Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias geológicas
versión On-line ISSN 2007-2902versión impresa ISSN 1026-8774
Rev. mex. cienc. geol vol.30 no.2 Ciudad de México ago. 2013
Impact of a domestic wastewater treatment plant on groundwater pollution, north Jordan
Impacto de una planta de tratamiento de aguas residuales domésticas en la contaminación de agua subterránea, norte de Jordania
Mutewekil M. Obeidat1*, Muheeb Awawdeh2, and Hussein Al-Mughaid3
1 Department of Humanities, Faculty of Science and Arts, Jordan University of Science and Technology, P.O. Box 3030, 22110 Irbid, Jordan. * mobeidat@just.edu.jo
2 Department of Geography and Geographic Information Systems, King Abdulaziz University, Jeddah, Saudi Arabia.
3 Department of Applied Chemistry, Faculty of Science and Arts, Jordan University of Science and Technology, P.O. Box 3030, 22110 Irbid, Jordan.
Manuscript received: June, 4, 2012
Corrected manuscript received: January 10, 2013
Manuscript accepted: January 13, 2013
ABSTRACT
An assessment of groundwater pollution in the area surrounding a domestic wastewater treatment plant in northern Jordan has been carried out. Groundwater in the study area is classified as alkaline earth water with increased portions of alkalis and prevailing chloride, tending to shift to alkaline water with the dominance of chloride. This trend indicates mixing between the two end members of fresh Ca2+-HCO3- water and saline Na+-Cl- water. Spatially, the highest concentrations of the hydrochemical parameters were found in close proximity of the Al Ramtha Wastewater Treatment Plant (RWWTP). Nitrate, which is the most common human-introduced pollutant into groundwater resources, was used to evaluate pollution of phreatic groundwater in the study area. Its concentration (as NO3-) ranges between 1 mg/L and 366 mg/L, with an average of 79 mg/L. A total of 71% of the samples present nitrate concentrations exceeding the threshold value for anthropogenic sources (20 mg/L), and more than 50% in excess of World Health Organization (WHO) standards for drinking water (50 mg/L). The most importantfactors affecting the magnitude of ground water pollution are depth to groundwater table, aquifer transmissivity (hydraulic conductivity), lineaments density, and distance from treatment plant with calculated correlation coefficients of -0.51, 0.65, 0.70, and -0.75 to nitrate concentration, respectively.
Key words: groundwater, pollution, wastewater, nitrate, threshold value, WHO standards, Jordan.
RESUMEN
Se llevó a cabo una evaluación de la contaminación del acuífero en el área que rodea una planta de tratamiento de aguas residuales domésticas en el norte de Jordania. El agua de este acuífero se clasifica como alcalina-térrea con proporciones de álcalis que se incrementan, prevaleciendo los cloruros, y con tendencia a cambiar a agua alcalina con predominio de cloruros. Esta tendencia indica una mezcla entre dos miembros de agua Ca2+-HCOf y agua salina de Na+-Cl-. Espacialmente, las concentraciones más altas de los parámetros hidroquímicos se encontraron en las cercanías de la planta de tratamiento de aguas residuales Al Ramtha (RWWTP, por sus siglas en inglés). El nitrato, que es el contaminante más común introducido por el hombre en los recursos acuíferos, fue usado para evaluar la contaminación del acuífero en el área de estudio. Su concentración (como NO3-) varía entre 1 mg/L y 366 mg/L, con un promedio de 79 mg/L. Setenta y un porciento de las muestras presenta concentraciones que exceden el valor de umbral para fuentes antropogénicas (20 mg/L), y más del 50% excede los estándares para agua potable (50 mg/L) según la Organización Mundial de la Salud (OMS). Los factores más importantes que afectan la magnitud de la contaminación de los acuíferos son la profundidad del nivel freático, la transmisividad del acuífero (conductividad hidráulica), densidad de alineamientos y distancia de la planta de tratamiento, con coeficientes calculados de correlación con la concentración de nitratos de -0.51, 0.65, 0.70y -0.75, respectivamente.
Palabras clave: agua subterránea, contaminación, aguas residuales, nitratos, valor de umbral, estándares OMS, Jordania.
INTRODUCTION
The geochemical processes help understanding changes in water quality due to water-rock interactions and anthropogenic influences (Helena et al, 1999). The geochemical properties of groundwater depend also on the chemistry of recharge water as well as geochemical processes occurring in the subsurface (Kumar et al., 2006). Geochemical processes are responsible for the seasonal and spatial variation in groundwater quality (Matthess, 1982). Groundwater chemistry evolution is caused by interaction with aquifer material or mixing of groundwater along flow paths (Toth, 1984; Locsey and Cox, 2003). Poor water quality has adverse effects on humans as well as other forms of life (WHO, 1993). Recently, there has been a global tendency of groundwater quality deterioration due to human-induced contamination (Bathrellos et al, 2008; Ma et al, 2009; Rouabhia et al, 2009). The most intensive contamination occurs in rapidly urbanized areas, where intensive exploitation of groundwater for industrial and domestic applications takes place, ultimately leading to a high downward gradient. These conditions may fasten the migration of surface contaminants to the aquifer (Jeong, 2001). Even in areas where overpumping of groundwater does not take place, contamination occurs where a natural downward water gradient exists. This is particularly the case in long-term sources of contamination influence, which dates back as far as previous centuries, such as the influence of unsewered urban and rural areas (Dragon, 2008). Nitrate is the most frequently introduced pollutant into groundwater systems (Spalding and Exner, 1993; Babiker et al., 2004). The adverse health effects of high nitrate levels in drinking water are well-known such as methemoglobinemia, gastric cancer, and non-Hodgkin's lymphoma (Ward et al., 1994; Fan and Steinberg, 1996; Knobeloch et al, 2000). Groundwater with nitrate concentration exceeding the threshold of 20 mg/L is considered contaminated as result of human activities (Spalding and Exner, 1993). According to the World Health Organization (WHO, 1993), the maximum acceptable nitrate concentration ( as NO3-) for drinking water is 50 mg/L. Nitrate contamination has been commonly linked to agricultural activities and the use of fertilizers. However, non-agricultural activities can contribute more nitrates to aquifers, especially those underlying urban areas (Wakida and Lerner, 2005). Potential sources of nitrate in groundwater include: fertilizers, septic tank effluent, municipal sewage, animal feedlots, decaying vegetation, and atmospheric deposition (Spalding and Exner, 1993; Wilhelm et al., 1996). Once nitrate reaches the groundwater, it migrates through advection and dispersion (Almasri, 2007). In the zone of saturation, nitrate undergoes, mostly, denitrification, depending on the properties and prevailing conditions (Tesoriero et al., 2000; Shamrukh et al., 2001). A nitrate fate and transport model in groundwater (NFTM) can be developed and used, in conjunction with a soil nitrogen model, to simulate the effectiveness of current and future agricultural practices and/or other management options to control nitrate occurrences in groundwater (Mercado, 1976; Ling and El-Kadi, 1998; Kyllmar et al., 2004; Almasri, 2007).
Groundwater is the major source of water in Jordan for different purposes, including domestic, agricultural, and industrial. However, because of the rapid development of living standards, high population growth, and massive migrations in the region, the demand of water has increased dramatically. This has led to depletion of groundwater resources and saltwater intrusion deteriorating its quality (El-Naqa and Al-Shayeb, 2009; MWI, 2001). The problem is intensified by the fact that Jordan is a water-poor country. The present study was initiated to assess the quality of B4 aquifer which forms the upper most aquifer of the study area. More specifically, the study is focused on assessing: (1) groundwater pollution in the vicinity of Al Ramtha Wastewater Treatment Plant (RWWTP) using pollution indicators, mainly nitrate, and (2) the impacts of RWWTP on the underlying groundwater quality.
DESCRIPTION OF THE STUDY AREA
Geological and hydrogeological setting
The study area is a sub-basin of the jordanian Yarmouk river basin, located in northern Jordan, between 590967.7-621161.3 N and 393638.3-414127.7 E (JTM, Jordan Transverse Mercator; Figure 1). Table 1 shows the lithostratigraphic units in the study area (WAJ, 1989). Geologically, Wadi Shallala Formation (B5) of early Middle-early Late Eocene age and Umm Rijam Formation (B4) of Paleocene age underlay the study area (Figure 2). The former consists of chalk, chalky limestone and marl with chert intercalations, and crops out in Kharja and north Saham areas. Umm Rijam Formation consists of alternations of limestone, chalk, and chert. It crops out in the Ramtha area (study area), northern Irbid, and Yarmouk river. Wadi Shallala and Umm Rijam formations underlie a basalt at the Mzereeb discharge area in Syria (WAJ, 1989). The subsurface lithology comprises Mesozoic units with two important formations: Wadi As Sir Formation (A7) of Turonian age, and Amman Formation (B2) from Santonian-Campanian period. The former one consists of limestone and dolomite with chert nodules. The later is built up of chert, marl, limestone, tripoli, and phosphatic chert and limestone. Due to their hydraulic interconnection, the two formations (B2 and A7) are considered as one aquifer in the study area and throughout Jordan with a significant groundwater potential. The Amman-Wadi As aquifer forms the middle aquifer system in the study area. The Muwaqqar Formation (B3, aquiclude/aquitard) of Maastrichtian age separates the upper aquifer system (B5/B4) from the middle aquifer system (B2/A7). The Muwaqqar Formation consists of bituminous marl and marly limestone, and has a thickness between 300 and 360 m in the study area. The lower aquifer system involves Paleozoic rocks and consists of sandy rocks, mainly Disi sandstone aquifer which is currently exploited in south Jordan. In the study area, the upper aquifer system consists of Umm Rijam aquifer, where the groundwater is stored under phreatic conditions. The aquifer (B4) is highly fractured and characterized by cavernous and karstic features. Figure 2 shows the hydrogeological setting in northern Jordan. The B5/B4 aquifer has a hydraulic conductivity in the range of 10-4 and 10-6 m/s with an average 5x10-5 m/s, and the B2/A7 aquifer has a hydraulic conductivity in the range of 10-3 and 10-7 m/s with an average of 2x10-5 m/s (Margane et al., 1999).
Climate and land use
The climate in the study area is of Mediterranean type, which is characterized by a cool, rainy winter, and a hot, dry summer. The average annual rainfall (1976-2002) at Al Ramtha rainfall station was 213.8 mm. The mean annual minimum and maximum temperatures are 10.7 °C and 23.7 °C, respectively. Land use/land cover (LULC) in the study area was mapped using Landsat satellite images and supervised classification with ENVI software. As shown in Figure 3, the predominant land use/land cover in the study area is agricultural (45.6%) followed by rangeland (31.6%), urban (12.8), bare land and forest (5% each).
Al Ramtha wastewater treatment plant (RWWTP)
RWWTP was established in 1988 about 4 km northwest of Al Ramtha city. The plant was operated as stabilization ponds. In 2003, it was converted to activated sludge as treatment option with a hydraulic design capacity of 5400 m3/day and operating capacity of 3492 m3/day. The average treated wastewater for the years 1991-2003 was 1916 m3/day (Figure 4). The plant was built up by a sequence of anaerobic ponds, successive facultative lagoons, and final maturation ponds, three of them each. The average BOD5 (five-day biological oxygen demand) from March 2000 to October 2001 (organic load) of the influent was 861.3 mg/L, and the average BOD5 of the effluent for the same period was 231 mg/L, with only about 73% treatment efficiency (Table 2). Based on the BOD5 content, the effluent quality before plant upgrading does not comply withjordanian standards of reclaimed wastewater. Based on the total suspended solids content (TSS), the influent of the plant can be classified as strong (UNDOTCFD, 1985). The descriptive statistics of the effluent and influent quality of plant after conversion to activated sludge are compiled in Table 2. The average BOD5 of the influent and effluent is 956.6 and 12.5 mg/L, respectively with a removal efficiency of 98.7%, indicating that the quality of the treated wastewater was highly improved after the conversion of the plant, and that the effluent quality after plant upgrading complies with the jordanian standards of reclaimed wastewater. The effluent water is used to irrigate clover in the area surrounding the plant.


MATERIAL AND METHODS
The methods described by APHA (1998) were followed during field and laboratory work. Three hundred and eleven samples were analyzed in the present study, representing 12 wells tapping the upper aquifer system (B4) and one spring emerging from the same aquifer (Figure 1). The samples involve those taken by the authors during the present study in November 2009 and analyzed samples retrieved from the Water Authority of Jordan (WAJ, 2008). Electrical conductivity (EC), temperature and pH were measured in situ using portable devices. Prior to sample collection, well purging was performed for those wells which were not pumped at the time of sampling. The goal was to ensure that the water sample truly represents the properties and conditions of the subsurface environment. Water was pumped from the well until the temperature, EC and pH became constant. The collected samples were analyzed for major cations and anions, total hardness (TH), and total dissolved solids (TDS). Concentrations of Na+ and K+ were determined by using flame photometer. As for Ca2+, Mg2+, HCO3- and Cl- concentrations, these were determined by volumetric titration method, and SO42-and NO3- spectro-photometrically. The total dissolved solids (TDS) content was calculated using the following equation (APHA, 1998):

The total hardness (TH) was calculated according to the following equation (Todd, 1980):
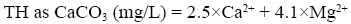
Samples with an error of less than 5% in the cation-anion balance were exclusively used for interpretation. Additionally, influent and effluent quality of RWWTP was taken from the open files of WAJ. Statistical analysis was carried out using SPSS 13.0 for Windows (Gerber and Finn, 2005).
RESULTS
Hydrochemical composition
The univariate statistics of the hydrochemical parameters of the Umm Rijam (B4) groundwater in the study area and WHO (1993) standards are compiled in Table 3, and averages of hydrochemical parameters for sampling point are presented in Table 4. The moderate to high variability (standard deviation and coefficient of variation) of the parameters indicates spatial and temporal variation in groundwater quality for the study area. The highest coefficient of variation (CV) was found for K+ and NO3- with CV above 1, followed by Cl- and SO42-with CV above 0.5. Electrical conductivity ranges between 550 and 3200 μS/cm with an average of 1364 uS/cm. The lowest value was found in March 1989 for well AD1210, and the highest value was recorded in July 1995 for well AD1296. TDS is in the range of 393-1669 mg/L with an average of about 707 mg/L. Based on the classification of Davis and DeWiest (1967), about 33% of the samples is classified as brackish water. About 33% percent of the samples have a TDS content in excess of WHO standards. TH ranges between 190 and 648.7 mg/L with an average of 357.3 mg/L. Calcium and magnesium are responsible for water hardness; hence, groundwater in the study area was categorized based on the classification of Sawyer and McCarty (1967), where about 51% of the samples is classified as hard, and about 49% very hard. More than 13% of the samples exceed WHO standards. Sodium concentration varies between 31 and 306.6 mg/L with an average of 140.7 mg/L. About 24% of the analyzed samples shows sodium concentration exceeding the maximum permissible limit set by WHO standards. Chloride concentration varies from about 32 to 909 mg/L with an average of 240 mg/L. More than 41% and 3% of the samples have chloride and bicarbonate concentrations, respectively, in excess of the WHO standards. The wide range of sulfate and nitrate concentrations (13—414 mg/L and 1-366 mg/L, respectively) indicates point source of pollution (Kumar et al., 2009). Lowest concentrations were reported for the wells AD3000, AD3008, and AD3045 in the northern part of the study area. The highest concentration was reported for well AD1296, in close proximity of the RWWTP. About 71% of the samples has nitrate concentration more than 20 mg/L, the threshold value of anthropogenic source (human affected value, HAV), and more than 50% of the samples present nitrate concentrations exceeding the WHO standards of 50 mg/L.
Groundwater classification
The hydrochemical parameters of the B4 groundwater are presented graphically using Piper (Langguth, 1966), and Durov (1948) diagrams. The trilinear presentation (Piper diagram) enables it to classify the groundwater into the following groups (Figure 5):
1. Alkaline earth water with increased portion of alkalies with prevailing chloride. The vast majority of the samples belongs to this group. This group can be related to the fourth group of Rimawi and Udluft (1985), which represents fresh-brackish water of non-contemporary and almost ancient recharge, ascending from deep aquifers along major faults, or it corresponds to fresh water mixed with saline water or fresh water which has passed through evaporates. However, five wells (AD1208, AD1209, AD1213, AD1296, and AD3028) tend to shift towards the second group. This indicates mixing between two end members: one fresh Ca+2-HCO3- water and one saline Na+-Cl- water (Panagopoulos et al., 2005)
2. Alkaline water with prevailing chloride (AD1210, AD1251)
3. Alkaline earth water with increased portion of alkalies with prevailing hydrogen carbonate (AD3000)
The hydrochemical parameters were plotted using Durov diagram (Lloyd and Heathcote, 1985) (Figure 5) whereby groundwater from the study area falls in Fields 5 and 6, indicating mixing between raw fresh groundwater and polluting treated/untreated domestic wastewater.
In this study, Pearson correlation coefficients for hy-drochemical parameters of B4 groundwater were calculated to correlate variables (Table 5). According to Giridharan et al. (2008), geochemical parameters showing correlation coefficient >0.7 are considered to be strongly correlated whereas coefficients between 0.5 and 0.7 show moderate correlation. A strong correlation exists between electrical conductivity and Na+, Ca2+, Cl-, NO3-, and K+, indicating that these parameters play an important role in the salinization of groundwater in the study area. Nitrate concentration is strongly correlated with electrical conductivity (r = 0.84) indicating a common source. According to the classification from Douglas and Leo (1977), the correlation of parameters in the study area can be summarized as follows:

Highly competitive ions relationship: Ca2+ with Na+ and K+ has high positive correlations; Cl- with SO42- low positive correlation; SO42- with HCO3- and NO3- have low positive correlation.
Affinity ions relationship: Na+ with Cl- and NO3-; K+ with NO3- have high positive correlations.
None-competitive ions relationship: Ca2+ with Mg2+; Na+ with K+; Cl- with NO3- and HCO3- have moderate positive correlations.
Cluster analysis
Cluster analysis allows the grouping of groundwater samples on the basis of their similarities in chemical composition. This procedure attempts to identify relatively homogeneous groups of cases based on selected characteristics, using an algorithm that can handle large numbers of cases. The hydrochemical parameters in the study area were subjected to k-mean cluster analysis, which has resulted in three clusters. The final cluster centers of the hydrochemical parameters are presented in Table 6. Cluster 1 involves only one sample (AD1296) with an average nitrate concentration of 193 mg/L, exceeding WHO standards for drinking water. Cluster 2 comprises 46.1% of the samples with average nitrate concentration of 69 mg/L, exceeding WHO standards. Cluster 1 and 2 can be classified as brackish water. Cluster 3 comprises 46.1% of the samples with average nitrate concentration less than WHO standards but more than the threshold value for anthropogenic sources, and with lowest TDS (freshwater). The regional distribution of the three clusters is presented in Figure 6. Clusters 1 and 2 are found in the area surrounding RWWTP, whereas cluster 3 is mainly located in the northern part of the study area.

The three clusters and the effluent of RWWTP were plotted using a compositional diagram (Figure 7). The straight line, which extrapolates to zero, indicates mixing between two end members: treated wastewater (saline water) and freshwater (cluster 3), which has ultimately evolved to clusters 1 and 2 due to long-term impact of the treatment plant. The Piper diagram reveals that the three clusters are: alkaline earth water with increased portion of alkalies with prevailing chloride. However, clusters 1 and 2 tend to shift towards an alkaline water type with prevailing chloride.

Temporal fluctuation of groundwater quality
Global studies have shown that changes in groundwater quality depend on several factors such as water-interaction, residence time of groundwater, seepage of polluted river water, mixing of groundwater with pockets of saline water, and human activities (Giridharan et al., 2008; Umar et al. 2006). Fluctuations in hydrochemical concentrations in the study area indicate seasonal changes in groundwater quality. High concentrations were reported during summer season (june, july), and low concentrations during rainy season (january, february, march). This can be attributed to the dilution effects by recharging water. Moreover, the influent quality of RWWTP shows seasonal fluctuations. The TDS of the influent in winter varies from 916 to 1176 mg/L, whereas from 1068 to 2068 mg/L in summer. Figure 8 shows the temporal variation of electrical conductivity and nitrate for well AD1251 from 1985-2008. This well is located at a distance of less than 2 km downstream of the RWWTP. Electrical conductivity and nitrate concentration increased from 900 fiS/cm and 34 mg/L in February 1985 to 1140 μS/cm and 44.5 mg/L in June 1987, respectively. Both increased from 1260 uS/cm and 59.3 mg/L in October 1988 to 2420 μS/cm and 149 mg/L in September 2002, and decreased to 1760 μS/cm and 122 mg/L in March 2004, followed by a constant period. This can be attributed to the upgrading of RWWTP, which was operated as stabilization ponds before 2003 with low treatment efficiency. The results of the performed ANOVA test (Gerber and Finn, 2005) for AD1251 well indicate significant differences between nitrate concentration before and after the construction of RWWTP. The calculated F-value (12.92948) is greater than critical F (4.078546) at a degree of freedom of 42, and the p value of 0.000861 is less than 0.05. A similar constellation can be observed for the electrical conductivity. Total coliform and Escherichia coli (E. coli) values of 1600 and 500 MPN/100 ml were reported for those wells located close to the RWWTP, respectively. Total coliform bacteria (excluding E. coli) occur in both sewage and natural waters, and some of these bacteria are excreted in humans and animals feces. E. coli occurs in high numbers in human and animal feces, sewage and water subject to recent faecal pollution (WHO, 2008).
Spatial variation of groundwater quality
The spatial distributions of EC, Cl-, and NO3- follow a single pattern with highest values for those wells located close to RWWTP (Figure 9). Nitrate concentration in the study area was correlated with aquifer transmissivity (r = 0.65), and depth to water table (-0.51). High concentration of nitrate is found in the area surrounding RWWTP, where aquifer transmissivity is more than 320 m2/day, and depth to water table is less than 20 m below ground surface; this increases aquifer's vulnerability to contamination. A study carried out by Changyuan et al. (2004) on the effects of wastewater irrigation on nitrate in groundwater in the North China plain, found that most groundwater wells with a depth of less than 40 m have a nitrate concentration of more than 50 mg/L. Enwright and Hudak (2009) found a moderate correlation (r = -0.392) between nitrate concentration and well depth in the High Plains aquifer, Texas.
Lineaments refer to linear features detected on aerial photographs and satellite images, which presumably have a geological origin (Campbell, 1996). Nitrate concentrations in the study area were correlated with lineaments density (Figure 10), as lineaments are closely related to groundwater flow and contaminants migration. The higher the lineaments density, the more vulnerable is the groundwater to contamination (Mabee et al., 1994). A strong correlation (r = 0.70) exists between lineaments density and nitrate concentration. The pollution plume decreases away from the plant, towards the north, south, and east. A significant correlation exists between nitrate concentration and distance from the treatment plant (r = -0.75). Jakhrani et al. (2009) found a significant correlation (r = -0.74) between distance and nitrate concentration for a treatment plant, near Hyderabad city.
The Amman-Wadi As Sir (B2/A7) aquifer is currently undergoing overpumping, and it contains low nitrate levels (Obeidat et al., 2012). Though, leaky conditions through B3 aquitard could take place, and ultimately contaminating the B2/A7 groundwater. There are many potential sources of groundwater salinity such as halite dissolution, retention or reflux of evaporatively concentrated seawater, shale membrane filtration, and/or evaporation of no-marine fluids (Grobe and Machel, 2002), and each source has distinct chemical characteristics and well-known ionic ratio (Kumar et al., 2009). Improper treatment and disposal of domestic wastewater could be one of the major sources of salinization in the aquifer (Metcalfe and Eddy, 2000). Irrigation with wastewater, which is generally more saline than regional groundwater, increases the rate of salinization of shallow groundwater (Kass et al., 2005). The results of saturation index calculations show that the groundwater in the study area is generally equilibrated to slightly oversaturated with respect to calcite, aragonite, and dolomite, indicating that these minerals are important controls on water chemistry (Table 3). Moreover, human activities played an important role in the evolution of the groundwater quality in the study area.
CONCLUSIONS
Hydrogeological, statistical and graphical approaches have shown that Al Ramtha wastewater treatment plant (RWWTP) is the major source of groundwater contamination in the Al Ramtha area, northern Jordan. High levels of nitrate were found in those wells located in close proximity of the treatment plant with more than 50% of the studied samples having concentration above the maximum permissible limit of WHO drinking water quality standards (50 mg/L). Depth to groundwater, hydraulic conductivity, distance from the plant, and lineaments density dictate the aquifer's vulnerability to contamination. Hydrochemical analysis has revealed that groundwater in the study area has a fresh-brackish, and hard to very hard composition. Groundwater chemistry has evolved from Ca-HCO3 fresh water type to Na-Cl brackish water type, due to long-term influences of human activities. Delineating protection zones for groundwater resources in the study area is a very crucial matter, and will help mitigating pollution. Modeling of contaminant transport is an open issue for future research.
ACKNOWLEDGMENTS
The authors are deeply grateful to Dr. P. Birkle, Scientific Editor of the Revista Mexicana de Ciencias Geologicas, Dr. T. Darwish, Lebanon, and one anonymous reviewer for reviewing the manuscript, and their fruitful comments and suggestions.
REFERENCES
Almasri, M.N., 2007, Nitrate contamination of groundwater: a conceptual management framewrok: Environmental Impact Assessment Review, 27, 220-242. [ Links ]
American Public Health Association (APHA), 1998, Standard methods for the examination of water and wastewater: Washington, American Public Health Association, American Water Works Association, Water Environment Federation, 20th ed. [ Links ]
Babiker, I.S., Mohamed, M.A.A., Terao, H., Kato, K., Ohta, K., 2004, Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system: Environmental International, 29, 1009-1017. [ Links ]
Bathrellos, G.D., Skilodimou, H.D., Kelepertsis, A., Alexakis, D., Chrisanthaki, I., Archonti, D., 2008, Environmental research of groundwater in the urban and suburban areas of Attica region, Greece: Environmental Geology, 56, 11-18. [ Links ]
Campbell, J., 1996, Introduction to remote sensing: New York, The Guilford Press, 2nd ed., 622 pp. [ Links ]
Changyuan, T., Jianyao, C., Njun, S., 2004, Long-term effect of wastewater irrigation on nitrate in groundwater in the North China Plain, in Steenvoorden J., Endreny, T. (eds.), Wastewater Re-use and Groundwater Quality Proceedings Symposium HS04: IAHS Publications, Red Book Series, 285, 34-40. [ Links ]
Davis, S.N., DeWiest, J.M., 1967, Hydrogeology: New York, John Wiley and Sons, 463 pp. [ Links ]
Douglas, E.B., Leo, W.N., 1977, Geochemical relationships using partial correlation coefficient: Water Resources Bulletin, 13, 843-846. [ Links ]
Dragon, K., 2008, The influence of anthropogenic contamination on the groundwater chemistry of a semi-confined aquifer (the Wielkopolska Buried Valley Aquifer, Poland): Water Resources Management, 22(3), 343-355. [ Links ]
Durov, S.A., 1948, Natural waters and graphical representation of their composition: Doklady Akademii Nauk, Union of Sovietic Socialist Republics, 59, 87-90. [ Links ]
El-Naqa, A., Al-Shayeb, A., 2009, Groundwater protection and management strategy in Jordan: Water Resources Management, 23, 2379-2394. [ Links ]
Enwright, N., Hudak, P.F., 2009, Spatial distribution of nitrate and related factors in the high Plains aquifer, Texas: Environmental Geology, 58, 1541-1548. [ Links ]
Fan, A.M., Steinberg, V.E., 1996, Health implications of nitrate and nitrite in drinking water: an update on methemoglobinemia occurrence and reproductive and developmental toxicity: Regulatory Toxicology and Pharmacology, 23, 35-43. [ Links ]
Gerber, S., Finn, K.V., 2005, Using SPSS for Windows: Data analysis and Graphics: New York, United States of America, Springer, 2nd ed., 227 pp. [ Links ]
Giridharan, L., Venugopal, T., Jayaprakash M., 2008, Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India: Environmental Monitoring and Assessment, 143, 161-178. [ Links ]
Grobe, M., Machel, H., 2002, Saline groundwater in the Musterland Cretaceous Basin, Germany: clues to its origin and evolution: Petroleum Geology, 19, 307-322. [ Links ]
Helena, B., Vega, M., Barrado, E., Pardo, R., Fernandez, L., 1999, A case of chemical characterization of an alluvial aquifer influenced by human activities: Water, Air, and Soil Pollution, 112, 365-387. [ Links ]
Jakhrani, A.Q., Samo, S.R., Nizam, I., 2009, Impact of wastewater effluents on physico-chemical properties of groundwater: Sindh University Research Journal, Science Series, 41-1, 75-82. [ Links ]
Jeong, C.H., 2001, Effect of land use and urbanization on chemistry and contamination of groundwater from Taejon area, Korea: Journal of Hydrology, 253, 19-210. [ Links ]
Kass, A., Gavriele, I., Yechiele, Y., Vengosh, A., Starinsky, A., 2005, The impact of freshwater and wastewater irrigation on the chemistry of shallow groundwater: a case study from the Israeli Coastal Aquifer: Journal of Hydrology, 300, 314-331. [ Links ]
Knobeloch, L., Salna, B., Hogan, A., Postle, J., Anderson, H., 2000, Blue babies and nitrate-contaminated well water: Environmental Health Perspectives, 108, 7, 675-678. [ Links ]
Kumar, M., Ramanathan, A.L., Rao, M.S., Kumar, B., 2006, Identification and evaluation of geochemical processes in the groundwater environment of Delhi, India: Environmental Geology, 50, 1025-1039. [ Links ]
Kumar, M., Sharma, B., Ramanathan, A.L., Rao, M.S., Kumar, B., 2009, Nutrient chemistry and salinity mapping of the Delhi aquifer, India: source identification perspective: Environmental Geology, 56, 1171-1181. [ Links ]
Kyllmar, K., Martensson, K., Johnsson, H., 2004, Model-based coefficient method for calculation of N leaching from agricultural fields applied to small catchments and the effects of leaching reducing measures: Journal of Hydrology, 304, 1-4, 343-354. [ Links ]
Langguth, H.R., 1966, Grundwasserverhaltnisses im Bereich des Velberter Sattels (Rheinisches Schiefergebirge): Dusseldorf, Deutschland, Minister fur Ernaherung und Gesundheit, 127 pp. [ Links ]
Ling, G., El-Kadi, A., 1998, A lumped parameter model for N transformation in the unsaturated zone: Water Resources Research, 34(2), 203-212. [ Links ]
Lloyd, J.W., Heathcote, J.A., 1985, Natural inorganic chemistry in relation to groundwater: an introduction: Oxford, Clarendon Press, 296 pp. [ Links ]
Locsey, K.L., Cox, M.E., 2003, Statistical and chemical methods to compare basalt- and basement rock-hosted groundwater: Atherton Tablelands, North eastern Australia: Environmental Geology, 43, 698-713. [ Links ]
Ma, J., Ding, Z., Wei, G., Zhao, H., Huang, T., 2009, Sources of water pollution and evolution of water quality in the Wuwei basin of Shiyang river, Northwest China: Journal of Environmental Management, 90, 1168-1177. [ Links ]
Mabee, S., Hardcastle, K., Wise, D., 1994, A method of collecting and analyzing lineaments for regional scale fractured bedrock aquifer studies: Groundwater, 32, 6, 884-894. [ Links ]
Margane, A., Hobler, M., Subah, A., 1999, Mapping of groundwater vulnerability and hazards to groundwater in the Irbid area, N Jordan: Zeitschrift Angewandte Geologie, 45, 175-187. [ Links ]
Matthess, G., 1982, The properties of groundwater: New York, Wiley, 406 pp. [ Links ]
Mercado, A., 1976, Nitrate and chloride pollution of aquifers: a regional study with the aid of a single-cell model: Water Resources Research, 12, 4, 731-747. [ Links ]
Metcalfe and Eddy Inc., 2000, Integrated aquifer management plan: Final Report: Gaza Coastal Aquifer Management Program, USAID, Contract No. 294-C-00-99-00038-00. [ Links ]
Ministry of Water and Irrigation (MWI), 2001, Groundwater management action plan for the Amman-Zarqa Basin highlands: Internal report, 64 pp. [ Links ]
Obeidat, M.M., Awawdeh, M., Abu Al-Rub, F., 2012, Multivariate statistical analysis and environmental isotopes of Amman/Wadi Sir (B2/A7) groundwater, Yarmouk River Basin, Jordan: Hydrological Processes, doi: 10.1002/hyp.9245. [ Links ]
Panagopoulos, G., Lambrakis, N., Katagas, C., Papoulis, D., Tsolis-Katagas, P., 2005, Water-rock interaction induced by contaminated groundwater in a karst aquifer, Greece: Environmental Geology, 49, 300-313. [ Links ]
Rimawi, O., Udluft, P., 1985, Natural water groups and their origin of the shallow aquifers complex in Azraq depression, Jordan: Geologisches Jahrbuch, C38, 17-38. [ Links ]
Rouabhia, A., Fehdi, Ch., Baali, F., Djabri, L., Rouabhi, R., 2009, Impacts of human activities on quality and geochemistry of groundwater in the Merdja area, Tebessa, Algeria: Environmental Geology, 56, 1259-1268. [ Links ]
Sawyer, C.N., McCarty, P.L., 1967, Chemistry and sanitary engineers: New York, McGraw-Hill, 2nd ed., 518 pp. [ Links ]
Shamrukh, M., Corapcioglu, M., Hassona, F., 2001, Modeling the effect of chemical fertilizers on ground water quality in the Nile Valley Aquifer, Egypt: Ground Water, 39, 1, 59-67. [ Links ]
Spalding, R.F., Exner, M.E., 1993, Occurrence of nitrate in groundwater-a review: Journal of Environmental Quality, 22, 392-402. [ Links ]
Tesoriero, A., Liecscher, H., Cox, S., 2000, Mechanism and rate of denitrification in an agricultural watershed: electron and mass balance along ground water flow paths: Water Resources Research, 36, 6, 1545-59. [ Links ]
Todd, D.K., 1980, Groundwater hydrology: New York, Wiley, 552 pp. [ Links ]
Toth, J., 1984, The role of regional gravity flow in the chemical and thermal evolution of groundwater, in Proceedings of the 1 st Canadian/ American Conference on Hydrogeology, Practical Applications of Groundwater Geochemistry: Banff, Alberta, Canada, B. Hitchon, E. Wallick (eds.), Worthington, Ohio, National Water Well Association, 3-39. [ Links ]
Umar, R., Muqtada, M., Khan, A., Absar, A., 2006, Groundwater hydrochemistry of a sugarcane cultivation belt in parts of Muzaffarnagar district, Uttar Pradesh, India: Environmental Geology, 49, 999-1008. [ Links ]
United Nations Department of Technical Cooperation for Development (UNDOTCFD), 1985, in FAO (1992): Wastewater treatment and use in agriculture: FAO Irrigation and Drainage Paper 47. [ Links ]
Wakida, T.F., Lerner, D.N., 2005, Non-agricultural sources of groundwater nitrate: a review and case study: Water Research, 39, 3-16. [ Links ]
Ward, M.H., Zahm, S.H., Blair, A., 1994, Dietary factors and non-Hodgkin's lymphoma in Nebraska (United States): Cancer Causes and Control, 5, 422-432. [ Links ]
Water Authority of Jordan (WAJ), 1989, Yarmouk Basin: Water Resources Study: Amman, Jordan, 222 pp. [ Links ]
Water Authority of Jordan (WAJ), 2008, Open files, Amman, Jordan, Electronic stored analysis. [ Links ]
Wilhelm, S.R., Schiff, S.L., Robertson, W.D., 1996, Biogeochemical evolution of domestic waste water in septic systems: 2. Application of conceptual model in sandy aquifers: Ground Water, 34, 853-864. [ Links ]
World Health Organization (WHO), 1993, Guidelines for drinking-water quality: Geneva, 2nd edition, 1, 188 pp. [ Links ]
World Health Organization (WHO), 2008, Guidelines for drinking water quality: Geneva, 3rd edition, 1, 515 pp. [ Links ]














