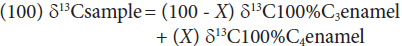INTRODUCTION
Values for δ13C and δ18O in fossil and current cetartiodactyls, perissodactyls, and proboscideans tend to reflect diet and habitats (Cerling and Harris, 1999; MacFadden et al, 1999; Feranec and MacFadden, 2000; Pérez-Crespo et al., 2009, 2012a, 2012b, 2014; Gutiérrez-Bedolla et al, 2016), and can be used as proxies for the paleoenvironmental reconstruction of the sites.
In Mexico, more than 25 species of Pleistocene Equus horses have been reported (see further discussion in Alberdi et al, 2014). In the Rancholabrean North American Land Mammal Age (NALMA) (ca. 160 ka to 9.5 ka; Bell et al, 2004), horses were represented by three species in west-central and north-central Mexico: Equus mexicanus Hibbard, 1955; E. conversidens Owen, 1869; and E. cedralensis Alberdi et al., 2014. These species had similar morphologies relating them to the genus Equus, however noticeable differences exist in some cranio-dental measurements, limb proportions, and body mass: the species E. mexicanus was large, had robust limbs, and a high body mass; E. conversidens was medium-sized, had robust limbs, and an intermediate body mass; and E. cedralensis was small-sized, had slender limbs, and a low body mass (Marín-Leyva, 2011; Alberdi et al, 2014).
We have assumed that these three horse species were sympatric since they have been found together in at least three localities in Mexico, one in the north-central region studied by Alberdi et al. (2014) in Cedral, San Luis Potosí, and two in the west-central region reported by Marín-Leyva (2011) in La Cinta-Portalitos and La Piedad-Santa Ana, in the states of Michoacán and Guanajuato (Figure 1). Based on that assumption, we proposed that the morphological and ecomor-phological differences in size, limb proportions, and body mass could have provided these sympatric species the ability of living in different microhabitats and of having different diets by means of sharing the same macrohabitat through resource use and partitioning.
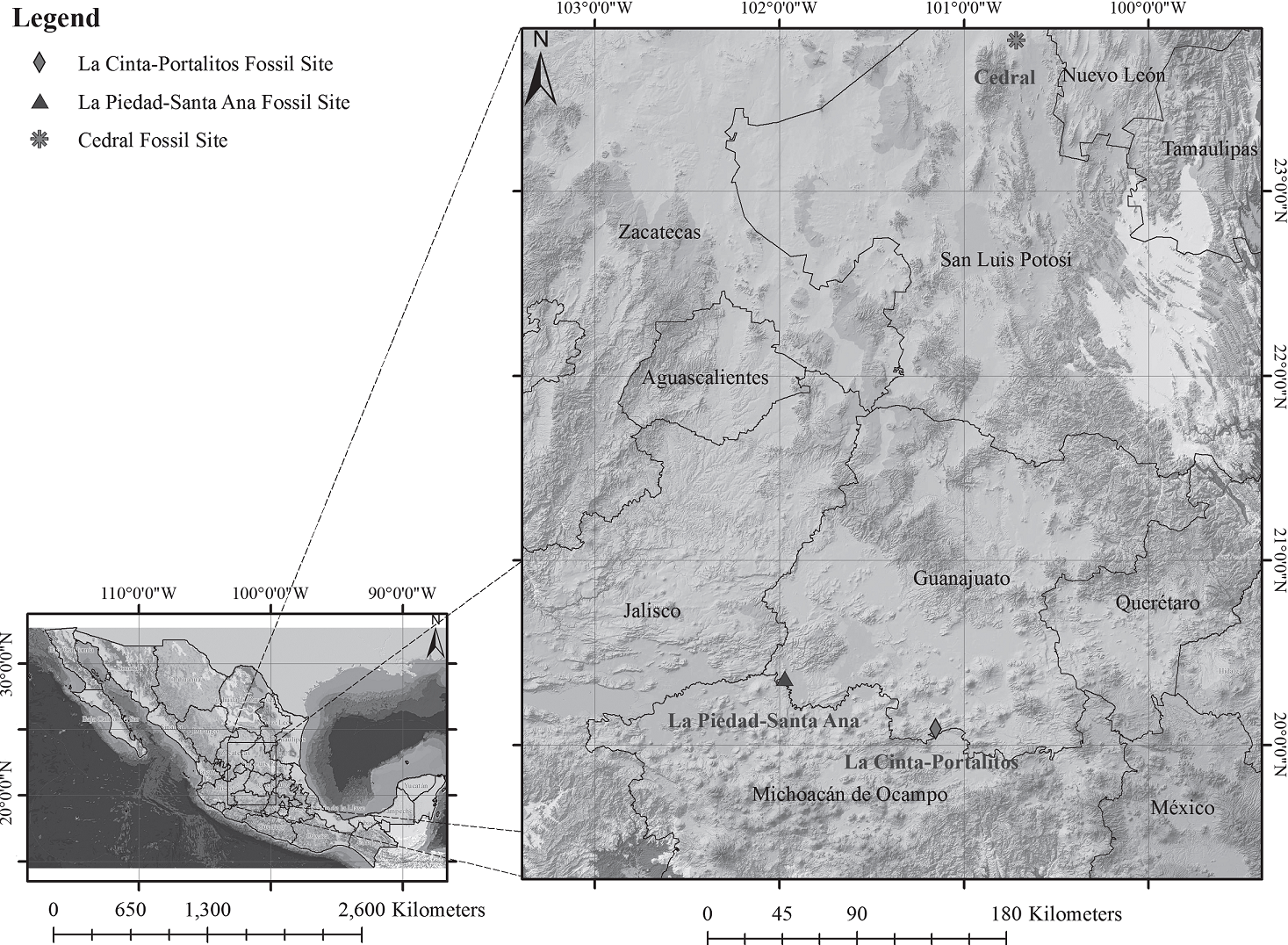
Figure 1 Geographical location of La Cinta-Portalitos and La Piedad-Santa Ana areas in the states of Michoacán and Guanajuato, west-central Mexico, and the Cedral area in the state of San Luis Potosí, north-central Mexico.
The two known localities of Equus in west-central Mexico were dated by Marín-Leyva (2011) as Late Pleistocene: La Cinta-Portalitos, with presence of a paleolake and characterized by an heterogeneous geomorphology and diversity of ecological niches; and La Piedad-Santa Ana, with a fluvial system and more homogeneous geomorphology and habitat diversity. Both sites are about 100 kilometers apart and are located in the Lerma-Chapala basin within the Trans-Mexican Volcanic Belt morphotectonic province (sensuFerrusquía-Villafranca et al., 2010) (Figure 1). These sites provide an excellent opportunityto evaluate the diet and habitat of two populations of the three above-mentioned sympatric horse species by means of stable carbon and oxygen isotopes, with the objective of finding the degree of dietary adaptability and resource partitioning that would reflect the environmental conditions of the localities they used to feed upon, also providing support to the habitat predicted from the morphology and ecomorphology.
Diet and habitat analysis using stable isotopes
The main reason why stable isotopes extracted from fossils can be used for research on past ecological or environmental conditions is because plants and surface waters in terrestrial landscapes show different isotopic proportions that are transferred to the consumer organisms (Fricke, 2007).
Based on the fact that there are three metabolic pathways in plants for photosynthesis and carbon fixation -C3 or Calvin-Benson cycle metabolism (O'Leary, 1981; Lee-Thorp et al, 1989; Cerling et al., 1997; Koch et al, 1998), C4 or Hatch-Slack cycle metabolism (Bender, 1971; Smith and Epstein, 1971; Vogel, 1978; Ehleringer et al, 1986, 1991; Cerling et al., 1993), and CAM or crassulacean acid metabolism (Decker and de Wit, 2005; Andrade, et al., 2007)- the stable isotopes of carbon can be used for inferring dietary preferences and type of habitat of Pleistocene herbivore and carnivore mammals (for example: Koch et al, 1998; MacFadden et al., 1999; Feranec and MacFadden, 2000; Palmqvist et al, 2003; Feranec, 2004; Pérez-Crespo et al., 2009, 2012a, 2012b, 2014).
Large ungate mammals incorporate to their tooth enamel the carbon contained in the plant they consume with an additional enrichment of δ13C of 14 ‰ ± 0.5 ‰ (Cerling and Harris, 1999). Mammals feeding exclusively on c3 plants have δ13C values of from -19‰ to -9‰, while those consuming only C4 plants have δ13C values between -2 ‰ and +2 ‰. Animals with a mixed diet will show δ13C values intermediate to those in C3 and C4 plants (Hofmann and Stewart, 1972; MacFadden and Cerling, 1996). Because of this, the relative proportion of C3 and C4 plants in the diet of a fossil animal allows for obtaining data about the ecological behavior of extinct species (Koch et al, 1998).
Stable isotopes of oxygen are also used for obtaining data on environmental conditions (temperature and precipitation) from mammal tooth enamel (Longinelli and Nuti, 1973; Kolodny et al., 1983; Longinelli, 1984; D’Angela and Longinelli, 1990; Hoppe et al., 2005). Oxygen isotopes are useful because mammal teeth mineralize under a constant temperature (∼37 °c) in balance with corporal water, so that the water used for drinking determines the values of δ18O present in the body (Luz et al.,1984; Bryant and Froelich, 1995; Kohn, 1996; Hoppe et al., 2005).
The isotopic composition of oxygen in the teeth of mammals is related to oxygen entering and leaving the body (Bryant and Froelich, 1995; Kohn, 1996; Sponheimer and Lee-Thorp, 2001). The main oxygen sources are atmospheric O2, oxygen from liquid water, and oxygen from food (Sponheimer and Lee-Thorp, 2001). The main ways in which the body gets rid of oxygen are: breathing (CO2), excretion of liquid water, and emissions of water vapor. This is interesting because animals have different physiological mechanisms for relieving heat stress (Wong et al., 1988).
For 18O we follow Hoppe et al. (2005) conception of horses being obligate drinkers, so that δ180 values are controlled by the isotopic composition of the water they drink. Some studies show that the δ18O values in bones and teeth of modern equids from different latitudes correlate with the δ18O values of local water (Bryant et al., 1994; Sánchez et al., 1994; Delgado et al., 1995); however other studies show that in extreme conditions, such as in arid zones, modern horses from a single population can display a wide range of δ18O values from 4.5 ‰ to 6.5 ‰ (Hoppe et al., 2004a), while in temperate environments this same value is of approximately of 5 ‰ (Hoppe et al., 2005).
REGIONAL SETTING
The two areas where materials herein studied were collected are within the hydrological region of Lerma-Chapala and were dated as Late Pleistocene. The area of La Cinta-Portalitos (hereafter abbreviated as LC-PT) is located between the villages of La Cinta, Michoacán, and Portalitos, Guanajuato, within the Cuitzeo basin. The studied area La Piedad-Santa Ana (hereafter abbreviated as LP-SA) is located within the Lower Lerma basin between the towns of La Piedad de Cabadas, Michoacán, and Santa Ana Pacueco, Guanajuato (Figures 1, 2). Vertebrates from the latter two localities were assigned to the Rancholabrean NALMA dated at ca. 160 ka to 9.5 ka (Bell et al., 2004; García-Zepeda, 2006; Díaz-Sibaja, 2013) because there are no radio-metric ages of 14C for both sites, and therefore, an accurate temporary location is lacking for samples. The faun al assemblage is varied and extensive, comprising numerous vertebrate taxa, including amphibians and reptiles, of which 14 are large mammals weighting above 44 kg. The perissodactyls are the most numerous and important components of the terrestrial ecosystems, followed by proboscideans, cetartio dactyls, and rodents.
Figure 2 Simplified stratigraphic columns: a) La Cinta-Portalitos, and b) La Piedad-Santa Ana. With stratigraphic data from Marín-Leyva (2011) and Díaz-Sibaja (2013). Geomorphological maps modified from Marín-Leyva et al. (2016).
The LC-PT area (Figures 1, 2a) has a heterogeneous geomorphology that is subdivided in four zones: NE, with shield-type volcanic edifices with normal faults, part of the northern portion of Cuitzeo graben (Israde-Alcántara et al., 2010a); NW, with monogenetic and shield-type volcanic edifices; SW, with presence of the oldest volcanic rocks; and S, with drainage altered by agricultural activity and a portion of the extant lake (Marín-Leyva, 2011). Six lithological and paleontological facies are recognized for this locality: Facies I, lacustrine, clay with diatoms, low energy and stable lake; Fades II, igneous, volcanic sand, medium energy; Facies III, fluvial-lacustrine, sand and clay, medium energy; Biofacies IV, fluvial-lacustrine, microconglomerate, medium energy with micro and macro vertebrate fossils; Facies V, lacustrine, clay with diatoms, low energy; and Facies VI, soils, silt, low energy (Figure 2a). All the vertebrate remains are located in one single stratigraphic layer that form the Biofacies IV with a time resolution according to Behrensmeyer et al. (1992) of 103-101 years. The associated large fauna includes mammoth (Mammuthus columbi), giant bison (Bison latifrons), ancient bison (Bison antiquus), camel (Camelops hesternus), llama (Hemiauchenia sp.), white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), deer (Odocoileus lucasi), and tayas-suid (Platygonus sp.). Other vertebrates include the rodents Microtus sp., Neotoma sp., Sigmo don sp., and spermophilus sp.,the snake Elaphe guttata, and the amphibians Lithobates pipiens and Ambystoma sp. (García-Zepeda, 2006; Pérez-González and Gоdínez-García; Marín-Leyva, 2008,2011; Plata-Ramírez, 2012; Díaz-Sibaja, 2013; Díaz-Sibaja et al., 2014; Gutiérrez-Bedolla et al., 2016).
The site of LC-PT was dated indirectly by Marín-Leyva (2011) using stratigraphie layers, sedimentation rates, and 18 accelerator mass spectroscopy (AMS) 14C dates from Israde-Alcántara et al (2010b), who found that the vertebrate remains from LC-PT could have an age of between 32, 565 and 18,500 years before present (BP). However, these dates assigned to the fossil site need to be taken carefully because they were not made directly on the fossils.
The LP-SA area (Figures 1, 2b) has a homogeneous geomorphology that is subdivided in four zones: NW, part of the Lerma river between highland and hills; W, part of the Chápala plains; SW, with the volcanic edifice of Cerro Grande; SE, part of the Lerma river great plains. For this site, six lithological and paleontological facies are recognized: Facies I, fluvio-lacustrine, sand with clay, medium to high energy; Facies II, igneous, volcanic sand, medium energy; Biofacies III, micro and macro-conglomerate, high energy with macrovertebrate fossils; Facies IV, fluvial, sand and silt, medium to high energy; Facies V, lacustrine, clay with diatoms, low energy; and Facies VI, soils, clay, silt with sand, low to medium energy (Figure 2b). Like in the fossil site of LC-PT, all vertebrate remains from LP-SA are located in one single stratigraphie layer that forms the Biofacies III with a time resolution according to Behrensmeyer et al. (1992) of 100-104 years. In this site there is also presence of mammoth, giant bison, ancient bison, camel, llamas and white-tailed deer (Plata-Ramirez, 2012; Díaz-Sibaja, 2013; Díaz-Sibaja et al.,2014; Gutiérrez-Bedolla et al, 2016).
The fossil sites contain similar taxa richness, including marker species characteristic of Rancholabrean age, like Bison latifrons and Bison antiquus (Bell et al., 2004). According to Díaz-Sibaja (2013) and the ranges of radiocarbon ages for these two species of Bison, the sites of LC-PT and LP-SA could have a relative age of between 51±1.5and8.39±0.14ka BP.
A similar fauna composition is found in both localities, a fact that contrasts with the different environments in which they inhabited (lacustrine in LC-PT and fluvial in LP-SA). Several paleoenvironmental studies including analyses of pollen and diatoms have been carried out in LC-PT (Israde-Alcántara et al., 2002, 2010b; Caballero et al., 2010), but no paleoenvironmental reconstructions are available for LP-SA.
MATERIALS AND METHODS
Samples, enamel preparation, and statistical analyses
Individual premolars and molars belonging to the genus Equus collected from the Biofacies IV in LC-PT and Biofacies III in LP-SA were assigned to species according to morphometry of cranio dental characteristics following Marín-Leyva (2011) and Alberdi et al (2014), and specimens of the same taxon were grouped as a population. Samples of the teeth used in this study are presented in Figure 3.
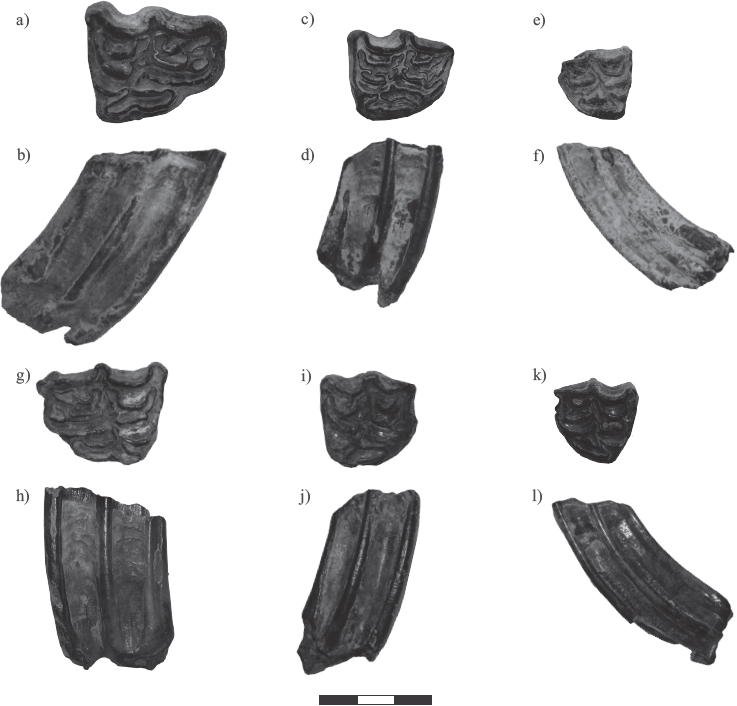
Figure 3 Photographs of occlusal and labial views (in that order) for third upper molars of the horse species Equus mexicanus, E. conversidens, and E. cedralensis from La Cinta-Portalitos and La Piedad-Santa Ana localities in west-central Mexico, a) and b) UM 104; с) and d) UM 165; e) and f) UM 40; g) y h) CPOEI 256; i) and j) CPOEI 266; k) and 1) UM 567. Scale bar 3 cm. For further information see Table SI of the supplementary material.
Only five samples were available for our study, although Hoppe et al. (2005) recommended a larger sample size of more than nine specimens per population. Therefore, according with Hoppe et al. (2005) our results could be regarded as preliminary. However, we consider our data to be meaningful for comparison with other samples of the same species from Cedral, San Luis Potosí (Pérez-Crespo et al, 2009). For this study we chose the fourth upper premolar (P4), and the third upper molar (M3), because these teeth mineralize from ∼16 to 54 and ~ 18 to 58 months after nursing, respectively (quasi-contemporaneous) (Hoppe et al., 2004b).
We followed the technique proposed by Koch et al. (1997) for enamel extraction and pretreatment. Previous X-ray diffraction (XRD) studies show that there are no diagenetic alterations in the enamel of the analyzed teeth (García-Reyes, 2004). Using a Dremel power drill with a dentist bit, 20 mg of enamel were obtained from each premolar and molar. In order to assure that the bulk sample represents material mineralized during an entire year of growth, the enamel was collected from a region encompassing ∼3 to ∼4 cm vertically (Hoppe et al., 2004b). The enamel sample was powdered with an agate mortar and pistil, and sieved to obtain the finest powder; 10 ml of 30 % hydrogen peroxide were added and the samples incubated for two hours at room temperature. The peroxide was then decanted and the sample was washed three times with distilled water.
After washing, a 1 M acetic acid and sodium acetate solution was added, leaving it to incubate for nine hours at room temperature. The solution was then decanted and the sample was again washed three times with distilled water. Finally, any remaining water was eliminated by addition of ethyl alcohol and samples were dried in an oven at 90°C for 12 h.
Samples were sent to the Laboratorio Universitario de Geoquímica Isotópica (LUGIS) at the Instituto de Geología of Universidad Nacional Autónoma de México (UNAM), and assayed with a Finnigan MAT 253 mass spectrometer with a dual inlet system, and GasBench auxiliary equipment with a GC Pal autosampler with a temperature-controlled aluminum plate adjoined to the mass spectrometer (Révész and Landwehr, 2002). Standards were analyzed in duplicate to accomplish a higher precision. Results were reported as δ18OVPDB and δ13 CVPDB, and they were normalized using NBS-19, NBS-18 and LS VEC to the Vienna Pee Dee Belemnite (VPDB) scale in accordance with the corrections described by Coplen (1988) and Werner and Brand (2001). For this technique, the standard deviation was 0.2 ‰ for oxygen and 0.2 ‰ for carbon.
Statistical analysis
Sample data (δ13C and δ18O) of the three species (E. mexicanus, E. conversidens, andE. cedralensis) were analyzed statistically, first by obtaining the mean, standard deviation, minimum, maximum, and range, and afterwards by applying an analysis of variance (ANOVA). To statistically distinguish differences between groups, paired comparisons were made using the Tukey-Kramer honest significant difference (HSD) test for all pairs. Probability level for the statistical test was 95% (p < 0.05); JMP 7.0 SAS Institute software programs were utilized.
The formula proposed by Koch et al. (2004) and Koch (2007) was used to estimate the percentage of C3 and C4 in the diet:
where enamel δ13С100%С3 value is-12.5 ‰ and enamel δ13С100%С4 value is 2.5 ‰, corresponding to estimates for the Late Pleistocene (Koch et al., 2004; Koch, 2007), and X is the percent of c4 plants in the diet (%C4). We also analyzed the δ13C and δ18O values obtained by Pérez-Crespo et al. (2009) for Cedral (CE) horses in order to find out if horses from the two localities in west-central Mexico had the same diet as in the locality from north-central Mexico. In order to transform the 618OVPDB values into 618OVSM0W values, the equation in Faure (1977) was used.
RESULTS
Analysis of δ13C values
In Tables 1 and 2 we show for each studied taxon the mean, standard deviation, maximum, minimum, range, variance, standard error, and the lower and upper confidence intervals of δ13с and %c4 values. The individual data values are available in Table SI of the supplementary material.
Table 1 Summary of values for δ13C expressed in ‰ VPDB.
| Site | N | Mean | SD | Min | Max | Range | Variance | Std Err | L CI | U CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. cedralensis | LC-PT | 5 | 0.32 | 1.05 | -1.24 | 1.68 | 2.91 | 1.10 | 0.47 | -0.98 | 1.63 |
| E. conversidens | LC-PT | 5 | -0.91 | 0.90 | -1.93 | 0.03 | 1.97 | 0.80 | 0.40 | -2.02 | 0.20 |
| E. mexicanus | LC-PT | 5 | -2.45 | 0.77 | -3.22 | -1.50 | 1.72 | 0.60 | 0.35 | -3.41 | -1.49 |
| E. cedralensis | LP-SA | 5 | -0.61 | 1.64 | -3.23 | 1.05 | 4.28 | 2.69 | 0.73 | -2.64 | 1.43 |
| E. conversidens | LP-SA | 5 | -0.49 | 1.54 | -2.71 | 1.34 | 4.05 | 2.37 | 0.69 | -2.40 | 1.42 |
| E. mexicanus | LP-SA | 5 | -0.86 | 0.56 | -1.70 | -0.39 | 1.32 | 0.31 | 0.25 | -1.55 | -0.17 |
Notes and abbreviations: LC-PT = La Cinta-Portalitos; LP-SA = La Piedad-Santa Ana; N = sample number; SD= standard deviation; Min = minimum; Max = maximum; Ran = range; Std Err = standard error; L CI = lower confidence interval; U CI = upper confidence interval. Values are expressed in ‰ VPDB.
Table 2 Summary of values for % of C4 plants consumed.
| Species | Site | N | Mean | SD | Min | Max | Range | Variance | Std Err | L CI | U CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. cedralensis | LC-PT | 5 | 85.49 | 7.01 | 75.10 | 94.53 | 19.43 | 49.08 | 3.13 | 76.79 | 94.19 |
| E. conversidens | LC-PT | 5 | 77.27 | 5.97 | 70.44 | 83.55 | 13.11 | 35.61 | 2.67 | 69.86 | 84.68 |
| E. mexicanus | LC-PT | 5 | 67.01 | 5.16 | 61.88 | 73.36 | 11.48 | 26.63 | 2.31 | 60.60 | 73.41 |
| E. cedralensis | LP-SA | 5 | 79.28 | 10.94 | 61.77 | 90.30 | 28.53 | 119.77 | 4.89 | 65.69 | 92.87 |
| E. conversidens | LP-SA | 5 | 80.04 | 10.26 | 65.24 | 92.26 | 27.03 | 105.30 | 4.59 | 67.30 | 92.79 |
| E. mexicanus | LP-SA | 5 | 77.57 | 3.70 | 71.97 | 80.76 | 8.79 | 13.72 | 1.66 | 72.97 | 82.17 |
Notes and abbreviations: C4% = percentage of C4 plants consumed based on Equation 1 (100) δ13Csample = (100-X) δ13C100%C3enamel + (X) δ13C100%C4enamel, where enamel δ13C100%C3 value is -12.5 ‰ and enamel δ13C100%C4 value is 2.5 ‰, corresponding to estimates for the Late Pleistocene (Koch et al. 2004, 2007); LC-PT = La Cinta-Portalitos; LP-SA = La Piedad-Santa Ana; N = sample number; SD= standard deviation; Min = minimum; Max = maximum; Ran = range; Std Err = standard error; L CI = lower confidence interval; U CI = upper confidence interval. Values are expressed in ‰ VPDB.
The mean δ13C value for E. cedralensis is 0.32 ‰ (%C4, 85.49) in samples from LC-PT, and -0.61 ‰ (%C4, 79.28) in samples from LP-SA. The mean δ13C values for the populations of E. conversidens are -0.91700 (%C4) 77.27) for LC-PT and -0!49 700 (%C4, 80.05) for LP-SA. For the populations of E. mexicanus, the mean value of δ13C is -2.45 (%C4, 67.0) for LC-PT and -0.86 ‰ (%C4, 77.57) for LP-SA (Figure 4a).
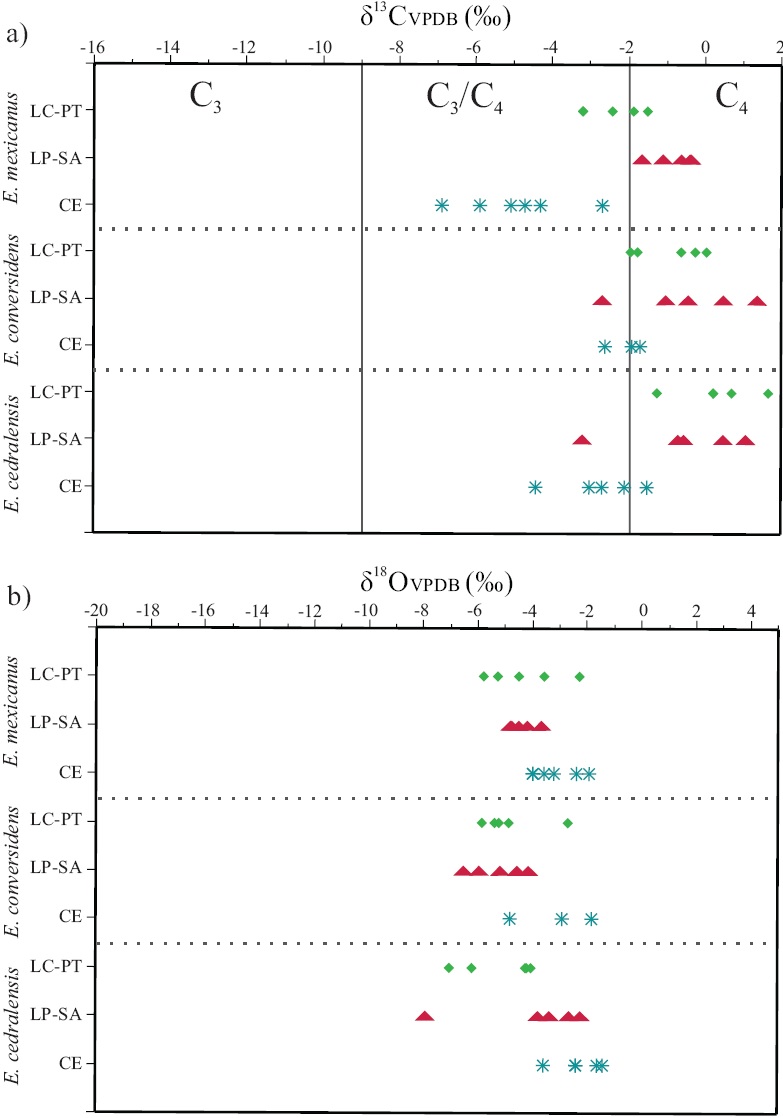
Figure 4 Comparison between individual values of δ13C (a) and δ18O (b) for the species under study and the population from Cedral (Pérez-Crespo et al., 2009). species abbreviations: Equus cedralensis = E.ce; E. conversidens = E.co; E. mexicanus = E.mx. Site abbre^ations: La Cinta-Portalitos = LC-PT; La Piedad-Santa Ana =LP-SA; Cedral = CE. Symbols: diamonds = LC-PT; triangles = LP-SA; asterisks = CE. Values are expressed in ‰ VPDB.
Analysis of variance (ANOVA) showed significant differences between δ13C values of the three species from the two Mexican west-central localities (degrees of freedom: 5; F ratio: 3.1390, ρ > F: 0.0255). The Tukey-Kramer HSD paired comparisons indicated significant differences for the populations of Equus cedralensis and E. mexicanus from LC-PT, whereas the rest of the populations presented similar values for both species with no statistical difference (Figure 4a).
The ANOVA (degrees of freedom: 8; F Ratio: 10.3935, p > F: 0.0001) including conspecifìc populations from the site in north-central Mexico (Cedral, San Luis Potosí) and the paired comparisons made using Tukey-Kramer HSD, showed that one of the populations of E. cedralensis from LC-PT had significantly different values of δ13C relative to the population of the same species from Cedral. The populations of E. conversidens from west-central and north-central Mexico have similar mean values of δ13C, but are significantly different from populations of E. cedralensis and E. mexicanus from Cedral. The two popdations of E. mexicanus from west-central Mexico are significantly different in δ13с values from the conspecifìc population from north-central Mexico (Figure 4a).
Analysis of δ18O values
In Table 3 we show the mean, standard deviation, maximum, minimum, range, variance, standard error, and lower and upper confidence intervals of δ18O values. The individual data values are available in Table SI of the supplementary material.
Table 3 Summary of values for δ18O expressed in ‰ VPDB.
| Species | Site | N | Mean | SD | Min | Max | Range | Variance | Std Err | L CI | U CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. cedralensis | LC-PT | 5 | -5.14 | 1.40 | -7.05 | -4.02 | 3.04 | 1.96 | 0.63 | -6.88 | -3.40 |
| E. conversidens | LC-PT | 5 | -4.80 | 1.24 | -5.85 | -2.68 | 3.17 | 1.54 | 0.55 | -6.34 | -3.26 |
| E. mexicanus | LC-PT | 5 | -4.28 | 1.40 | -5.77 | -2.28 | 3.50 | 1.96 | 0.63 | -6.02 | -2.54 |
| E. cedralensis | LP-SA | 5 | -4.03 | 2.27 | -7.94 | -2.28 | 5.66 | 5.14 | 1.01 | -6.85 | -1.22 |
| E. conversidens | LP-SA | 5 | -5.33 | 0.97 | -6.58 | -4.22 | 2.36 | 0.94 | 0.43 | -6.53 | -4.13 |
| E. mexicanus | LP-SA | 5 | -4.44 | 0.47 | -4.84 | -3.71 | 1.13 | 0.22 | 0.21 | -5.02 | -3.85 |
Notes and abbreviations: LC-PT = La Cinta-Portalitos; LP-SA = La Piedad-Santa Ana; N = sample number; SD= standard deviation; Min = minimum; Max = maximum; Ran = range; Std Err = standard error; L CI = lower confidence interval; U CI = upper confidence interval. Values are expressed in ‰ VPDB.
The mean δ18O value for E. cedralensis from LC-PT 18-5.14‰ and from LP-SA is -4.03 ‰· The mean δ18O value for the populations of E. conversidens from LC-PT is -4.80 ‰, and from LP-SA is -5.33 ‰. For the populations of E. mexicanus, mean δ18O values of -4.28 ‰ for LC-PT, and -4.44 700 for LP-SA were obtained (Figure 4b).
No significant differences in δ18O values were found between populations of E. cedralensis, E. conversidens, and E. mexicanus from the two Mexican west-central localities (ANOVA: degrees of freedom: 5; F Ratio: 0.6590,p > F: 0.6578). The paired comparisons with Tukey-Kramer HSD indicated that the populations of these three species have similar mean values of δ18O (Figure 4b).
The ANOVA (degrees offreedom: 8; F Ratio: 3.0280, p > F: 0.0108) including the same species populations from the site in north-central Mexico (Cedral, SLP) and the paired comparisons made using Tukey-Kramer HSD, showed that the populations of E. cedralensis from LC-PT and E. conversidens from LP-SA had a significant difference in the values of δ18O with the population of E. cedralensis and E. conver-sidens from Cedral. The rest of the species and populations presented similar values of δ18O (Figure 4b).
DISCUSSION
δ13с and diet
The mean δ13C values of the two populations of small-sized slender horse species Equus cedralensis from west-central Mexico indicate that they had similar diets, mostly composed of c4 plants. The population from LC-PT had a diet with a high consumption of C4 plants (85.49 %); in fact, this population ingested the highest levels of C4 plants of all analyzed taxa. The population from LP-SA also had a diet with a high consumption of C4 plants (79.28 %), with the exception of one individual recording a lower consumption of C4 plants (61.77 %), which could indicate a tendency to a mixed feeder diet (C3/C4). In comparison with the populations under study, the populations of E. cedralensis from Cedral exhibit a mixed feeder diet with consume tendency to C4 plants (Pérez-Crespo et al., 2009), indicating that in the north-central locality this species consumed a different percentage of C3 (34.94 %) and c4 (65.06%) plants.
For the medium-sized, robust species Equus conversidens, the two populations from west-central Mexico had similar diets, mostly composed by c4 plants. The population from LC-PT had a diet with a high consumption of C4 plants (77.27 %). The population from LP-SA also had a diet with a high consumption of C4 plants (80.04 %), with the exception of one individual that ate 65.23 % of C4 plants, thus showing a tendency to a mixed feeding diet (C3/C4). The comparison of the study populations with the one from Cedral analyzed by Pérez-Crespo et al. (2009) showed that in both regions E. conversidens had a diet mainly composed by C4 plants; however, the diet of populations from Cedral included more C3 plants, which also evidences the capacity of this species to ingest C3 and C4 plants.
In the case of the large and robust horse species E. mexicanus, the populations from west-central Mexico had a different type of diet. The population from LC-PT had a C3/C4 mixed feeder diet with tendency to consume C4 plants, with 32.99 % ingestion of C3 plants and 67.01 % of C4 plants, while the population from LP-SA had a diet with a higher consumption of C4 plants of 77.57 %. This difference in diet could be explained by a more eclectic dietary behavior, different environmental conditions for each site, or that horse specimens of one locality had different radiometric age than those from the other, and other possible explanations. The comparisons of the populations under study with that from Cedral indicate that E. mexicanus may have had different dietary behaviors within its geographic distribution. In LP-SA the species was mainly a C4 feeder, in LC-PT it was а С3/C4 mixed feeder with a tendency to a C4 diet, and in Cedral it was а С3/C4 mixed feeder with 50 % consumption of each carbon-type plants; this could indicate an eclectic dietary behavior in the consumption of plants of E. mexicanus related with the vegetation available in the region they occupied.
The different feeding styles observed in sites from west-central and north-central Mexico (mainly composed by C4,C3/C4 with a tendency to C4) and 50 % C3/C4) indicates that, during the Late Pleistocene, E. mexicanus was the horse species with the greatest diet diversity and more eclectic dietary behavior. Similarly, the horse with the second most variable diet observed in the three sites was E. cedralensis, with a diet mainly composed by C4 plants in both west-central sites, and a C3/C4 diet in the north-central Mexico site. Finally the horse with the more homogenous dietary behavior observed in both regions was E. conversidens with a diet mainly composed of C4 plants.
Our results agree with previous reports stating that not all Late Pleistocene Mexican horses from these areas were exclusive C4 plant eaters, because these species can ingest plant material having different levels of abrasiveness (i.e., from trees, shrubs and grasses) (Barrón-Ortiz et al., 2014; Marín-Leyva et al, 2016), and different hypotheses were proposed to explain that dietary heterogeneity: 1) Dietary variability in some species like E. mexicanus and E. cedralensis related to an eclectic dietary behavior observed in other Equus populations by MacFadden et al (1999), Perez-Crespo et al. (2009), and Prado et al. (2011); 2) Resource partitioning (similar sympatric species compete for resources) like that shown by Koch et al. (1998); and 3) Extant (Hoppe et al, 2004a) and fossil (Kaiser, 2003) horses diet depending upon the type and amount of food available for eating, which would be dependent on environmental conditions.
Resource partitioning in west-central Mexico
Our preliminary results suggest two different diet types in horse species from the LC-PT locality, one with high consumption of C4 plants for E. cedralensis and E. conversidens, and one with consumption of C3/C4 plants for E. mexicanus. We also found a high percentage range of ingestion of C4 plants between populations (for example between Equus cedralensis and Equus mexicanus the range is 18.48 %). This could evidence resource partitioning between horse species in order to avoid competition, which would be supported by the existence in this area of a more diverse environment providing a more ample diversity of available food sources from either C3 or C4 plants. The latter result is corroborated by the two different dietary behaviors found by Marin-Leyva et al (2016) in the same site using meso- and microwear analysis.
The three horse species in LP-SA showed a diet with a low percentage range of ingestion of C4 plants between populations (for example, between Equus conversidens and Equus mexicanus the range is 2.47 %). Only two individuals, each of E. cedralensis and E. conversidens, showed a diet of mixed C3/C4 feeder (UM 567: 61.67 %C4 and CPOEI 267: 23 %C4); an evidence discarding the existence in the area of resource partitioning between horse species. This could be due to a high abundance of C4 plants permitting the coexistence of the three horse species, an explanation corroborated by the single dietary behavior found in the same locality by Marín-Leyva et al (2016) using meso- and microwear analyses.
δ18O and water source, elevation, and precipitation
On the basis of the mean, range and similarities of δ18O values we found in populations of E. cedralensis, E. conversidens and E. mexicanus from west-central Mexico (Table 1), it can be inferred that these extinct horses drank water from a similar source and presented a similar variability of δ18O as that observed in extant horses (Hoppe 11 2004a, 2005).
In fossil gomphotheres that lived in South America, Sánchez et al. (2004) and Pérez-Crespo et al (2016) observed that δ18O values in enamel decreased at higher altitudes, suggesting that low δ18O values would be characteristic of high elevation areas. Based on the observations of Sánchez et al. (2004), our results would indicate that the δ18O values in the studied horses might correspond to high altitude areas. This relation between low δ18O values and higher altitude is associated to the continuous condensation of atmospheric vapor and rain out of the condensed phase, which takes place when air masses climb up along the slopes of high mountains and cool off as a consequence of adiabatic expansion, causing δ18O enrichment of precipitated water, relative to δ18O values in remainder vapor (Gonfìantini et al., 2001). We however observed some individuals from LP-SA with δ18O values that would correspond to low elevation areas like: CPOEI 252: -2.28 ‰ and CPOEI 265: -2.70 ‰,both identified as E. cedralensis. This could indicate that elevation did not affect the δ18O values in this region, or that individuals with extreme δ18O values were migrants. Another two explanations for this observation would be that we analyzed different moments of mineralization in the teeth, similar to the findings of Pérez-Crespo et al. (2012b) for some populations of Mammuthus columbi from Mexico, or that the dynamics of enrichment and depletion of δ18O is similar in these two localities belonging to the Lerma-Chápala basin, located less than 100 km away, and at a similar elevation (Priego et al., 2003).
The differences found in the mean δ18O values between populations of E. cedralensis and E. conversidens, but not of E. mexicanus, from westcentral and north-central Mexico, indicates that horses were drinking water from isotopically distinct reservoirs and could be explained by different factors affecting the dynamics of enrichment and depletion of δ18O in surface water, such as altitude, latitude, temperature, distance from the sea, rainy seasons, and the effects in precipitation patterns caused by the complex Mexican topography (Sánchez et al., 2004; Metcalfe, 2006; Wassenaar et al., 2009). Another factor that should be taken into account is the amount effect (related to the volume of precipitation). This amount effect may in tropical regions cause that isotopic values of oxygen in rainwater become more negative in the summer, because of the large amount of rainfall during this season both from hurricanes and monsoons (Dansgaard, 1964). Bradbury (1997,2000) noted that, during the Last Glacial Maximum (LGM), the western area of the Trans-Mexican Volcanic Belt experienced increased humidity and precipitation due to the migration to the south of the intertropical convergence zone, as well as to an increased intensity and frequency of northern winds that caused an extensive regime of rains similar to a monsoon (Caballero et al., 2010). Bernal et al. (2011) and Lachniet et al. (2013, 2014), based on δ18O values from stalagmites, demonstrated that during the Late Pleistocene (the last 22 ka) rain water δ18O in western Mexico was modulated by the amount effect, and had a large variability. Those records also support a wet LGM and discard the suggestion of Caballero et al. (2010) that such conditions might have been the result of enhanced westerlies and polar incursions (locally known as "nortes").
Due to the minimal distance between the two west-central sites and their similar altitude above sea level, the variability found in the enamel δ18O values of the west-central populations, in comparison with the north-central horses, could be more related to the amount effect than to the altitude. However, given the lack of datings it is not possible to resolve whether the differences in the oxygen isotopic values observed in horses are due to heavy rains in this area during the Late Pleistocene, or to age differences between horse populations.
Ecomorphology and diet
According to the ecomorphological characteristics of each horse species, E. cedralensis and E. conversidens are assumed to have lived in open environments on compacted soils (Marín-Leyva, 2011; Alberdi et aL, 2014), therefore exhibiting diets with high level of con sumption of C4 plants. This hypothesis is corroborated in all studied horse populations from western-central and north-central Mexico, with the exception of E. cedralensis from Cedral, which exhibited a diet with 35 % C3 plant consumption. Marín-Leyva (2011) and Alberdi et al. (2014) assumed that E. mexicanus lived in more closed environments on un-compacted soil and, therefore, its diet would show an increased ingestion of C3 plants. This hypothesis was corroborated in two populations displaying а С3/C4 mixed diet, one from the west-central locality LC-PT, and another one from north-central Mexico (Cedral). This assumption was, however, not corroborated in the population of E. mexicanus from one of the west-central localities (LP-SA) that displayed a consumption of 77.5 % of C4 plants. The observed ecomorphological and dietary characteristics of the studied horse species could evidence that these ecomorphological characteristics give horses a different degree of capacity for living in different niches, therefore avoiding competition for food resources; however, depending on the horses' needs they could live in different environments and eat different types of plants, like did the populations studied by Hoppe et al (2004a). More research needs to be made on the relation of body mass, limb proportions and diet to arrive to more robust conclusions.
Habitat
Our preliminary results from stable isotopes of 13C and 18O analyses indicate two possible dietary behaviors in LC-PT (C4 grazer and C3/C4mixed feeder), and the individual δ13C values indicate minimal and maximal presence of C4 plants of 61.87 % and 94.52 %, respectively, indicating a mixture in the dietary plant components resulting from a highly heterogeneous environment with open and closed vegetation types containing variable proportions of grasses, herbs, shrubs and trees. The latter assumption is corroborated by the two types of diet found by Marín-Leyva et al (2016), which indicates the presence of vegetation elements with different physical properties. This finding is in accordance with the varied fossil faun al association of the locality and the wide range of feeding styles reflected by the record of forest dwelling browsers such as Odocoileus (Koch et al.,, 1998), grazer taxa adapted to more open areas such as Bison (Koch et al., 2004; Pérez-Crespo et al., 2014), and ecologically intermediate taxa such as Mammuthus columbi, Camelops and Hemiauchenia (Koch et al., 1998; Feranec and MacFadden, 2000; Feranec, 2007; Higgins and MacFadden, 2009; Pérez-Crespo et al., 2012b, 2014; Gutiérrez-Bedolla et al, 2016). Further, the presence of amphibians registered by Pérez-González and Gоdínez-García (2007) suggests the presence of permanent water bodies in the area. The isotopic compositions of the studied fossil horse teeth are also consistent with vegetation data inferred from pollen studies for the site made by Israde-Alcántara et al. (2010b), who found an ample variety of vegetation types and flora, with presence of grasses (Poaceae), herbs, and shrubs (Chenopodiaceae, Amaranthaceae, Asteraceae, Ranunculaceae), and elements from riparian (gallery) forests (Salix sp.). Based on these facts, we deduced that the habitat at LC-PT was highly heterogeneous with a mixture of mixed forests in higher altitude areas, grassland or savanna in the open areas, and wetlands surrounding a paleolake.
In contrast, our preliminary results from LP-SA indicate a drier and more seasonal ecosystem with more open scenarios and a higher diversity of C4 plants in this locality, where the dominant feeding type was c4 grazer, with the exception of two individuals with C3/C4 diets. The individual δ13C values indicates maximal and minimal presences of c4 plants of 61.77 % and 92.26 %, respectively, which is corroborated by the single type of diet found by Marín-Leyva et al. (2016) indicating the abundance of highly abrasive plant components. These ρ aleo environmental inferences are confirmed by the abundance of fossil Bison in the site (Koch et al., 2004; Pérez-Crespo et al. 2014). Certainly, the two individuals with С3/C4 diets that we observed and the reports of other taxa (Odocoileus, Mammuthus columbi, Camelops and Hemiauchenia) in this fossil site (Koch et al., 1998; Feranec and MacFadden, 2000; Feranec, 2007; Pérez-Crespo et al., 2012b, 2014; Gutiérrez-Bedolla et al, 2016) are indicators of the presence of woody vegetation (shrubs and trees). This is compatible with the dietary homogeneity that we found in this site, since Equus species from LP-SA would have been probably restricted to the more open landscapes.
CONCLUSIONS
Oxygen and carbon isotopic analyses of fossil tooth enamel of three species of horses from two localities in west-central Mexico, indicate the presence of two feeding styles in La Cinta-Portalitos: one conformed by E. mexicanus with a diet composed by С3/C4 plants, and another represented by E. conversidens and E. cedralensis with high consumption of C4 plants, while for La Piedad-Santa Ana only one feeding group was observed to be present, which includes the three species (E. mexicanus, E. conversidens and E. cedralensis), with a diet mainly composed by C4 plants.
If our preliminary results are further corroborated, the horse species with the greatest diet diversity and more eclectic behavior during the Late Pleistocene in the west-central and north-central areas of Mexico was E. mexicanus, followed by E. cedralensis, and E. conver-sidens. This indicates that horses were not exclusive C4 plants eaters, likely due to dietary variability, resource partitioning, or type and amount of available food depending upon the environment.
The two feeding types in La Cinta-Portalitos could evidence some resource partitioning and environment diversity in this area, while for La Piedad-Santa Ana the presence of only one feeding type would indicate absence of resource partitioning and the abundance of a single type of food plant.
We found that the δ18O values for some species could vary among regions, whereas values for other species can be the similar within a region, and assumed this to be due to the dynamics of enrichment and depletion of δ18O in surface water in Mexico, a process that can be influenced by different geographic factors, the migratory movements of animals during their life, and the age at which the teeth are mineralized.
The ecomorphological characteristics of some horse populations could predict the type of diet; however those predictions are not true for other populations, indicating that relationships between body mass, limb proportions and diet are more complex, and that more research is needed to reach to more robust conclusions.
Using the type of diet and the associated taxa found in each locality as proxies for habitats, we found that La Cinta-Portalitos locality had a high habitat heterogeneity with forest in the higher areas, grassland or savanna in lower, open areas, and wetlands close to a lake. In contrast, La Piedad-Santa Ana was a more open scenario with a higher diversity of C4 plants and presence of woody vegetation including shrubs and trees.











 nueva página del texto (beta)
nueva página del texto (beta)