Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias geológicas
versão On-line ISSN 2007-2902versão impressa ISSN 1026-8774
Rev. mex. cienc. geol vol.27 no.3 Ciudad de México Dez. 2010
Discovery and characterization of a struvite layer in the Chalco paleolake, Mexico
Descubrimiento y caracterización de una capa de estruvita en el paleolago de Chalco, México
Teresa Pi1*, Socorro Lozano–García1, Margarita Caballero–Miranda2, Beatriz Ortega–Guerrero2, and Priyadarsi Roy1
1 Instituto de Geología, Universidad Nacional Autónoma de México, Cd. Universitaria, 04510 México D.F., México. *Correo electrónico: tpuig@geologia.unam.mx
2 Instituto de Geofísica, Universidad Nacional Autónoma de México, Cd. Universitaria, 04510 México D.F., México.
Manuscript received: April 24, 2010.
Corrected manuscript received: September 1, 2010.
Manuscript accepted: September 2, 2010.
ABSTRACT
Centimeter–sized crystals of struvite (MgNH4PO46H2O) were found during the drilling of a core from Chalco paleolake, Mexico for paleoecological and paleoclimatical research. Struvite is a biogenic mineral, unstable in most geological environments. The unusual size and preservation of these struvite crystals allowed us to perform a chemical and mineralogical characterization. This is the first record of struvite from a geological locality in Mexico, and one of the most remarkable in the world.
Key words: phosphate, X–ray diffraction, infrared spectra, thermogravimetric analysis, Rietveld refinement.
RESUMEN
Se describe la presencia de cristales centimétricos del mineral estruvita (MgNH4PO46H2O) en un núcleo de perforación del lago de Chalco, México, extraído en el marco de un estudio paleoecológico y paleoclimático. La estruvita es un fosfato de origen biogénico altamente inestable en muchos ambientes geológicos. Dada la rareza de encontrar cristales de origen natural tan bien preservados, se realizó una caracterización química y mineralógica de dicho mineral. Se reporta por primera vez la presencia de estruvita en el registro geológico de México, siendo la de Chalco una de las localidades más destacables del mundo.
Palabras clave: fosfato, difracción de rayos X, refinamiento Rietveld, análisis termogravimétrico, espectro de infrarrojo.
INTRODUCTION
Struvite is a hydrous magnesium–ammonium phosphate (MgNH4PO4·6H2O) found rarely in the geological record, but it is relatively abundant in some soils and modern lakes with phosphorus and ammonium input. Being a rather unstable and very soft mineral, it is difficult to find in ancient sediments, which have been subjected to diagenesis. Excluding the anthropogenic struvite found in sediments, lakes, archeological sites, etc., the main environment in which struvite is preserved is in guano accumulations in sediments or caves (Hutchison, 1950). Lacustrine struvite was discovered in 1980 by Johnson in sediments of Wild Cat Lake (eastern Washington). Recently formed guano accumulations on islands of the subantarctic Bounty Group are formed principally by struvite (Cullen 1988).
A core drilled for paleolimnological studies from Chalco paleolake (19°15'13" N, 98°58'39" W), near Mexico City, shows a 4 – 5 cm thick layer formed mainly by large (2 – 3 cm long) euhedral crystals of a very soft transparent–white mineral, identified at first sight as gypsum. After X–ray diffraction study, the mineral was identified as struvite. As the purity and size of these struvite crystals are very unusual, we initiated a mineralogical study presented here. Struvite was found at 80 m in the core. This is the first report of a geological occurrence of this mineral in Mexico.
GEOLOGICAL SETTING
The Chalco paleolake is situated at 2200 m a.s.l. in the Mexico basin, in the central part of Trans–Mexican Volcanic Belt (Figure 1). The Chalco sub–basin is a half graben (NNE–SSW) (Urrutia and Chávez, 1991) limited by the Sierra Nevada (E), the Sierra de Santa Catarina (N) and the Sierra de Chichinautzin (S). The lake of Chalco was formed during the Pleistocene after the emplacement of the Chichinautzin volcanic field (Ortega–Guerrero et al., 2000) and the surroundings are volcanic and volcanoclastic deposits (Bloomfield, 1975). The lacustrine plain of Chalco covers an area of about 120 km2.
Chalco was originally fed by the Amecameca and Tlalmanalco permanent rivers as well as by several seasonal streams and springs, but it was artificially drained by the end of 18th century. Now, the lake is reduced to a marshy area to the SW of Xico volcano (Caballero–Miranda, 1997).
The evolution of the lakes in the Mexico basin and the correlation of its changes with climatic parameters have been previously studied (Sears and Clisby, 1955; Bradbury, 1971, 1989). Some climatic reconstructions were made using microfossils like diatoms and ostracodes (Caballero–Miranda and Ortega–Guerrero, 1998), pollen and spores (Lozano–García et al., 1993; Lozano–García and Ortega–Guerrero, 1994) and mineral magnetic properties (Ortega–Guerrero et al., 2000). These studies are correlated with information obtained from the study of tephra layers and radiocarbon dating of organic matter (Caballero–Miranda, 1997; Ortega–Guerrero et al., 2000).
EXPERIMENTAL PROCEDURE
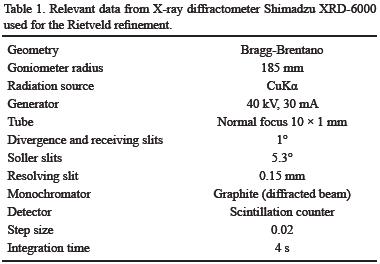
Two samples of struvite were ground with an agate pestle and mortar to <75 μm and mounted in aluminum holders for X–ray powder diffraction analysis. A Shimadzu XRD–6000 X–ray diffractometer equipped with a Cu tube and graphite monochromator was used. Phase identification was made with a PDF–2 database using Shimadzu software. Rietveld refinement of the data was done with TOPAS© Academic v. 4.1 software (http://www.topas–academic.net). Relevant data from the diffractometer used in the refinement are shown in Table 1.
Chemical analysis were performed on the fraction selected for X–Ray diffractometry. Total N and C were measured with a Perkin Elmer 2400 series II elemental analyzer using helium as carrier gas. The elemental composition of struvite was analyzed with a Siemens 3000 X–ray fluorescence spectrometer. The powder was fused with a 50:50 mixture of lithium metaborate plus lithium tetraborate in a Claisse® oven. Calibration of major elements was done with international reference materials using routine procedures for minerals and rocks.
Isotopic composition of nitrogen and carbon were obtained using a Delta Plus XL isotope ratio mass spectrometer. The δ15N values are reported relative to air; reproducibility was within 0.2 ‰ based on multiple analyses of reference materials (IAEAN1, USGS 25 y USGS 26). δ13C values are reported relative to Vienna PDB ; reproducibility was within 0.15 ‰ based on multiple analyses of reference materials (NBS22, NBS18, IAEA CH6, IAEA CH7).
Fourier Transform infrared (FT–IR) spectrum was recorded on a Bruker Tensor 27 infrared interferometer. The spectral range of the instrument was 4000–650 cm–1 and the working resolution was better than 1 cm–1.
Thermogravimetric and differential thermal analyses were done with a Mettler Toledo 851e and STAR 8.1 software. Samples were weighed in open alumina panels of 70 (il. Heating was performed between 25 °C and 200 °C at rates of 1 °C min–1 and 20 °C min–1 in air atmosphere.
X–ray diffraction, X–ray fluorescence, elemental and isotopic analyses were made at Instituto de Geología, Universidad Nacional Autónoma de México. Infrared analysis was made at Instituto de Investigaciones Metalúrgicas, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, Mexico. Thermogravimetric–differential thermal analyses were done at USAI of Facultad de Química, Universidad Nacional Autónoma de México.
RESULTS AND DISCUSSION
Crystal morphology
The struvite crystals are euhedral (1–3 cm long) and colorless or gray (Figure 2). The habit of the struvite crystals is tabular and similar to crystals described by Wevers et al. (1981) from archaeological sites of the Middle Ages in Amsterdam. All crystals are terminated by striated irregular faces. Twins were not observed.

X–ray powder diffraction (XRD)
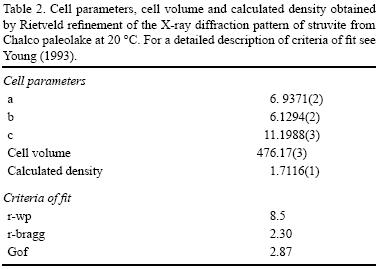
Diffraction patterns showed a pure struvite (MgNH4PO4·6H2O) phase that matched very well with PDF–2 reference sample 015–0762. Very minor phases, 0.9 % in weight, of newberyite (MgHPO4·3H2O) and 0.6 % in weight of monetite (CaHPO4) were calculated by Rietveld refinement done with Topas© Academic. This software implements the fundamental parameters approach (FPA, Cheary and Coelho, 1992). Instrumental data for FPA (full axial divergence model, Cheary and Coelho, 1998a, 1998b) are shown in Table 1. The specimen–dependent parameters which were refined were the zero error, displacement error, Chebyshev polynomial fitting for the background with six coefficients, cell parameters, crystallite size, microstrain, atomic coordinates and isotropic temperature factors. The obtained values for cell parameters and calculated density are summarized in Table 2. Chemical occupancy was fixed from X–ray fluorescence and elemental analyzer results (Table 3). not been resolved at the time of writing. We conclude that the exceptionally large crystals from Chalco paleolake are indeed struvite crystals without significant mineralogical transformation.
Chemical composition
Struvite is a low stability mineral that is easily altered by volatile loss, mainly H2O, but also N2 (Bhuiyan et al., 2008). Struvite may transform to newberyite as a result of NH4 volatilization (Boistelle et al., 1983). Heating struvite at temperatures as low as 50 °C can cause irreversible transformations. Due to the inevitable drying of samples at 110 °C before X–ray fluorescence sample preparation (pearl fusion), the complete chemical composition was derived from three analytical procedures, namely, X–ray fluorescence (XRF) for cations, Elemental Analyzer for both C and N and loss of weight after heating for water. Each type of analysis was done in a different aliquot, but for XRF we used the same powder previously measured by XRD.
Table 3 shows the chemical composition obtained from combining the aforementioned methods. Total percentage weight was adjusted to 100% in order to account for differences between the calculation procedure for the three analytical methods (oxides, elements and volatiles). There is good agreement between the theoretical struvite composition (Table 3) and our results, with only minor discrepancies for some elements, which can be adscribed to chemical impurities in struvite. It is also possible that they constitute independent minerals, not shown by the X–ray diffraction technique, as clays, amorphous silica or fluid inclusions.
Isotopic composition
The δ15N value of struvite is +9.24 %o and their δ13C is –17.4 %o. Lakes with this isotopic composition of N are eutrophised with natural nutrient inputs, as for example in Karkul Lake on Kodiak Island Alaska (Finney et al., 2002), or by human activities as for example in Baldeggersee lake in Switzerland (Teranes and Bernasconi, 2000).
Struvite is a mineral formed by the decomposition in a wet environment of guano and other fecal substances. Stable isotopes of nitrogen are a powerful tool for estimating the trophic position of the organism in the food webs because the δ15N value of an animal tissue (including urine and fecal material) depends on its diet (DeNiro and Epstein, 1981). The δ15N value in a consumer is typically enriched by 2—4 %o relative to its diet (DeNiro and Epstein, 1981). Urinary urea has nitrogen isotopic composition 2 to 4% more negative that the diet of the organism (Ambrose, 1991). In this sense we consider that struvite with δ15N of 9 % was probably formed from the waste of animals with δ15N values on the range of 11 to13 %o.
In terrestrial ecosystems, the relation between diet and isotopic composition of N is less evident that in ocean food webs (Sharp, 2007). It is probable that the elevated values of δ15N indicate an organism with at least a partial animal diet, therefore a secondary consumer. Given the lacustrine environment of Chalco, it is possible that this secondary consumer was a bird. Kitchell et al. (1999), reported that some waterfowl excreted about 60% of their consumed nutrients in waters and that these animals are grouped in feeding aggregations during migration, producing water eutrophication.
The value of δ13C (–17.4%) is consistent with an organic origin for struvite. In herbivores, the C isotopic composition of excreta reflects the C isotopic composition (C3 or C4 plants) of their food source (Haines, 1976), but this correlation is very difficult to discern for omnivore organisms.
Infrared spectra
The fityk (Wojdyr, 2010) software was used for infrared data analysis. It is a free program for nonlinear least square curve fitting. Band fitting (Figure 4 and Table 4) was done using the minimum number of Gaussian functions needed to adjust the whole profile. The squared regression coefficient (r2) was 0.99988.
The infrared spectrum of struvite between 650 and 4000 cm–1 (15.4 – 2.5 μm) is shown in Figure 4. Intense bands at 747, 888, 980, 1430, 2341, 2889 and 2978 cm–1 are clearly seen. The last three are located at the center of a broad (2000 to 3800 cm–1) asymmetric band (Soptrajanov et al., 2004, Stejov et al., 2005). The assignment of bands is not simple because IR spectra of natural struvite is quite complicated compared to the synthetic phase; some bands are temperature and composition sensitive (Stejov et al., 2005) and significant overlap exists between the individual absorption lines.
TGA–DTA
The thermogravimetric analysis (TGA) of struvite was performed at two different heating rates, 1°C min–1 and 20°C min–1 (Figure 5) on the same powder used previously for X–ray diffraction. The TGA and DTGA curves for struvite are shown in Figure 5. These data indicate that mass loss at 1 °C min–1 heating rate began at a temperature of 42 °C, had a maximum at 75.7 °C and was essentially complete when the temperature reached about 200 °C. At this temperature, a loss of 47.9 % of the original mass has occurred. This mass loss corresponds to the following decomposition reaction for struvite (Bhuiyan et al., 2008) :
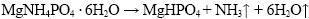
At a heating rate of 20 °C min–1 the onset of mass loss occured at 74.7 °C, with a peak at 113 °C and ended at about 151 °C. In comparison to the 1 °C min–1 heating rate, all temperatures were displaced to higher values. This behavior has been also observed by Frost et al. (2004) and Bhuiyan et al. (2008). In comparison to the data of the latter, our analyses showed a lower peak loss temperature at the same heating rate. This can be easily explained by the presence of small impurities (see above) and analyzed crystal size; both factors promote heat activated diffusion and consequent loss of volatiles. Bhuiyan et al. (2008) report also progressive amorphization of struvite with heating, from the first mass loss at about 40 °C to ~110 °C, when complete amorphization occurs. Therefore, the good crystal structure from Chalco paleolake struvite is indicative of an exceptional preservation.
Significance of struvite
The XRF, XRD, elemental analyses for N, infrared spectra and thermogravimetric analysis confirmed the presence of a 4–5 cm layer of struvite in the Chalco paleolake. Struvite is a magnesium phosphate mineral formed in anoxic humid sedimentary environments by decay or putrefaction of animal waste products. Its formation in present lakes is associated with an important input of nutrients (N, P) and eutrophication, either natural or anthropic, and is favored by elevated pH, low temperature (Ohlinger et al., 1999), high water salinity, dry climate, and low lake water level (Donovan and Grimm, 2007). In the Chalco paleolake, eutrophication and struvite formation were probably associated with large populations of migrating waterfowl.
The loss of ammonia in struvite determines the formation of newberyite, which takes place easily in both subaerial and subaqueous environments (Cohen and Ribbe, 1966; Boistelle et al., 1983). For this reason, the preservation of true struvite with only very minor transformation to newberyite (+ monetite) in the geological record is rare. We think that the described occurrence in Chalco has important palaeoecologic and palaeoclimatic information that needs a more detailed study, which is now in progress.
CONCLUSIONS
During the preliminary reconnaissance study of the core drilled in the Chalco paleolake, Mexico, centimeter–sized crystals of transparent struvite were found. This is the first geologic occurrence of this mineral in Mexico and one of the best examples of crystallized struvite ever found in the geological record. Struvite formed after guano is unstable in non–alkaline conditions and only occurs in an environment protected from the direct effects of climate (Cullen, 1988). The preservation of struvite crystals is not common and suggests lack of emergence or another cause that prevented the removal of ammonia and no exposure to oxygenated weathering conditions. We conclude that struvite was deposited during eutrophication in a Mg–rich lacustrine environment related to a dry period and large populations of migratory waterfowl.
ACKNOWLEDGEMENTS
We acknowledge Patricia Girón for the X–ray fluorescence analyses, Edith Cienfuegos for the isotopic determinations of N and C, Kumiko Shimada for the elemental determination of N and C, Remedios Cisneros Magaña for the infrared spectra determination and Margarita Portilla for thermogravimetric analysis. This research was supported by UNAM–DGAPA IN2206093 and IN–DGAPA118109. Authors thanks to Joseph J. Donovan of West Virginia University, USA and Ray L. Frost of Queensland University of Technology of Brisbane, Autralia, whose comments and suggestions greatly improved the manuscript.
REFERENCES
Ambrose, S.H., 1991, Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial fooodweb: Journal of Archaeological Science, 18, 293–317. [ Links ]
Bhuiyan, M.I.H., Mavinic, D.S., Koch, F.A., 2008, Thermal decomposition of struvite and its phase transition: Chemosphere, 70(8), 1347–1356. [ Links ]
Bloomfield, K., 1975, A late Quaternary monogenetic volcanic field in Central México: Geologische Rundschau, 64, 476–497. [ Links ]
Boistelle, R., Abbona, F., Lundager–Madsen, H.E., 1983, On the transformation of struvite in newberyite in aqueous systems: Physics and Chemistry of minerals, 9(5), 212–222. [ Links ]
Bradbury, J.P., 1971, Paleolimnology of lake Texcoco, México, Evidence from diatoms: Limnology and Oceanography, 16(2), 180–200. [ Links ]
Bradbury, J.P., 1989, Late Quaternary lacustrine paleoenviroments in the Cuenca de México: Quaternary Science Reviews, 8(1), 75–100. [ Links ]
Caballero–Miranda, M., 1997, The last glacial maximum in the Basin of Mexico: the diatom record between 34.000 and 15.000 years BP from lake Chalco: Quaternary International, 43–44, 125–136. [ Links ]
Caballero–Miranda, M., Ortega–Guerrero, B., 1998, Lake levels since about 40.000 years ago at Lake Chalco, near México City: Quaternary Research, 50(1), 69–79. [ Links ]
Cheary, R.W., Coelho, A.A., 1992, A fundamental parameters approach of a X–Ray line–profile fitting: Journal of Applied Crystallography, 25(2), 109–121. [ Links ]
Cheary, R.W., Coelho, A.A., 1998a, Axial divergence in a conventional X–Ray powder diffractometer. I. Theoretical foundations: Journal of Applied Crystallography, 31(6), 851–861. [ Links ]
Cheary, R.W., Coelho, A.A., 1998b, Axial divergence in a conventional X–Ray powder diffractometer. II Implementation and comparison with experiment: Journal of Applied Crystallography, 31(6), 862–868. [ Links ]
Cohen, L.H., Ribbe, P.H., 1966, Magnesium phosphate mineral replacement at Mono Lake, California: American Mineralogist, 51, 1755–1765. [ Links ]
Cullen, D.J., 1988, Mineralogy of nitrogenous guano on the Bounty Islands, SW Pacific Ocean: Sedimentology, 35(3), 421–428. [ Links ]
DeNiro, M.J., Epstein, S., 1981, Influence of diet on the distribution of nitrogen isotopes in animals: Geochimica et Cosmochimica Acta, 45(3), 341–351. [ Links ]
Donovan, J.J., Grimm, E.C., 2007, Episodic struvite deposits in a Northern Great Plains flyway lake: indicators of mid–Holocene drought?: The Holocene, 17(8), 1155–1169. [ Links ]
Finney, B.P., Gregory–Eaves, I., Douglas, M.S.V., Smol, J.P., 2002, Fisheries productivity in the Northeastern Pacific Ocean over the past 2200 years: Nature, 416, 729–732. [ Links ]
Frost, R.L., Weier, M.L., Erickson, K.L., 2004, Thermal decomposition of struvite: Journal of Thermal Analysis and Calorimetry, 76(3), 1025–1033. [ Links ]
Frost, R.L., Weier, M.L., Martens, W.N., Henry, D.A., Mills, S.J., 2005, Raman spectroscopy of newberyite, hannayite and struvite: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 62(1–3), 181–188. [ Links ]
Haines, E.B., 1976, Relation Between the Stable Carbon Isotope Composition of Fiddler Crabs, Plants, and Soils in a Salt Marsh: Limnology and Oceanography, 21(6), 880–883. [ Links ]
Hutchinson, G.E., 1950, Survey of contemporary knowledge of biogeochemistry 3. The biogeochemistry of vertebrate excretion: Bulletin of the American Museum of Natural History, 96, 553 pp. [ Links ]
Johnson, C.R., 1980, Mineralogy and Geochemistry of Wildcat Lake, Whitman County, Washington: Washington, Washington State University, M.S. Thesis, 131pp. [ Links ]
Kitchell, J.F., Schindler, D.E., Herwig, B.R., Post, D.M., Olson, M.H., 1999, Nutrient Cycling at the landscape scale: the role of diet foraging migration by geese at the Bosque del Apalache National Wildlife Refuge, New Mexico: Limnology Oceanography, 44(3), 828–836. [ Links ]
Lozano–García, M.S., Ortega–Guerrero, B., Caballero–Miranda, M., Urrutia–Fucugauchi, J., 1993, Late Pleistocene and Holocene paleoenviroments of Chalco lake, Central México: Quaternary Research, 40(3), 332–342. [ Links ]
Lozano–García, M.S., Ortega–Guerreo, B., 1994, Palynological and magnetic susceptibility records of lake Chalco, central México: Paleogeography, Paleoclimatology, Palaeoeocology, 109(2–4), 177–191. [ Links ]
Ohlinger, K.N., Young T.M., Schroeder, E.D., 1999, Kinetics effects on preferential struvite accumulation in wastewater: Journal of Environmental Engineering, 125(8), 730–737. [ Links ]
Ortega–Guerrero, B. , Thompson, R., Urrutia–Fucugauchi, J., 2000, Magnetic properties of lake sediments from lake Chalco, central México, and their paleoenvironmental implications: Journal of Quaternary Science, 15(2), 127–140. [ Links ]
Sears, B.H., Clisby, K.H., 1955, Palynology in southern North America Part IV: Pleistocene climate in Mexico: Bulletin of the Geological Society of America, 66(5), 521–530. [ Links ]
Sharp, Z., 2007, Principles of Stable Isotope Geochemistry: New Jersey, Pearson Prentice Hall, 344 pp. [ Links ]
Soptrajanov, B., Stefov, V., Lutz, H.D., Engelen, B., 2004, Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (Struvite) and its isomorphous analogues, in Faulques, E.C., Perry, D.L., Yeremenko, A.V. (eds.), NATO Science Series II: Mathematics, Physics and Chemistry: Spectroscopy of Emerging Materials: Netherlands, Springer, 165, 299–308. [ Links ]
Stefov, V., Soptrajanov, B., Kuzmanovski, I., Lutz, H.D., Engelen, B., 2005, Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues, III: Spectra of protiated and partially deuterated magnesium ammonium phosphate hexahydrate: Journal of Molecular Structure, 752, 60–67. [ Links ]
Teranes, J.L., Bernasconi, S.M., 2000, The record of nitrate utilization and productivity limitation provided by 515N values in lake organic matter; a study of sediment trap and core sediments from Baldeggersee, Switzerland: Limnology and Oceanography, 45(4), 801–813. [ Links ]
Urrutia, J.F., Chávez, R.S., 1991, Gravity modeling of lake basin structure: the lakes of Xochimilco and Chalco, southern basin of Mexico, in 64th Annual International Meeting: United States of America, Society of Exploration Geophysics (SEG) Annual Proceedings, Expanded Abstracts Book, 94, 655–657. [ Links ]
Wevers, J.M.A.R., Kars, H., Schuiling, R.D., 1981, A struvite occurrence in Amsterdam: Bulletin de Mineralogie 104(5), 686–689. [ Links ]
Wojdyr M., 2010, Fityk: a general–purpose peak fitting program: Journal of Applied Crystallography, 43, 1126–1128. [ Links ]
Young, R.A., 1993, The Rietveld Method: United States, Oxford University Press, 312 pp. [ Links ]














