Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias geológicas
versión On-line ISSN 2007-2902versión impresa ISSN 1026-8774
Rev. mex. cienc. geol vol.28 no.2 Ciudad de México ago. 2011
Paleobiogeography of trilophodont gomphotheres (Mammalia: Proboscidea). A reconstruction applying DIVA (Dispersion–Vicariance Analysis)
Paleobiogeografía de gonfoterios trilofodontos (Mammalia: Proboscidea). Una reconstrucción por medio de la aplicación de DIVA (análisis de dispersión–vicarianza)
María Teresa Alberdi1,*, José Luis Prado2, Edgardo Ortiz–Jaureguizar3, Paula Posadas3, and Mariano Donato1
1 Departamento de Paleobiología, Museo Nacional de Ciencias Naturales, CSIC, José Gutiérrez Abascal 2, 28006, Madrid, España. E–mail: * malberdi@mncn.csic.es
2 INCUAPA, Departamento de Arqueología, Universidad Nacional del Centro, Del Valle 5737, B7400JWI Olavarría, Argentina.
3 LASBE, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque S/N°, B1900FWA La Plata, Argentina.
Manuscript received: June 28, 2010.
Corrected manuscript received: December 17, 2010.
Manuscript accepted: January 28, 2011.
ABSTRACT
The objective of our paper was to analyze the distributional patterns of trilophodont gomphotheres, applying an event–based biogeographic method. We have attempted to interpret the biogeographical history of trilophodont gomphotheres in the context of the geological evolution of the continents they inhabited during the Cenozoic. To reconstruct this biogeographic history we used DIVA 1.1. This application resulted in an exact solution requiring three vicariant events, and 15 dispersal events, most of them (i.e., 14) occurring at terminal taxa. The single dispersal event at an internal node affected the common ancestor to Sinomastodon plus the clade Cuvieronius – Stegomastodon. A vicariant event took place which resulted in two isolated groups: (1) Amebelodontinae (Africa – Europe – Asia) and (2) Gomphotheriinae (North America). The Amebelodontinae clade was split by a second vicariant event into Archaeobelodon (Africa and Europe), and the ancestors of the remaining genera of the clade (Asia). In contrast, the Gomphotheriinae clade evolved mainly in North America. A dispersal event expanded the range of the common ancestor to Sinomastodon plus the clade Cuvieronius – Stegomastodon to include Asia again. A new vicariant event split North America and Asia resulting in the isolation of Sinomastodon in the latter, and the ancestor of the clade Cuvieronius – Stegomastodon in the former. Finally, these two genera reached South America in two independent dispersal events. This biogeographic history has been driven by sea–level changes. During the low sea–level episodes, trilophodont gomphotheres expanded its geographical distribution by means of dispersion events, and during high sea–level episodes suffered vicariant events.
Key words: sea–level changes, dispersal, event–based biogeography method, DIVA, Cenozoic.
RESUMEN
El objetivo del trabajo fue analizar el modelo de distribución de los gonfoterios trilofodontos, aplicando un método biogeográfico basado en eventos. Se ha tratado de interpretar la historia biogeográfica de los gonfoterios trilofodontos en el contexto de la evolución biológica de los continentes que ellos habitaron durante el Cenozoico. Para reconstruir esta historia biogeográfica se ha utilizado el programa DIVA 1.1. Sus resultados indican tres eventos vicarantes y 15 dispersivos, la mayoría de ellos (i.e., 14) acontecidos en los taxones terminales. El único evento dispersivo en un nodo interno afectó al ancestro común de Sinomastodon más el clado Cuvieronius – Stegomastodon. Un primer evento vicariante dio lugar a dos grupos aislados: (1) Amebelodontinae (África – Europa – Asia) y (2) Gomphotheriinae (Norte América). El clado Amebelodontinae, por un segundo evento, se escindió en Archaeobelodon (África y Europa) y los ancestros de los géneros restantes del clado (Asia). En contraste, el clado Gomphotheriinae evolucionó principalmente en Norte América. Un evento dispersivo expandió el rango del ancestro común de Sinomastodon más el clado Cuvieronius – Stegomastodon para incluir Asia de nuevo. Un nuevo evento vicariante separó a Norte América y Asia, dando lugar al aislamiento de Sinomastodon en Asia, y al del ancestro del clado Cuvieronius – Stegomastodon en Norte América. Finalmente, estos dos géneros alcanzan América del Sur en dos eventos dispersivos independientes. Esta historia biogeográfica ha sido dirigida por los cambios en el nivel del mar. Durante los momentos donde los niveles del mar fueron más bajos los gonfoterios trilofodontos expandieron su distribución geográfica por dispersión, mientras que en los momentos en los que los niveles fueron más altos sufrieron eventos de vicariancia.
Palabras clave: cambios en el nivel del mar, dispersión, método biogeográfico basado en eventos, DIVA, Cenozoico.
INTRODUCTION
Throughout most of the Cenozoic Era, the Proboscidea were among the largest land mammals of the Earth. The earliest known record of a proboscidean, Eritherium azzouzorum, is from the middle Paleocene land–mammal bearing sediments of northern Africa (Gheerbrant, 2009). Most Paleogene proboscideans did not look very "elephant–like", because they were pig–sized and nearly trunk–less and tusk–less. But in the course of their evolution the proboscideans became larger, the trunk became longer, and the tusks and the cheek teeth, became larger (Göhlich, 1999). By the middle Eocene to Oligocene some proboscideans had reached the body size of a modern tapir (e.g., Moeritherium). Others had reached even higher body masses (two tons) and show the typical columnar limbs of modern elephants (e.g., Palaeomastodon, Phiomia, see Shoshani, 1998).
According to Shoshani and Tassy (1996), the Proboscidea may have undergone three major radiation events. The first occurred during the Eocene and Oligocene, and affected the earliest proboscideans (e.g., anthracobunids, moeritheres, and deinotheres); the second occurred during the latest Oligocene and Miocene, and affected gomphotheres and stegodontids; finally, the third occurred from the latest Miocene to the Pleistocene, and affected the Elephantidae. All the taxa of the first radiation, except the American mastodon (Mammut americanum) had vertical tooth displacement, which is the usual method tooth replacement in Mammalia. Mammut americanum and the proboscideans depicted in the second and third radiations had a horizontal tooth displacement, a derived condition in which the size of the mandible is too short to accommodate all the enlarged premolars and molars at once. Proboscideans in the first radiation had low crowned teeth (brachyodont) with three or four plates in the upper third molar, and some taxa still had canine teeth. In the second radiation, upper third molars had up to seven plates and were brachyodont or hypsodont, and in the third radiation they had up to 30 plates and were hypsodont. Proboscideans in the first radiation were mostly browsers, whereas those in the second and third radiations were mostly grazers (Maglio, 1973).
The initial radiation of Elephantimorpha (i.e., Mammutidae and Elephantidae) that replaced the archaic Elephantiformes (i.e., Phiomidae, Paleomastodontidae) was centered in Africa and was primarily an event of the initial Neogene. During this period these proboscideans also expanded out of Africa, reaching all the continents except Australia and Antarctica (Göhlich, 1999; Prado and Alberdi, 2008). Their widespread distributions are probably related to their large body size: elephants require a large geographical range for resources, and they are capable of long–distance travel. In addition, some elephantimorph species (i.e., mammoths and mastodons) were clearly well adapted for living in cold climates, which indicates a certain degree of environmental flexibility (Sánchez et al., 2004).
The phylogenetic relationships of elephantimorphs to more archaic taxa were considered to be uncertain and were subject to considerable debate. Neogene proboscidean genera that do not fit easily into Stegodontidae, Elephantidae, or any other contemporaneous taxon, are usually placed in a group called gomphotheres. Most of these taxa were assigned to the "Bunomastodontidae" by Osborn (1936), and Simpson (1945) employed the term Gomphotheriidae to include the same group of taxa (Tobien et al., 1986, 1988). The family Gomphotheriidae is considered to be a long lived ancestral stock from which a succession of other groups originated. This family was widespread throughout all continents except, again, Australia and Antarctica, but North America played a significant role in its biogeography and diversity (Lambert, 1996). From the early Miocene to the Pleistocene this continent received numerous immigrant taxa from the Old World via Beringia and vice versa. The diversity of gomphotheres also reached its peak during this time, with six genera known from the middle Miocene (Gomphotherium, Rhynchotherium, Amebelodon, Serbelodon, Platybelodon, and Torynobelodon), though the number of genera declined during the late Miocene (Gomphotherium, Rhynchotherium, and Amebelodon) (Lambert and Shoshani, 1998). The gomphotheres were widespread throughout South America from the middle Pleistocene and became extinct at the end of the late Pleistocene (Prado et al., 2005; Reguero et al., 2007; Prado and Alberdi, 2008). Simpson and Paula Couto (1957) proposed that all of the gomphotheres known from South America derived from a single radiation in Central America.
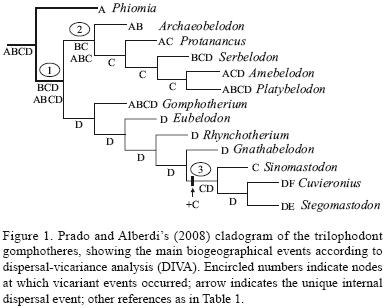
Resolving the systematics of gomphotheres has long been considered to be a difficult task because of their great intraspecific variation, as well as the general diversity of the group. During the past three decades, many proboscidean genera of uncertain taxonomic position, but which show the same the pattern of dentition as gomphotheres, have been classified in the family Gomphotheriidae. Several cladistic works on proboscideans have been published since the mid–1990s (Kalb et al., 1996; Shoshani, 1996; Tassy, 1990, 1994, 1996). Recently, Prado and Alberdi (2008) performed a cladistic analysis of the trilophodont Gomphotheriidae, using 12 genera as terminal taxa (Figure 1). According to these authors, these genera are members of a monophyletic group, separated from other genera of Proboscidea by one synapomorphy: trefoil shaped wear patterns on the occlusal surface of the teeth. The wear patterns vary from being a single trefoil to complex combinations of trefoils. Their cladogram rejects the hypothesis that consider Rhynchotherium (middle Miocene–Pliocene, North America) as a direct ancestor of South American gomphotheres, and supports that Sinomastodon (Late Miocene–Pleistocene, Asia) is the sister taxon of Cuvieronius and Stegomastodon (Pleistocene, North and South America) on the basis of the short mandibular symphysis and the absence of lower tusks. Additionally, Prado and Alberdi (2008) found high congruence between the stratigraphic record and the phylogenetic hypotheses.
A phylogeny and the knowledge of the geographical distributions of taxa are not, by themselves, sufficient to resolve complex histories of speciation and chorology for any given group (e.g., Brooks and McLennan, 2002; Green et al., 2002; Donoghue and Moore, 2003; Brooks et al., 2004; Halas et al., 2005). In the case of proboscideans, large–scale geological and/or environmental phenomena, like the changing configuration of continents and oceans, have affected their evolution and biogeography (e.g., Shoshani and Tassy, 1996, 2005; Shoshani, 1998). The Cenozoic configuration of continents and oceans has been strongly influenced by plate tectonic movements. The displacement of continents in the Southern Hemisphere during the middle Cenozoic, with the northward movement of the Indian and Australian continents, together with the counter clockwise rotation of Africa, closed down the Tethys Ocean. The history of the circum–Mediterranean area was strongly influenced by the Alpidic orogenies, which caused tectonic compression and fusion of numerous microplates between Europe and Africa. As a consequence of this compressive tectonic regime, Eurasia moved northwards and experienced considerable uplift (e.g., Tibetan Plateau, Alpine–Carpathian Chain, Anatolian Plate; see Kuhlemann, 2003). Simultaneously, the Eurasian ecosystems and landscapes were impacted by a complex pattern of changing seaways and land–bridges between the Paratethys Sea, the North Sea, and the Proto–Mediterranean Sea, as well as the western Indo–Pacific ocean (Popov et al., 2004). The geodynamic changes in landscapes and environments were further amplified by drastic climate changes during the Cenozoic.
Connections among the major Laurasian geographic provinces have changed over time, for example, with the widening of the Atlantic ocean, and the intervention of intercontinental seaways. During the Tertiary, several major dispersal pathways facilitated biotic exchange between the Old World and New World, but shifting latitudes and climates rendered these paths either more, or less, accessible to organisms with different physiological tolerances and dispersal capabilities. The dispersal of land mammals depends not only on the availability of physical connections but also on the presence of habitats that can support viable populations.
In this context, the objective of our paper was to analyze the distributional patterns of trilophodont gomphotheres, applying an event–based biogeographic method on the basis of Prado and Alberdi's (2008) cladogram. Additionally, we have attempted to interpret the biogeographical history of trilophodont gomphotheres in the context of the geological evolution of the continents they inhabited during the Cenozoic.
MATERIAL AND METHODS
Biogeographical analysis. The event–based methods approach is primarily a taxon biogeography research program, since it is focused on the distributional history of a particular taxon instead. As our objective was to analyze the distributional patterns of trilophodont gomphotheres, for our analysis we choose a method developed by Ronquist (1996), named dispersal–vicariance analysis (DIVA). Following Ronquist and Nylin (1990), Crisci et al. (2000, 2003) considered DIVA as an event–based method [but see Morrone (2005) for a different view of the use of taxonomic methods in historical biogeography].
DIVA is a biogeographic method that allows reconstruction of ancestral distributions, maximizing vicariant events and minimizing dispersal and extinction events, and allowing non–hierarchical area relationships. DIVA works by assuming that the distributions of each taxon in a phylogeny (terminals) and their ancestors (internals nodes) may be described in terms of a set of area units. If there has been a shift between the distribution of the ancestral and terminal taxa, it has occurred somewhere along the branch connecting them. To do that, DIVA uses a data matrix in which phylogenetic and distributional information of the taxon to be analyzed is included. Distributional information is encoded as the distribution of each terminal taxon of the phylogeny. Distributional areas are assigned to each internal node following two optimization rules: (a) the optimal distribution at any ancestral node cannot include any area not occupied by its descendents, and (b) the optimal set of areas for any ancestral node should include at least one area from each descendent node (Ronquist, 1997). Then, costs are assigned to changes (which represent events) between the distributional states in the descendants with respect to their immediate ancestors. Four events (or processes) are considered: vicariant speciation, dispersal, vicariance–independent speciation (i.e., 'duplication' of a lineage within an area), and extinction. The internal nodes are then assigned the distribution state through a series of optimizations that result in the lowest cost of biogeographic events over the whole area cladogram.
To reconstruct the biogeographic history of the Gomphotheriidae we used DIVA 1.1 (Ronquist, 1996), applying an exact search according to the dispersal–vicariance optimization proposed by Ronquist (1997). This software allows inference of the ancestral distribution of a taxon and thus permits the vicariance and dispersal events that account for the geographic history of the taxon under consideration to be evaluated. To do so, the software constructs a three–dimensional cost matrix derived from a simple biogeographical model (Ronquist, 1997). The input information is the phylogenetic and distributional information encoded on the taxon–area cladogram.
The historical biogeography of trilophodont Gomphotheriidae was analyzed in terms of the phylogeny proposed by Prado and Alberdi (2008). According to the geographical distribution of the taxa (Table 1), six areas were considered as geographic units: A) Africa; B) Europe; C) Asia; D) North America; E) South American eastern area; and F) South American Andean–Patagonian area.
RESULTS AND DISCUSSION
Applying DIVA to the cladogram of Prado and Alberdi (2008), the distributional pattern of trilophodont gomphotheres has only one exact solution, requiring 15 dispersal events. All possible ancestral distributions at each node are summarized on Figure 1.
According to DIVA, there are two possible ancestral distributions for trilophodont gomphotheres at the basal node. Based on fossil record we selected the more widespread one which included: Africa – Europe – Asia – North America (Figure 1). Proboscideans are usually considered to have been endemic to Africa during the Paleogene. Shoshani and Tassy (1996: fig. 34.6) suggest that the ancestors of modern elephants "inhabited the shores of the Tethys Sea during the Eocene. From there, some [descendants] have spread to Asia, Europe, and the New World". The recent discovery of Erithreum melakeghebrekristosi (a species that occupies an intermediate morphological stage between Phiomia and Palaeomastodon, meaning that all three are gomphotheres) in late Oligocene deposits of eastern Africa (Shoshani et al., 2006), helps us to better understand the biogeographical implications of the early proboscidean radiation between Africa and Arabia, as well as improve the analysis of the relationships among elephantimorphs, phiomiids and palaeomastodonts (Sanders et al., 2004; Prado and Alberdi, 2008). Nevertheless, the phylogenetic position of this species was uncertain and Shoshani et al. (2006) tentatively included it as family incertae sedis; for this reason Prado and Alberdi (2008) do not include this form in the cladogram.
The earliest known African gomphotheres (Gomphotherium sp.) occur in East Africa at Mfwangano and Mwiti (east Turkana, Kenya), both early Miocene localities (20–17 Ma). Primitive elephantoids, represented by genus Eozygodon, reached the Indo–Pakistani subcontinent during the earliest Miocene about 22–21 Ma (Tassy, 1989; Kalb et al., 1996; Lukas and Bendukidze, 1997), or perhaps earlier, as recently scanty evidence for the presence of elephantoids (referred as Elephantoidea indet.) was also found from the late Oligocene of Pakistan (Antoine et al., 2003). This implies that the first, short–lasting dispersal corridors had evolved already during the Aquitanian Age (around 25–23.8 Ma). The time of this proposed dispersal corresponds to a phase of lower temperatures (as a consequence of the preceding Mi–1 Glaciation of Antarctica), lower sea–levels, an acceleration of the Tibetan Plateau uplift, and the rifting of the Red Sea (Haq et al., 1987; Zachos et al., 2001).
According to DIVA, a vicariant event (node 1, Figure 1) took place resulting in two isolated groups: Amebelodontinae (Africa – Europe – Asia) and Gomphotheriinae (North America).This distribution could have been achieved during the earliest Miocene, during the previously mentioned environmental conditions (Haq et al., 1987; Zachos et al., 2001). Additionally, DIVA shows that Gomphotherium emigrated from North America to the Old World (Figure 1). The first gomphotheres in North America came from several widespread localities from the Great Plains during the early to middle Barstovian "Land–mammal Age" (16–14.5 Ma; Middle Miocene). Lambert and Shoshani (1998) suggest a rapid spread of gomphotheres during the early Barstovian, or perhaps that the arrival of gomphotheres in North America from the Old World took place earlier than currently thought (as is possible deduce from the fossil distribution in Figure 2). DIVA does not support a dispersal event. Consequently, this result implies that gomphotheres must have been present in North America before the early Barstovian.
Gomphotherium is recorded for the first time in Europe at the end of the Mammal Neogene Zone MN3 (20–17 Ma), at the same time that other immigrant proboscideans (i.e., the deinothere Prodeinotherium Ehik, 1930, and the mammutid Zygolophodon Vacek, 1877) first appear in the European record (Mein, 1975, 1999; Tassy, 1989; Koufos et al., 2003). According to Steininger (1999), this wave of proboscidean immigration is dated at 19–18.5 Ma. The northern expansion of early elephantid immigration into Western Europe, where they dispersed rapidly, started during the middle late Burdigalian Age (Early Miocene), an event previously referred to as the "Proboscidean Datum Event" by Madden and Van Couvering (1976; see also Tassy, 1989; Rögl, 1999). This is a time interval corresponding to the increased temperatures, and elevated sea–levels, of the "Mid–Miocene Climatic Optimum" (Haq et al., 1987; Zachos et al., 2001). Additionally, during the Early Miocene time (Burdigalian Age) the strong movements of the Savic tectonic phase changed the paleogeographic patterns in the circum–Mediterranean area. The rotation of Africa finally closed the gap between it and Eurasia, and the Arabian Peninsula collided with the Anatolian Plate. The so called "Gomphotheres land bridge" was established, and continental faunal exchange in both directions started around 19 Ma (Fortelius et al., 1996; Bernor et al., 1996). Subsequently, this event was recognized as being composed of multiple immigration events (Tassy, 1989, 1996; Koufos et al., 2003). In Asia, the earliest gomphotheres (Gomphotherium) come from the Bugti Hills (Pakistan) dated around 18.3 Ma (Jacobs et al., 1989).
According to DIVA, the Amebelodontinae clade was split by a second vicariant event (node 2, Figure 1): Archaeobelodon Tassy, 1984 was confined to Africa and Europe (in Africa around 19–16 Ma, and in Europe around 15.5–13 Ma following Pickford, 2003), and the ancestors of the remaining genera of the clade were confined to Asia. This vicariant event probably took place around the Early to Middle Miocene boundary (Burdigalian–Langhian). For a short time, the Mediterranean–Indo–Pacific seaway reopened. During this phase of open seaways, the Eurasian and African mammal dispersions were interrupted, and these seaways could explain the vicariant event that split Amebelodontinae.
Archaeobelodon is recorded for the first time in Europe during the Mammal Neogene Zone MN4. According to Steininger (1999), Archaeobelodon is part of a second wave of proboscidean immigration that took place between 18 and 17.5 Ma, a hypothesis not supported by DIVA. All other Amebelodontinae genera (i.e., Protanancus, Serbelodon, Amebelodon, and Platybelodon) had widespread distributions which are implied from a minimum of two areas for Protanancus (Africa and Asia) to a maximum of four areas for Platybelodon (Africa, Asia, Europe, and North America). All of these widespread distributions resulted from independent dispersal events for each genus, since all of their ancestral distributions are restricted to Asia. These dispersal events (eight of the 15 postulated according to DIVA results, Figure 1) occurred after the final closure of the circum–equatorial oceanic current system that caused worldwide cooling and an increased accumulation of the East Antarctic ice sheet during the Langhian (early Middle Miocene), around 15 Ma (Kennett, 1995). Later, a new marine regression (Serravallian, late Middle Miocene) reestablished the "Gomphotheres land bridge" (Eastern Mediterranean area: Balkan Peninsula, Aegeab Sea, Asia Minor and Middle East, Koufos et al., 2005). In addition, during the early Late Miocene (Tortonian) the sea–level fell drastically (see Haq et al., 1987), and it was probably during this time that Serbelodon, Amebelodon and Platybelodon reached North America via Beringia, where to be in contact with Gomphotheriinae (Figure 2).
The Late Cenozoic opening of the Bering Strait ended the separation of the Arctic and North Pacific oceans that had persisted for about 100 million years, since the Albian period of the middle Cretaceous (Marincovich et al., 1990). Since Hopkins (1967) published "The Bering Land Bridge", many geological and paleontological works concerning the Bering Strait and its adjacent areas have accumulated. The earliest known opening of Bering Strait is signaled by the presence in southern Alaskan Neogene strata of the marine bivalve mollusk Astarte, which had dwelled throughout the Cenozoic in the Arctic and North Atlantic oceans (Marincovich and Gladenkov, 2001).The periods of a land connection of the continents during the Pliocene are thought to have been at 4.8, 3.7, 2.5 and 2 Ma based on mammalian fossils, while marine connections between the Arctic and Pacific are suggested at around 4.2–3.0, 2.5 and 2.2 Ma, mainly based on transgressive facies of the land sections and shallow marine benthic fossils (Gladenkov et al., 1991).
According to DIVA (Figure 1), the other major group (Gomphotheriinae) had evolved in isolation in North America since the Middle Miocene. At this time, the ancestor of this group, which had reached North America via Beringia during the earliest Miocene, became isolated due to higher sea–levels that occurred during Middle Miocene (see Haq et al., 1987). Most ancestral distributions of the Gomphotheriinae clade were restricted to North America. Gomphotherium achieved a widespread distribution, colonizing Asia, Europe and Africa. This event probably took place during the aforementioned Tortonian sea–level fall. Thus, Gomphotherium crossed to Asia via Beringia by a migratory route that was the converse of that followed by Serbelodon, Amebelodon and Platybelodon. This dispersal of Gomphotherium from North America to Asia, Europe, and Africa, contradicts the "classical" hypothesis that proposes a dispersal in the opposite direction (e.g., Shoshani and Tassy, 1996).
A major biogeographic event of the Gomphotheriinae clade is represented by the dispersal of the ancestor of Sinomastodon plus Cuvieronius–Stegomastodon. This unique dispersal event occurred at an internal node. The ancestor of these three genera expanded its range from North America to North America plus Asia. This event took place via Beringia, most probably during the sea–level fall of the Messinian–Zanclean (latest Miocene–early Pliocene; see Haq et al., 1987).
At the end of the Zanclean the sea–level increased again, resulting in a new vicariant event (node 3, Figure 1) which affected this clade by splitting North America and Asia. Thus Sinomastodon evolved in isolation in Asia, being recorded in Early Pliocene sediments of China (Tobien et al., 1986; Tassy, 1996). In contrast, Sinomastodon's sister group (Cuvieronius–Stegomastodon) evolved in North America; this clade subsequently dispersed to South America during the Great American Biotic Interchange (GABI).
The GABI was a major event in late Cenozoic biogeography as taxa from North and South America moved across the land bridge that formed with the emergence of the Isthmus of Panama (Simpson, 1950, 1980; Patterson and Pascual, 1972; Webb, 1976, 1985, 1991; Morgan, 2002, 2005). Recent studies indicate that this event was complex and started during the Miocene (Cione and Tonni, 1995; Ortiz–Jaureguizar, 1997, 2001; Scillato–Yané et al., 2005; Woodburne et al., 2006; Reguero et al., 2007; Carlini et al., 2008a, 2008b), but the main phase of the GABI occurred from about 2.7–1.8 Ma (Gelasian, early Pleistocene), with laggards lasting until about 1.0 Ma (Calabrian, late Pleistocene). A later phase occurred from about 0.8 Ma to virtually modern times and resulted in mainly southern enrichment (Woodburne et al., 2006).
The new land bridge functioned as an ecologically selective dispersal corridor (Webb, 1978; Simpson, 1980). Biogeographic data indicate three major types of Plio–Pleistocene habitat corridors existed on the Panamanian land bridge: mesic tropical forest, mesic savanna, and xeric scrub savanna (Webb, 1978). During the humid interglacial phase, rain forests dominated the tropics, and the principal biotic movement was from Amazonia to Central America (south to north). During the more arid glacial phase, when savanna habitats predominated and extended well into tropical latitudes, the directional pattern reversed, and biotic forms moved from north to south (Webb, 1991).
Before the interchange, Cuvieronius (Gomphotheriidae), Mammuthus (Elephantidae), and Mammut (Mammutidae) were recorded in Florida and Honduras. There appears to be no obvious biological explanation why Mammuthus and Mammut, which might have been expected to cross the Panamanian land bridge, did not reach South America. The reason may be found in the diet and habitat preferences of these genera. Mammut have relatively low–crowned molars with zygodont crests. This dental morphology led to the recognition of mastodons as browsers (Webb et al., 1992). Mammoths (Mammuthus) have high–crowned molars with closely spaced enamel lophs coated with cement, which identifies them as grazers (Davis et al., 1985). Isotopic analyses confirm this hypothesis (MacFadden and Cerling, 1996). The gomphotheres from West Palm Beach, Florida, and from the middle Pleistocene of South America have δ13C values that are intermediate between the isotopic values for browsers and grazers (Koch et al., 1998; Connin et al., 1998). Mammoth and mastodon species were more specialized feeders than Cuvieronius, which was a mixed–feeder. Sánchez et al. (2004) propose that the different feeding preferences among mastodons, mammoths, and gomphotheres could explain why only the bunodont forms reached South America.
The some members of Gomphotheriinae crossed into South America during the GABI event; it apparently did so during the more arid glacial phase, when savanna habitats extended broadly through tropical latitudes (Prado et al., 2005). Cuvieronius and Stegomastodon reached South America in two independent dispersal events. Stegomastodon ranges from early Blancan to early Irvingtonian. Although the genus was considered as the more specialized grazer within the American gomphotheres, it has been redefined as a mixed feeder with tendencies toward both browsing and grazing (Prado et al., 2005). This feeding habit indicates that the genus may have been adapted to warm to temperate open grasslands.
According to Prado et al. (2005), Cuvieronius dispersed across the Andean corridor, whereas Stegomastodon dispersed along the eastern and Atlantic coastal areas of the continent. Cuvieronius hyodon is geographically restricted to the Andean Region in Ecuador, Peru, Bolivia, and Chile. It inhabited an arid landscape. This species seems to have been adapted to a temperate–cold climate, since in the inter–tropical zones it has been only found at the highest altitudes, while in Chile it expanded to the littoral zone. The latter surely offered similar living conditions, in terms of temperature, as the Andes corridor. Stegomastodon seems to have predominated in lower latitudes, where it occupied savannahs or xerophytic pasture areas, and consequently it would have been better adapted to warm or temperate climatic conditions. Stegomastodon waringi was recorded in the Santa Elena peninsula in Ecuador, and in Brazil and Uruguay (Alberdi et al., 2002, 2007; Gutiérrez et al., 2005). Stegomastodon platensis was recorded in the Middle to latest Pleistocene of Argentina, especially the Pampean Region, and also during the Late Pleistocene in Uruguay, Paraguay and Chile. All of these species became extinct at the end of the Pleistocene. The only exceptions in the Proboscidea were the African and Indian elephants. Owen–Smith (1987, 1999) has argued that the extinction of mega–mammals (more than 1000 kg) transformed a minor extinction pulse, that was affected by climate change, into a major extinction cascade because mega–mammals (such as proboscideans) were "keystone herbivore species" that had greatly raised diversity at the patch level. With the mega–mammals gone, natural processes such as woody regeneration and shrub invasions of grassy glades progressed unimpeded, thus reducing carving capacity for non–migratory grazers.
CONCLUSIONS
The application of DIVA resulted in an exact solution requiring three vicariant events, and 15 dispersal events, most of them (i.e., 14) occurring at terminal branches. The single dispersal event at an internal node affected the common ancestor to Sinomastodon plus the clade Cuvieronius – Stegomastodon.
The ancestral distribution for trilophodont gomphotheres included Africa – Europe – Asia – North America (Figure 1). This distribution could have been achieved during the earliest Miocene, a time of low sea–levels and low temperatures. A vicariant event took place which resulted in two isolated groups: (1) Amebelodontinae (Africa – Europe – Asia); and (2) Gomphotheriinae (North America). The Amebelodontinae clade was split by a second vicariant event: Archaeobelodon (Africa and Europe), and the ancestors of the remaining genera of the clade (Asia). In contrast, the Gomphotheriinae clade evolved mainly in North America. A dispersal event expanded the range of the common ancestor to Sinomastodon plus the clade Cuvieronius – Stegomastodon to include Asia again. A new vicariant event split North America and Asia resulting in the isolation of Sinomastodon in the latter, and the ancestor of the clade Cuvieronius – Stegomastodon in the former. Finally, these two genera reached South America in two independent dispersal events.
The biogeographic history of trilophodont gomphotheres has been driven by sea–level changes. During low sea–level episodes, trilophodont gomphotheres expanded their distribution by means of intercontinental dispersion events, and during high sea–level episodes they underwent vicariant events.
ACKNOWLEDGEMENTS
This paper was financed by grants of the DGICYT (CGL2004–00400/BTE and CGL2007–60790/BTE) to DGCYT of Spain, grant from the Universidad Nacional del Centro, and the Project PIP–02773, CONICET, PICTO 04–11503 ANPCYT and PICT 07–01563, to JLP; and grants of the ANPCyT (PICT 26298) and CONICET (PIP 5604) to EOJ, PP, and MD. EOJ, PP, and MD are scientific researchers of the CONICET (Argentina), whose continuous support they gratefully thanks. Stefan Gabriel revised the English text.
REFERENCES
Alberdi, M.T., Prado, J. L., Cartelle, C., 2002, El registro de Stegomastodon (Mammalia, Gomphotheriidae) en el Pleistoceno superior de Brasil: Revista Española de Paleontología, 17(2), 217–235. [ Links ]
Alberdi, M.T., Prado, J.L., Perea, D., Ubilla, M., 2007, Stegomastodon waringi (Mammalia, Proboscidea) from the Late Pleistocene of northeastern Uruguay: Neues Jarhbuch Geologie und Paläontologie, Abhandlungen, 243(2), 179–189. [ Links ]
Andrews, C.W., Beadnell, H.J.L., 1902, A preliminary note on some new mammals from the upper Eocene of Egypt: Cairo, Survey Department, Public Works Ministry, 1–9. [ Links ]
Antoine, P.O., Welcome, J. L., Marivaux, L., Baloch, I., Benammi, M.,Tassy, P., 2003, First Record of Paleogene Elephantoidea (Mammalia, Proboscidea) from the Bugti Hills of Pakistan: Journal of Vertebrate Paleontology, 23, 977–980. [ Links ]
Arambourg, C., 1945, Anancus osiris, un mastodonte nouveau du Pliocène inférieur d'Égypte: Bulletin de la Société Géologique de France, 5a serie, 7, 1–126. [ Links ]
Barbour, E.H., 1914, Mammalian fossils from Devil's Gulch: Nebraska Geological Survey, 4, 177–190. [ Links ]
Barbour, E.H., 1927, Preliminary notice of a new proboscidean Amebelodon fricki, gen. et sp. nov.: Bulletin of the Nebraska State Museum, 13, 131–134. [ Links ]
Barbour, E.H., Sternberg, G., 1935, Gnathabelodon thorpei, gen. et sp. nov. A new mud–grubbing mastodon: Bulletin of the Nebraska State Museum, 42, 395–404. [ Links ]
Bernor, R.L., Koufos, G.D., Woodburne, M.O., Fortelius, M., 1996, The evolutionary history and biochronology of European and southwest Asian late Miocene and Pliocene Hipparionine horses, in Bernor, R.L., Fahlbusch, V., Mittmann, H.W. (eds.), The Evolution of Western Eurasian Neogene Mammal Faunas: New York, Columbia University Press, pp. 307–338. [ Links ]
Borissiak, A.A., 1928, On a new mastodon from the Chokrak Beds (middle Miocene) of the Kuban region. Platybelodon danovi, n. gen., n. sp.: Annual of the Paleontological Society Russie, 7, 105–120. [ Links ]
Brooks, D.R., McLennan, D.A., 2002. The Nature of Diversity: An Evolutionary Voyage of Discovery: Chicago, University of Chicago Press, 315 pp. [ Links ]
Brooks, D.R., Dowling, A.P.G., van Veller, M.G.P., Hoberg, E.P., 2004, Ending a decade of deception: a valiant failure, a not–so–valiant failure, and a success story: Cladistics, 20, 32–46. [ Links ]
Burmeister, G., 1837, Handbuch der Naturgeschichte. Zum Gebrauch bei Vorlesungen entworfen: Berlin, Zweite Abteilung, Zoologie, T. C. F. Enslin, pp. 369–858. [ Links ]
Carlini, A.A., Zurita, A.E., Aguilera, O., 2008a, Additions to the knowledge of Urumaquia robusta (Xenarthra, Phyllophaga, Megatheriidae) from the Urumaco Formation (late Miocene), Estado Falcón, Venezuela: Paläontologische Zeitschrift, 82(2), 153–162. [ Links ]
Carlini, A.A., Zurita, A.E., Aguilera, O., 2008b, North American Glyptodontines (Xenarthra, Mammalia) in the Upper Pleistocene of Northern South America: Paläontologische Zeitschrift, 82(2), 125–138. [ Links ]
Cione, A.L., Tonni, E.P., 1995, Chronostratigraphy and "land mammal–ages": The Uquian problem: Journal of Paleontology, 69, 135–159. [ Links ]
Connin, S.L., Betancourt, J., Quade, J., 1998, Late Pleistocene C4 plant dominance and summer rainfall in the southwestern United States from isotopic study of herbivore teeth: Quaternary Research, 50, 179–193. [ Links ]
Crisci, J.V., Katinas, L., Posadas, P., 2000, Introducción a la Teoría y Práctica de la Biogeografía Histórica: Sociedad Argentina de Botánica, Buenos Aires, 170 pp. [ Links ]
Crisci, J.V., Katinas, L., Posadas, P., 2003, Historical Biogeography: An Introduction: Harvard University Press, Cambridge, 250 pp. [ Links ]
Davis, O.K., Mead, J.I., Martin, P.S., Agenbroad, L.D., 1985, Riparian plants were a major component of the diet of mammoths of southern Utah: Current Research in the Pleistocene, 2, 81–82. [ Links ]
Donoghue, M.J., Moore, B.R., 2003, Toward an integrative Historical Biogeography: Integrative and Comparative Biology, 43, 261–270. [ Links ]
Ehik, J., 1930, Prodinotherium hungaricum n.g., n. sp.: Geologica Hungarica, Series Paleontologica, 6, 1–24. [ Links ]
Falconer, H., 1868, Paleontological memoirs and notes of the late Hugh Falconer with a biographical sketch of the author: London, Editor C. Murchison, Hardwicke. [ Links ]
Fortelius, M., Werdelin, L., Andrews, P., Bernor, R.L., Gentry, A., Humphrey, L., Mittmann, H.W., Viratana, S., 1996, Provinciality, diversity, turnover, and paleoecology in land mammal faunas of the later Miocene of western Eurasia, in Bernor, R.L., Fahlbusch, V., Mittmann, H.W. (eds.), The Evolution of Western Eurasian Neogene Mammal Faunas: New York, Columbia University Press, pp. 414–448. [ Links ]
Frick, C., 1933, New remains of trilophodont–tetrabelodont mastodons: Bulletin of the American Museum of Natural History, 59, 505–652. [ Links ]
Gheerbrant, E., 2009, Palaeocene emergence of elephant relatives and the rapid radiation of African ungulates: Proceedings of the National Academy of Sciences of the United States of America, 106, 10717–10721. [ Links ]
Gladenkov, Y.B., Barinov, K.B., Basilian, A.E., Cronin, T.M., 1991, Stratigraphy and paleoceanography of Miocene deposits of Karaginsky Island, eastern Kamchatka, USSR: Quaternary Science Reviews, 10, 239–245. [ Links ]
Göhlich, U.B., 1999, Order Proboscidea, in Rössner, G.E., Heissig, K. (eds.), The Miocene Land Mammals of Europe: Germany, Verlag Dr. Friedrich Pfeil, Munich, pp. 156–168. [ Links ]
Green, M., van Veller, M.G.P., Brooks, D.R., 2002, Assessing modes of speciation: range asymmetry and biogeographical congruence: Cladistics, 18, 112–124. [ Links ]
Gutiérrez, M., Alberdi, M.T., Prado, J. L., Perea, D., 2005, Late Pleistocene Stegomastodon (Mammalia, Proboscidea) from Uruguay: Neues Jarhbuch Geologie und Paläontologie, Monatshefte, 11, 641–662. [ Links ]
Halas, D., Zamparo, D., Brooks, D.R., 2005, A historical biogeographical protocol for studying biotic diversification by taxon pulses: Journal of Biogeography, 32, 249–260. [ Links ]
Haq, B.U., Hardenbol, J., Vail, P.R., 1987, Chronology of fluctuating sea levels since the Triassic: Science, 235, 1156–1167. [ Links ]
Hopkins, D.M., 1967, The Bering Land Bridge: Stanford, Stanford University Press, 495 pp. [ Links ]
Jacobs, L.L., Flynn, L.J., Downs, W.R., Barry, J.C., 1989, Quo vadis, Antemus? The Siwalik muroid record, in Lindsay, E.H., Fahlbusch, V., Mein, P. (eds.), European Neogene mammal chronology: New York, Plenum Press, pp. 573–586. [ Links ]
Kalb, J.E., Froehlich, D.J., Bell, G.L., 1996, Palaeobiogeography of late Neogene African and Eurasian Elephantoidea, in Shoshani, J., Tassy, P. (eds.), The Proboscidea. Evolution and Palaeoecology of Elephants and their relatives: New York and Tokyo, Oxford University Press, pp. 117–123. [ Links ]
Kennett, J.P., 1995, A review of polar climatic evolution during the Neogene, based on the marine sediment record, in Vrba, E.S., Denton, G.H., Partridge, T.C., Burckle, L.H. (eds.), Paleoclimate and Evolution with Emphasis on Human Origins: New Haven and London, Yale University Press, pp. 49–64. [ Links ]
Koch, P.L., Hoppe, K.A., Webb, S.D., 1998, The isotopic ecology of late Pleistocene mammals in North America, Part 1. Florida: Chemical Geology, 152, 119–138. [ Links ]
Koufos, G.D., Zouros, N., Mourouzidou, O., 2003, Prodeinotherium bavaricum (Proboscidea, Mammalia) from Lesvos island, Greece; the appearance of deinotheres in the Eastern Mediterranean: Geobios, 36, 305–315. [ Links ]
Koufos, G.D., Kostopoulos, D.S., Vlachou, T.D., 2005, Neogene/Quaternary mammalian is eastern Mediterranean: Belgian Journal of Zoology, 135(2), 181–190. [ Links ]
Kuhlemann, J., 2003, Global Cenozoic relief formation and mountain uplift in convergent plate margins: Neues Jahrbuch Geologie und Paläontologie, Abhandlungen, 230, 215–256. [ Links ]
Lambert, W.D., 1996, The biogeography of the gomphotheriid proboscideans of North America, in Shoshani, J., Tassy, P. (eds.), The Proboscidea. Evolution and Palaeoecology of Elephants and their Relatives: New York and Tokyo, Oxford University Press, Oxford, pp. 143–148. [ Links ]
Lambert, W.D., Shoshani, J., 1998, Proboscidea, in Janis, C.M., Scott, K.M., Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America: I. Terrestrial, Carnivores, Ungulates and Ungulatelike Mammals: New York, Cambridge University Press, pp. 606–621. [ Links ]
Lukas, S.G., Bendukidze, O.G., 1997, Proboscidea (Mammalia) from the Early Miocene of Kazakhstan: Neues Jahrbuch Geologie und Paläontologie, Monatshefte, 11, 659–673. [ Links ]
MacFadden, B.J., Cerling, T.E., 1996, Mammalian herbivores communities, ancient feeding ecology, and carbon isotopes: A 10 million–year sequence from the Neogene of Florida: Journal of Vertebrate Paleontology, 16, 103–115. [ Links ]
Madden, C.T., Van Couvering, J.A., 1976, The Proboscidean Datum Event: early Miocene migration from Africa: Geological Society of America Abstracts with Programs, 992. [ Links ]
Maglio, V.J., 1973, Origin and evolution of the Elephantidae: Transactions of the American Philosophical Society of Philadelphia, NS 63, 1–149. [ Links ]
Marincovich, L.Jr., Gladenkov, A.Y., 2001, New evidence for the age of Bering Strait: Quaternary Science Review, 20, 329–335. [ Links ]
Marincovich, L.Jr., Brouwers, E.M., Hopkins, D.M., McKenna, M.C., 1990, Late Mesozoic and Cenozoic paleogeographic and paleoclimatic history of the Arctic Ocean basin, based on shallow–water faunas and terrestrial vertebrates: Geological Society of America, The Geology of North America, L, The Arctic Ocean Region, 403–426. [ Links ]
Mein, P., 1975, Résultats du Groupe de Travail des Vertébrés, in Senes, J. (ed.), Abstract of VI Congress of Regional Committee of Mediterranean Neogene Stratigraphy: Bratislava, VEDA, Publishing House of the Slovak Academy of Sciences, pp. 78–81. [ Links ]
Mein, P., 1999, European Miocene mammal biochronology, in Rössner, G.E., Heissig, K. (eds.), The Miocene Land Mammals of Europe: Munich, Germany, Verlag Dr. Friedrich Pfeil, pp. 25–38. [ Links ]
Morgan, G.S., 2002, Late Rancholabrean mammals from southernmost Florida, and the Neotropical influence in Florida Pleistocene faunas: Smithsonian Contributions to Paleobiology, 93, 15–38. [ Links ]
Morgan, G.S., 2005, The Great American Biotic Interchange in Florida: Bulletin of the Florida Museum of Natural History, 45, 271–312. [ Links ]
Morrone, J.J., 2005, Cladistic biogeography: identity and place: Journal of Biogeography, 32, 1281–1286. [ Links ]
Ortiz–Jaureguizar, E., 1997, La fauna de mamíferos de América del Sur y el gran intercambio biótico americano: un ejemplo de invasión natural a escala continental: Actas 1° Jornadas Nacionales y 61° Regionales sobre Medio Ambiente, La Plata, 134–141. [ Links ]
Ortiz–Jaureguizar, E., 2001, Cambios en la diversidad de los mamíferos sudamericanos durante el lapso Mioceno superior–Holoceno: el caso pampeano, in Meléndez, G., Herrera, Z., Delvene, G., Azanza, B. (eds.), ¿Los Fósiles y la Paleogeografía? 5: Zaragoza, Publicaciones del SEPAZ (Universidad de Zaragoza), pp. 397–403. [ Links ]
Osborn, H.F., 1923, New subfamily, generic, and specific stages in the evolution of the Proboscidea: American Museum Novitates, 99, 1–4. [ Links ]
Osborn, H.F., 1936, Proboscidea. A Monograph of the Discovery, Evolution, Migration and Extinction of the Mastodons and Elephants, Vol. 1—Moeritherioidea, Deinotheiroidea, Mastodontoidea: The American Museum of Natural History, New York, 1, 1–802. [ Links ]
Owen–Smith, R.N., 1987, Pleistocene extinctions: the pivotal role of megaherbivores: Paleobiology, 13, 351–62. [ Links ]
Owen–Smith, R.N., 1999, The interaction of humans, megaherbivores, and habitats in the late Pleistocene extinction event, in MacPhee, R.D.E. (ed.), Extinctions in Near Time: Causes, Contexts, and Consequences: New York, Kluwer Academic/Plenum, pp. 57–69. [ Links ]
Patterson, B., Pascual, R., 1972, The fossil mammal fauna of South America, in Keast, A., Erk, F.C., Glass, B. (eds.), Evolution, Mammals and Southern Continents: Albany, University of New York Press, pp. 247–309. [ Links ]
Pickford, M., 2003, New Proboscidea from the Miocene strata in the lower Orange River Valley, Namibia: in Pickford, M., Senut, B., (eds.), Geology and Palaeobiology of the central and southern Namib, volume 2, Palaeontology of the Orange River Valley, Namibia: Geological Survey of Namibia, Memoir, 19, 207–256. [ Links ]
Pohlig, H., 1912, Sur une vieille mandibule de "Tetracaulodon ohiotocum>> Blum., avec défense in situ: Bulletin de la Société Belge Géologique, 26, 187–193. [ Links ]
Popov, S.C., Rögl, S., Rozanov, A.Y., Steininger, F.F., Shcherba, I.G., Kovac, M., 2004, Lithological–paleogeographic maps of the Paratethys: Courier Forschung–Institut Senckenberg, 250, 1–46. [ Links ]
Prado, J.L., Alberdi, M.T., 2008, A cladistic analysis among trilophodont gomphotheres (Mammalia, Proboscidea) with special attention to the South American genera: Palaeontology, 51, 903–915. [ Links ]
Prado, J.L., Alberdi, M.T., Azanza, B., Sánchez, B., Frassinetti, D., 2005, The Pleistocene Gomphotheriidae (Proboscidea) from South America: Quaternary International, 126–128, 21–30. [ Links ]
Reguero, M.A., Candela, A.M., Alonso, R.N., 2007, Biochronology and biostratigraphy of the Uquía Formation (Pliocene–early Pleistocene, NW Argentina) and its significance in the Great American Biotic Interchange: Journal of South American Earth Sciences, 23, 1–16. [ Links ]
Rögl, F., 1999, Circum–Mediterranean Miocene paleogeography, in Rössner, G.E., Heissig, K. (eds.), The Miocene Land Mammals of Europe: Germany, Verlag Dr. Friedrich Pfeil, Munich, pp. 39–48. [ Links ]
Ronquist, F., 1996, Manual DIVA version 1.1. Computer program for MacOS and Win32. http://diva.sourceforge.net/ [ Links ]
Ronquist, F., 1997, Dispersal–vicariance analysis: a new approach to the quantification of historical biogeography: Systematic Biology, 46, 195–203. [ Links ]
Ronquist, F., Nilyn, S., 1990, Process and pattern in the evolution of species association: Systematic Zoology, 39, 323–344. [ Links ]
Sánchez–Chillón, B., Prado, J.L., Alberdi, M.T., 2004, Feeding ecology, dispersal, and extinction of South American Pleistocene gomphotheres (Gomphotheriidae, Proboscidea): Paleobiology, 30, 146–161. [ Links ]
Sanders, W.J., Kappelman, J., Rasmussen, D.T., 2004, New large–bodied mammals from the late Oligocene site of Chilga, Ethiopia: Acta Palaeontologica Polonica, 49(3), 365–392. [ Links ]
Scillato–Yané, G.J., Carlini, A.A., Tonni, E.P., Noriega, J.I., 2005, Palaeobiogeography of the late Pleistocene pampatheres of South America, in Rabassa, J., Carlini, A.A. (eds.), Quaternary Paleontology and Biostratigraphy of Southern South America: Journal of South American Earth Sciences, 20, 131–138. [ Links ]
Shoshani, J., 1996, Para– or monophyly of the gomphotheres and their position within Proboscidea, in Shoshani, J., Tassy, P. (eds.), The Proboscidea. Evolution and Palaeoecology of Elephants and their Relatives: Oxford, Oxford University Press, pp. 149–177. [ Links ]
Shoshani, J., 1998, Understanding proboscidean evolution: a formidable task: Trends in Ecology & Evolution, 13, 480–487. [ Links ]
Shoshani, J., Tassy, P., 1996, Summary, conclusions, and a glimpse into the future, in Shoshani, J., Tassy, P. (eds.), The Proboscidea. Evolution and Palaeoecology of Elephants and their Relatives: Oxford, Oxford University Press, pp. 335–348. [ Links ]
Shoshani, J., Tassy, P., 2005, Advances in proboscidean taxonomy & clasiffication, anatomy & physiology, and ecology & behavior: Quaternary International, 126–128, 5–20. [ Links ]
Shoshani, J., Walter, R.C., Abraha, M., Berhe, S., Tassy, P., Sanders, W.J., Marchant, G.H., Libsekal, Y., Ghirmai, T., Zinner, D., 2006, A proboscidean from the late Oligocene of Eritrea, a 'missing link' between early Elephantiformes and Elephantimorpha, and biogepgraphic implications: Proceedings of the National Academy of Sciences , USA, 103(46), 17296–17301. [ Links ]
Simpson, G.G., 1945, The principles of classification and classification of mammals: Bulletin of the American Museum of Natural History, 85, 1–350. [ Links ]
Simpson, G.G., 1950, History of the fauna of Latin America: American Science, 38, 261–389. [ Links ]
Simpson, G.G., 1980, Splendid isolation. The Curious History of South American Mammals: New Haven and London, Yale University Press, 266 pp. [ Links ]
Simpson, G.G., Paula Couto, C., 1957, The mastodonts of Brazil: Bulletin of the American Museum of Natural History, 112, 125–190. [ Links ]
Steininger, F.F., 1999, The continental European Miocene. Chronostratigraphy, geochronology and biochronology of the Miocene "European Land Mammal Mega–zones (ELMMZ)" and the Miocene "Mammal–zones (MN–zones)", in Rössner, G.E., Heissig, K. (eds.), The Miocene Land Mammals of Europe: Munich, Germany, Verlag Dr. Friedrich Pfeil, pp. 9–24. [ Links ]
Tassy, P., 1984, Le mastodonte à dents étroites, le grade trilophodonte et la radiation initiale des Amebelodontinae, in Buffetaut, E., Mazin, J.M., Salmon, E. (eds.), Actes du Symposium Paléontologique Georges Cuvier: France, Impressions le Serpentaire, Montbéliard, pp. 459–473. [ Links ]
Tassy, P., 1989, The "Proboscidean Datum Event:" how many proboscideans and how many events?, in Lindsay, E.H., Fahlbusch, V., Mein, P. (eds), NATO ASI Series, New York and London, Plenum Press, pp. 237–253. [ Links ]
Tassy, P., 1990, Phylogénie et classification des Proboscidea (Mammalia): historique et actualité: Annales de Paléontologie, 76, 159–224. [ Links ]
Tassy, P., 1994, Gaps, parsimony, and early Miocene elephantoids (Mammalia), with a reevaluation of Gomphotherium annectens (Matsumoto, 1925): Zoological Journal of the Linnean Society, 112, 101–117. [ Links ]
Tassy, P., 1996, Who is who among the Proboscidea?, in Shoshani, J., Tassy, P. (eds.), The Proboscidea. Evolution and Palaeoecology of Elephants and their relatives: Oxford, Oxford University Press, pp. 39–48. [ Links ]
Tobien, H., Chen, G.F., Li, Y.Q., 1986, Mastodonts (Proboscidea, Mammalia) from the late Neogene and early Pleistocene of the People's Republic of China. Part. 1. Historical account: the genera Gomphotherium, Choerolophodon, Synconolophus, Amebelodon, Platybelodon, Sinomastodon: Mainzer Geowissenschaftliche Mitteilungen, 15, 119–181. [ Links ]
Tobien, H., Chen, G.F., Li, Y.Q., 1988, Mastodonts (Proboscidea Mammalia) from the late Neogene and early Pleistocene of the People's Republic of China. Part. 2. The genera Tetralophodon, Anancus, Stegotetrabelodon, Zygolophodon, Mammut, Stegolophodon. Some generalities on the Chinese mastodonts: Mainzer Geowissenschaftliche Mitteilungen, 17, 95–220. [ Links ]
Vacek, M., 1877, Über österreichische Mastodonten und ihre Beziehungen zu den Mastodon–Arten Europas: Abhandlunge der Kaiserlich–Königlichen geologischen Reichsanstalt, 7(4), 1–45. [ Links ]
Webb, S.D., 1976, A history of savanna vertebrates in the New World. Part I: North America: Annual Review of Ecology and Systematics, 8, 355–380. [ Links ]
Webb, S.D., 1978, A history of savanna vertebrates in the New World. Part II: South America and the Great Interchange: Annual Review of Ecology and Systematics, 9, 393–426. [ Links ]
Webb, S.D., 1985, Late Cenozoic mammal dispersals between the Americas, in Stehli, F.G., Webb, S.D. (eds.), The Great American Biotic Interchange: New York and London, Plenum Press, pp. 357–386. [ Links ]
Webb, S.D., 1991, Ecogeography and the Great American Interchange: Paleobiology, 17, 266–280. [ Links ]
Webb, S.D., Dunbar, J., Newsom, L., 1992, Mastodon digesta from North Florida: Florida Geological Survey Special Publication, 10, 1–59. [ Links ]
Woodburne, M.O., Cione, A.L., Tonni, E.P., 2006, Central American provincialism and the Great American Biotic Interchange, in Carranza–Castañeda, O., Lindsay, E.H. (eds.), Advances in late Tertiary vertebrate paleontology in Mexico and the Great American Biotic Interchange: Publicación Especial del Instituto de Geología y Centro de Geociencias de la Universidad Nacional Autónoma de México, 4, 73–101. [ Links ]
Zachos, J., Pagani, M., Sloan, L., Thomas, E., Billups, K., 2001, Trends, rhythms, and aberrations in global climate 65 Ma to Present: Science, 292, 686–693. [ Links ]














