Palacios-Fest and Dettman, 2001). Using the water and air temperature relationship at Lake El Yeso as an analog, the mean summer air temperature at Térapa during OIS 3 could not have been more than 5°C cooler than today (Figure 4) in order to support the tropical ostracode taxa. This estimate seems reasonable based on temperature reconstructions for the last glacial period from southern California, southern Arizona, and southern Texas that collectively suggest a reduction in annual temperatures of about 4°C to 6°C, with associated errors of ± 0.5°C to ±1.1°C depending on the study (Stute et al., 1992; Anderson et al., 2002; Holmgren et al., 2006; Kulongoski et al., 2009).
To our knowledge, the Térapa locality is only the third reported fossil occurrence of F. caudata in northern Mexico, with the other two being from Late Pleistocene sediments deposited in Laguna Babicora, Chihuahua (Figure 1; Palacios-Fest et al.,2002), and juvenile valves that have been reported from extensive but undated sediments at Comarca Lagunera (Paleolake Irritia) of Durango and Coahuila (Czaja et al., 2014). The modern distribution of F. caudata is confined to Canada and the continental United States (Forester et aL, 2005) where it lives in water with optimal and maximum temperatures of about 6°C to 12°C and 18°C, respectively (Curry, 1999). Although F. caudata can tolerate water temperatures above 20°C, it does not live in areas where such temperatures persist annually (Forester, 1991). The southward expansion of F. caudata into northern Mexico during the last glacial interval is most likely attributed to the southward expansion of polar air masses (e.g., Kutzbach and Wright, 1985; Kutzbach, 1987; Ortega-Ramirez et al., 1998), which was probably accompanied by a reduction in summer temperatures. Although F. caudata and C. arcuata are found together at Térapa, the optimal water temperatures reported for F. caudata are several degrees cooler than the lower survival limit of С. arcuata (Forester, 1991). The most likely explanation is that the life cycles of these two ostracodes were seasonally offset, with F. caudata completing its life cycle during the cooler spring or fall months and c. arcuata completing its life cycle during the warmer summer months (e.g., Bowen, 1976; Pieri et al., 2007).
Surface water δ18O reconstruction and seasonality of precipitation
Correcting ostracode δ180 values for vital effects
Ostracodes molt eight or nine times over the course of their life cycle and calcify their new valves within a few hours to at most a few days after molting (Turpen and Angel, 1971; Chivas et al., 1983). Rather than providing a continuous isotopie record over weeks to years like mollusk shell or tooth enamel, the calcite in ostracode valves provides a "snapshot" of the δ18O composition of the host water at the time the ostracode molted.
Unlike inorganic calcite, the oxygen isotope value in ostracode calcite (δ18OOST) does not appear to be in isotopie equilibrium with the surrounding water; for a given water temperature and δ18O value, δ18O qst is usually more positive than the expected equilibrium value (e.g., Pérez et al., 2013), partially because of the presence of a poorly understood "vital effect" (e.g., von Grafenstein et al, 1999). The δ18O vital effect in ostracodes is species specific, but is probably relatively similar within any given genus (von Grafenstein et al., 1999). Numerous natural calibration studies on a variety of Fabaeformiscandona species report vital effects of about +2‰ to +3‰ (e.g., von Grafenstein et al., 1999; Keatings et al. 2002; Wetterich et al., 2008; Belmecheri et al., 2010). Xia et al. (1997) reported a slightly lower vital effect of about + 1‰ from laboratory cultured F. rawsoni. However, the ostracode valves from this experiment were less massive than the valves of naturally occurring E rawsoni and this vital effect may not be accurate. We favor correcting the F. caudata δ18O values using vital effects of +2‰ and +3‰ because of the larger naturally calibrated data set.
Correcting the I. bradyi δ18O results (δ18OILY) is more problematic because a vital effect for Ilyocypris has not been clearly established. Published vital effects for various species of Ilyocypris are as high as about +2‰ (Develle et al. 2010) and as low as about +0.2‰ (Schwalb et al. 2002; Belis and Ariztegui, 2004; Mischke et al., 2008; Lawrence et al., 2008). For the purposes of this paper we corrected the I. bradyi δ18O values using vital effects of 0‰ and +2‰.
To our knowledge, there are no data on vital effects specifically for E. meadensis calcite. Li and Liu (2010) demonstrated that there is no vital effect in cultured E. mareotica at 10°C and a small negative vital effect at both 15°C and 19°C. Based on our optimal water temperature estimate (see below), the E. meadensis δ18O values were not corrected for any vital effect.
The δ18O value of ostracode calcite (δ18OOST) is dictated by the water temperature and the δ180 value of the water (δ18OSW) in which the ostracode calcified its valves (von Grafenstein et al., 1999). Our δ18OOST (VPDB) values were converted to surface water δ18O values (δ18OSW; VSMOW) using the calcite-water fractionation data of Friedman and O'Neil (1977) and the optimal (6°C to 12°C) and maximum (18°C) modern water temperature constraints for F. caudata (Curry, 1999), which was the most common ostracode in the Térapa sediments (Table 1) and comprised the bulk of the δ18OOST results (Figures 4 and 6; Table 2). The 6°C range in optimal water temperatures will produce about a 1.5‰ VSMOW range in the reconstructed δ18OSW estimates, which is sensitive enough for the purpose of this study.
Reconstructing surface water δ18O values at Térapa
The ostracode-based surface water δ18O reconstruction for Térapa (δ18OSW-OST; VSMOW), using the optimal temperature range for F. caudata (6°C to 12°C) and assuming vital effects of 0‰ for both Ilyocypris and Eucypris and +2‰ to +3‰ for F. caudata, is -10 ± 1‰ (Figure 7). Increasing the temperature of calcification to the upper temperature limit for F. caudata (18°C) increases the δ18OSW-OSTw estimate to about -8 ± 1‰, with one maximum value of about -6‰ (Figure 7). More negative δ18OSW-OST estimates of about -12‰ at water temperatures of 6°C to 12°C and about-10‰ at a water temperature of 18°C are produced when the δ180ΙLΎ values are corrected for a +2‰ vital effect.
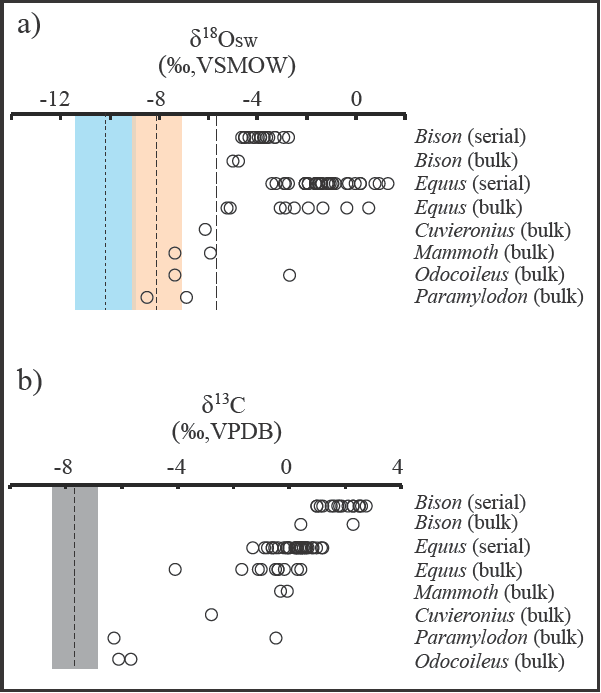
Figure 7 Comparisons of (a) reconstructed δ18OSW values (‰, VSMOW) of surface/meteoric water based on ostracode calcite and herbivore tooth enamel (open circles), and (b) the range of δ13C values in ostracode calcite and herbivore tooth enamel (open circles) at Térapa, Sonora, Mexico, during mid-OIS 3. Open circles in (a) and (b) are values derived from bulk and serial analysis of enamel from the teeth of large herbivores collected throughout the deposit (Nunez et al., 2010). Finest dashed vertical line and faint blue box in (a) are the mean and 1σ standard deviation, respectively, of δ18OSW values calculated from all ostracode calcite samples corrected for various vital effects and calcified over the preferred water temperature range of 6°c to 12°C. Medium dashed vertical line and faint orange box in (a) are the mean and 1σ standard deviation, respectively, of δ18OSW values calculated from all ostracode calcite samples corrected for various vital effects and calcified at the maximum water temperature estimate of 18°C. Coarsest dashed vertical line in (a) is the highest reconstructed δ18OSW value (see text for discussion). Medium dashed vertical line and grey box in (b) are the mean and 1σ standard deviation, respectively, of δ13C values from all ostracode calcite samples.
The reconstructed δ18OSW-OST values for Térapa at 40-43 ka (Figure 7) indicate that the isotopie composition of the Térapa impoundment was dominated by winter precipitation and runoff. This result agrees with other speleothem (Szabo et al., 1994; Wagner et al., 2010; Asmerom et al., 2010) and numerous paleolake/paleowetland studies in the southwestern U.S. and northwestern Mexico (Ortega-Guerrero et al., 1999; Anderson et al., 2002; Kirby et al., 2006; Allen et al., 2009; Pigati et al., 2009, 2011; Roy et al., 2010, 2012; Garcia et al., 2014; Reheis et al., 2015) that suggest increased winter moisture at about 40-45 ka, the time the Térapa sediments were deposited.
Comparing ostracode-based and tooth enamel-based surface water δ18О reconstructions for Térapa
We also compared our δ18OOST with both bulk and serial δ18O values from herbivore tooth enamel (δ18OΕΝ) previously recovered from Térapa (Nunez et al., 2010). Enamel δ180 values are often used to reconstruct paleo-water δ18O values (e.g., Widga et al., 2010; Metcalfe et al., 2011; Pérez-Crespo et al.,2012), and Térapa affords the opp or-tunity to compare δ18OSW reconstructions from the same site based on two commonly used archives; ostracode calcite and tooth enamel. The δ18OΕΝ values (VPDB) from Nunez et al (2010) were converted to surface water values (δ18OSW-EN) VSMOW) using the equations provided in their results section.
Several studies have demonstrated that the δ18OΕΝ values from large herbivores are strongly positively correlated with δ18OSW (Huertas et al., 1995; Kohn et al., 1996; Hoppe et al., 2004). The strength of the correlation between δ18OSW and δ18OΕΝ increases with the body size of the herbivore because larger animals are preferentially obligate drinkers (e.g., Bryant and Froelich, 1995; Hoppe et al, 2004). Smaller herbivores are better able to meet their water requirement through the food that they eat which weakens or eliminates the correlation between δ18OSW and δ18OEN in small animals (Huertas et al., 1995). Instead, the δ18OEN in small herbivores correlate better with humidity as it relates to evaporation and water stress on vegetation (Huertas et al., 1995). Leaves, especially in arid environments, can have δ180 values as much as 20‰ higher than the water absorbed at the plant root (Förstel, 1978; Kohn et al., 1996).
In order to generate the best comparison, we excluded the bulk tooth δ18OΕΝ data from two small herbivores (peccary; +0.9‰; capybara, -5.3‰; Nunez et al. (2010)) and a very high outlier from a llama (+4.4‰; Nunez et al. (2010)). This produced an average bulk δ18OSW-EN estimate of about -4 ± 3‰ (n = 19). This value is dominated by the results from numerous horse teeth that cluster with consistently high δ18OSW_EN values (-3 ± 3‰; n = 10). Nunez et al. (2010) produced serial δ18OΕΝ values from a bison and a horse tooth that captured 1 and 2 years of hydrologie information, respectively. When converted to δ18OSW-EN values, the serial bison and horse teeth results are also on the order of -4‰ to +2‰. We recognize that both the bulk and serial horse δ18OSW_EN values may be slightly too high because of a potential offset between δ18OΕΝ and δ18OSW that has been reported for modern horses (Hoppe et al., 2004), but that does not change the observation that the majority of δ18OSW-EN estimates at Térapa are roughly 4‰ to 12‰ higher than the δ18OSW-OST estimate (Figure 7).
The dissimilarity between δ18OSW-OST and δ18OSW-EN at Térapa was unexpected and intriguing. The δ18OSW-OST and δ18OSW-EN values lead to very different paleohydrologic interpretations; the δ18OSW-OST estimate favors winter-derived moisture, whereas the δ18OSW-EN estimate favors significant amounts of monsoon-derived moisture or possibly a very evaporative environment. If we assume that the δ18OSW-EN estimate is correct, then to generate the measured δ18OOST values would require either the absence of any vital effects and calcification in 18-20°c water, or the presence of vital effects and calcification in >28°c water. Both of these options seem unreasonable based on the available literature regarding δ18O systematics in ostracode calcite (e.g., von Grafenstein et al., 1999) and the ecological tolerances of the ostracodes used in this study (Forester, 1991; Curry, 1999) which are admittedly skewed towards ostracode occurrences at higher latitudes than Térapa. Diagenetic alteration of the δ18OΕΝ values is not a likely cause for the discrepancy because alteration typically changes δ18OΕΝ by about ±1‰ (Kohn et al, 1999), which is much less than the offset between the δ18OSW-OST and δ18OSW-EN values reported here. The most likely explanation for the offset is that the ostracodes consistently calcified their valves during the spring or early summer when the runoff feeding the impoundment was still dominated by winter precipitation. Additionally, the high δ18OΕΝ values from the large mammals may suggest that the animals consistently drank from evaporated water sources (e.g., Hoppe et al., 2004; Wigda et al., 2010) or incorporated a higher than expected proportion of evaporated leaf-water in their diet (e.g., Feranec and MacFadden, 2006; Zanazzi and Kohn, 2008; Pérez-Crespo et al., 2016), or perhaps the animals (especially the horses) migrated through the Térapa area. If the large herbivores are migrants then their δ18OΕΝ values may inelude contributions from non-local water sources (e.g., Pérez-Crespo et al., 2012).
Ostracode δ13C values and paleovegetation reconstruction
The δ13С value in ostracode calcite (δ13COST) faithfully records the δ13C value of dissolved inorganic carbon (δ13CDIC) of the water the ostracode calcified its valves in (von Grafenstein et al., 1999). The isotopie fractionation of carbon between aqueous carbon dioxide (CO2) and inorganic calcite produces a calcite δ13C value that, over the range of 10°C to 25°C, is approximately +12‰ higher than the δ13C value of the dissolved CO2 in the host water (Romanek et al., 1992).
In this study, we are interested in using δ13COST values from Ilyocypris bradyi, an ostracode with a strong affinity for streams and flowing water (Curry, 1999; Quade et al., 2003), to reconstruct the δ13CDIC value of the streams feeding the Térapa impoundment, and from that, broadly estimating the relative proportion of paleovegetation types present in the Térapa watershed at 40-43 ka.
Variables that influence ostracode δ13C values
The δ13CDIC value of springs and streams is largely derived from respired soil CO2 (δ13CSOIL), which itself is derived from the surrounding plant community, although contributions from water-rock interactions, for instance the dissolution of marine carbonate bedrock (δ13C ≈ 0‰), may be present in groundwater as well. Terrestrial vegetation can be subdivided into C4,C3, and Crassulacean Acid Metabolism (CAM) groups, with each group having a representative range of δ13C values. Grasses and other plants that use the C4 photosynthetic pathway generally have higher δ13C values (-13 ± 2‰) than woody plants and shrubs that use the C3 photosynthetic pathway (-28 ± 3‰; O'Leary, 1981). Plants that use the CAM photosynthetic pathway (e.g. cactus, agave, yucca) have intermediate δ13C values (-13‰־ to -27‰; O'Leary, 1981) that overlap with C3 and C4 plants. Obligate CAM plants have δ13C values closer to C4 plants, whereas facultative CAM plants tend to have δ13C values that are closer to C3 plants (Griffiths, 1992). Thus, the δ13С value of CAM plants overlaps those of C3 and C4 plants, rendering them isotopically invisible as a group. As a result, our discussion of plant communities is restricted to an estimate of the apparent abundances of C3 and C4 plants, recognizing that the C3 category may contain contributions from facultative CAM plants and that the c4 category may contain contributions from obligate CAM plants. And finally, aquatic vegetation typically has very low δ13C values (-25 to - 30 ‰; Keeley and Sandquist, 1992).
Further complicating the issue is the fact that some ostracode species live within the top few cm of sediment (epifaunal; Decrouy et al., 2012) and may calcify their valves within the sediment. Epifaunal species may record the δ13CDIC value of sediment pore water rather than of the overlying water body (Decrouy et al., 2012). Consequently, δ13COST of epifaunal ostracodes may be lower than expected because the valves were calcified in an environment where decaying organic matter often leads to pore water that is enriched in respired 12C (e.g., Keeley and Sandquist, 1992; Decrouy et al., 2011).
Reconstructing ground cover at Térapa
The modern flora of the Sonoran Desert consists of roughly 50% annuals and 50% perennials (e.g., Venable and Pake, 1999). The winter annual flora is populated exclusively by C3 plants whereas the summer annual flora is dominated by C4 plants (summer annual C3:C4 = 35:65; Mulroy and Rundel, 1977). Perennials are comprised mostly of c3 and CAM plants (Drennan and Nobel, 1997). Packrat middens dating to the last glacial period (60 to ∼13 ka) indicate that much of the Sonoran Desert landscape between about 300 and 1,300 m a.s.l. was dominated by pinyon-juniper-oak woodlands and chaparral (Van Devender, 1990). Similar packrat midden studies and the isotopie analysis of megaher-bivore (e.g, mammoth, bison, horse) tooth enamel have suggested that summer monsoon precipitation supported C4 grasslands throughout much of the southwestern U.S. and northern Mexico during the last glacial period (e.g., Connin et al., 1998; Holmgren et al., 2006, 2007).
The average δ13C value (VPDB) for I. bradyi (δ13СІLУ) is about -8‰ (Figure 6; Table 2). Reducing this value by 12‰ (e.g., Romanek et al., 1992) produces a δ13C value (VPDB) for dissolved aqueous CO2 of about -20‰. Subtracting an additional 4‰ for the kinetic fractiona-tion of C02 in soil profiles (Cerling, 1984) the Δ13C0st value could have been derived from a landscape with a δ13CSOIL value of about -24‰.
The C3 and C4 vegetation at Térapa was assigned end member values of -28‰ and -13‰, respectively, based on previous studies in similar desert environments (Mooney et al., 1989; Biedenbender et al., 2004). If Térapa had a δ13CSOIL value of about -24‰ then that would suggest ground cover with a C3:C4 ratio of about 75:25.
Comparing ostracode-based and tooth enamel-based paleovegetation reconstructions for Térapa
At face value, a C3-dominated flora surrounding Térapa at 40-43 ka contrasts with the enamel results of Nunez et al. (2010), where they concluded that C4 grasses and perhaps obligate CAM plants were ab un-dant around Térapa and comprised a significant portion of the diet of even typically obligate browsing herbivores (i.e., Odocoileus; Figure 7). The discrepancy between our C3-dominated interpretation and Nunez et al. (2010) C4-rich interpretation (Figure 7) may be explained by several factors. First, the δ13CILY and δ13СЕN values are derived from two very different processes. The δ13СІLУ values likely reflect the averaged δ13Cs01l composition of the surrounding watershed during the season that the ostracodes preferentially calcified their valves, whereas the δ13CEN values undoubtedly incorporate a selective dietary preference for each animal (e.g., Feranec and MacFadden, 2006). Second, the δ18OOST values suggest a winter-dominated precipitation regime. If so, then the δ13CDIC of runoff was probably dominated by a winter vegetation signal that would have been skewed towards C3 plants. Similarly, we suspect that the streams that fed the Térapa impoundment drained the highlands to the east (Figure 2) and may have had δ13CDIC values that were skewed towards the low values typical of a woodier C3-dominated mountainous ecosystem. And finally, I. bradyi may have calcified in 12C-enriched microhabitats such that their δ13C values would be lower than the bulk stream value. This would suggest that additional factors that should have increased the δ13CDIC value, such as the absorption of atmospheric CO2 (δ13C ≈ -6.7‰; Köhler et al., 2010) or the decay of C4 organic material that might have washed into the streams, were outweighed by the decay of aquatic vegetation within the streams. A combination of these seasonal and habitat-specific factors likely produced δ13CILY values that significantly underestimate the abundance of C4 plants on the landscape. In spite of their aquatic origin and likely seasonal bias, the δ13СІLУ values still require about 25% input from c4 plants, suggesting that C4 plants were perhaps abundant near Térapa, as Nunez et al. (2010) concluded.
CONCLUSIONS
A fossil ostracode fauna and stable isotope (δ18O and δ13С) analyses have been used to reconstruct the paleoenvironment during the deposition of a unique megafauna vertebrate assemblage at Térapa, Sonora, Mexico, duringmid-OIS 3 (about 40-43 ka). The fossil ostracode fauna was comprised of 12 genera and 13 species, species that reflect flowing water and more quiescent conditions were found intermixed. Most of the ostracode species are common, wide- spre ad temperate species, but two, C. arcuata and Stenocypris sp., have tropical affinities. The mix of temperate and tropical ostracode species mirrors the mix of temperate and tropical vertebrate fossils recovered from the site. Based on the presence of tropical ostracode species, summer temperatures at Térapa at 40-43 ka were at most ∼5°C cooler than at present. The fresh-water ostracode fauna and low δ18OOST values suggest that the water body at Térapa was through-flowing. Our ostracode-based TDS estimate for the impoundment is on the order of 200-600 mg־L 1.
The δ18OOST and δ13CILY values from throughout the deposit suggest that winter precipitation dominated the hydrologie cycle and that the surrounding vegetation community was dominated by C3 plants. The δ18OSW-OST estim for Térapa at 40-43 ka was approximately -10 ± 2‰ VSMOW, indicating that increased winter precipitation dominated the surface runoff. This finding is consistent with other paleoclimatic studies from the southwestern U.S. and northern Mexico at 45-40 ka. The δ18OSW-OST estimate is roughly 4‰ to 12‰ lower than the δ18OSW-EN estimates derived from teeth of large herbivores collected from the same site, however. The δ13СІLУ results suggest that C3 plants dominated the landscape (perhaps 75%), although associated herbivore tooth enamel suggests a strong C4 (i.e., grass) component was present. Because of the season and aquatic setting in which the ostracode valves were likely calcified, the δ13COST values probably underestimate the proportion of C4 plants that were on the landscape.
The Térapa vertebrate site is an excellent example of how different isotopie archives can produce very different paleoenvironmental interpretations from the same deposit. Térapa provides a cautionary example of how using a single isotopie archive to reconstruct desert paleohydrology may be misleading. When used individually, the ostracode and enamel δ18O and δ13C values suggested opposing precipitation and vegetation regimes. The ostracode results favor a winter-dominated hydrology and a C3-rich vegetation community whereas the tooth enamel results favor a summer-dominated or more evaporative hydrology and a c4-rich vegetation community. A more complete interpretation emerged when both datasets were used together. Interpretive differences arose because the processes that produced the δ18O and δ13C values in the ostracode calcite and herbivore enamel captured paleoenvironmental information at different spatial, temporal, and seasonal scales.











 nueva página del texto (beta)
nueva página del texto (beta)



